Open Access Article
Open Access ArticleHierarchical branched α-MnO2: one-step synthesis and catalytic activity†
Hengfa Liu,
Bentian Zhang,
Wanping Li,
Gao Cheng,
Jiaxi Han,
Bang Lan,
Ming Sun * and
Lin Yu
* and
Lin Yu
Key Laboratory of Clean Chemistry Technology of Guangdong Regular Higher Education Institutions, School of Chemical Engineering and Light Industry, Guangdong University of Technology, Guangzhou, 510006, P. R. China. E-mail: sunmgz@gdut.edu.cn; Fax: +86-20-39322231; Tel: +86-20-39322202
First published on 2nd October 2017
Abstract
Hierarchical pine tree-like α-MnO2 architectures are prepared by a facile one-step method without any template. The hierarchical α-MnO2 is composed of branched α-MnO2 short nanorods assembled onto the backbone of an α-MnO2 long nanowire. Time-dependent experiment shows that the growth process of the branched α-MnO2 nanostructures is the formation of β-MnOOH and then the transformation to a α-MnO2 branched structure in a step by step manner. Owing to its specific structure features, the hierarchical pine tree-like α-MnO2 exhibits superior catalytic performance in the catalytic combustion of dimethyl ether with a light-off temperature (T10) at 169 °C and a complete conversion temperature (T90) of 235 °C, which is far better than commercial MnO2.
1 Introduction
The rational design and preparation of hierarchical structures has great significance because of their morphology and size dependent properties. The hierarchical architectures possess multifunctional and collective properties, and have found wide applications in heterogeneous catalysis, photo-catalysis and electrochemistry.1 Therefore, much efforts have been focused on this special functional material.As a transition metal oxide with rich sources and wide usages, the MnO2 material has attracted great interest due to its excellent physical and chemical properties.2–4 And such properties are highly dependent on the morphology and crystalline structure of MnO2. To date, many efforts have been made to synthesize MnO2 with different morphology and crystal phase aiming to explore better activities. Also, there are some reports on the hierarchical MnO2 based architectures. Careful study the reported papers we can find that: (1) the preparation of hierarchical MnO2 usually involves multistep process,5–7 or using template assistance,8–11 or with metal ions assistance;12–14 (2) the morphology of the obtained MnO2 materials is mainly confined to microsphere via hydrothermal process which is energy consuming, for example, α-MnO2,15–17 γ-MnO2,12,14,18,19 δ-MnO2,20,21 OMS-1,22 ε-MnO2.23 The hierarchical MnO2 with other shape instead of sphere is rare except the star-like24 and the branched α-MnO2,11,25,26 the β-MnO2 nanopincer,27 star-like ε-MnO2,28 and the δ-MnO2 hollow nanobox;29 Therefore, it is still a challenge to develop an easy and temple-free method to prepare hierarchical MnO2 with new morphology other than the commonly obtained sphere-like shape. The complexity and creativity in structure may bring new properties to the MnO2 functional materials,18 and further favoring for their potential application.
Herein, we present a facile method to prepare the α-MnO2 hierarchical architecture with pine tree-like morphology. The formation is accomplished by one-step reaction between MnCO3 and (NH4)2S2O8 in H2SO4 solution at room temperature. The strategy has the merit of one-step synthesis at room temperature without any template or chelating ligand. As a typical example, the prepared pine tree-like α-MnO2 has been evaluated as catalyst for the catalytic combustion of dimethyl ether (DME), and exhibited better performance than that of the commercial MnO2.
2 Experimental
The MnCO3 were obtained from Aladdin, the (NH4)2S2O8 and condensed H2SO4 (98 wt%) were bought from Guangzhou Chemical Agent Factory. They were used as received. In a typical synthesis, 20 mmol (NH4)2S2O8 and 20 mL H2SO4 were dissolved in 400 mL distilled water to form the transparent solution in a beaker, then 20 mmol MnCO3 was introduced. The solution was magnetically stirring at room temperature for 54 h. Afterward, the products were filtered and washed with ethanol solution and dried at 60 °C for further use.A Brucker D8-advance X-ray diffractometer was used to test the crystal structure of the products, operating at 40 kV, and 40 mA at a scan rate of 4° min−1. The morphology of the MnO2 were studied on a scanning electron microscopy (SEM, HITACHI SU8010) and a transmission electron microscopy (TEM, FEI, Tecnai G2 F20). The Mn 2p, O 1s spectra were obtained on the X-ray photoelectron spectroscopy (XPS, Thermo ESCALAB 250) equipped with a monochromatic Al Kα (1486.6 eV) X-ray source. The temperature programmed reduction of hydrogen (H2-TPR) of MnO2 was tested on Autochem 2920 (Micromeritics) instrument, 30 mg of sample was used and pretreated at 200 °C in a flow of Ar for 1 h. Afterwards, the catalyst was heated from 50 °C to 600 °C at a rate of 10 °C min−1 in a flowing 5 vol% H2/Ar mixture (50 mL min−1).
The catalytic combustion of dimethyl ether was performed in a continuous flow fixed-bed reactor (8 mm i.d.). The loading amount of MnO2 was 100 mg with 100 mg of quartz sands. A gas mixture of DME, oxygen and helium were co-feed into the reactor with the volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40, and the total gas hourly space velocity was 30
40, and the total gas hourly space velocity was 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1. The out-flow gas was analyzed by gas chromatograph (Agilent 6820).
000 h−1. The out-flow gas was analyzed by gas chromatograph (Agilent 6820).
3 Results and discussion
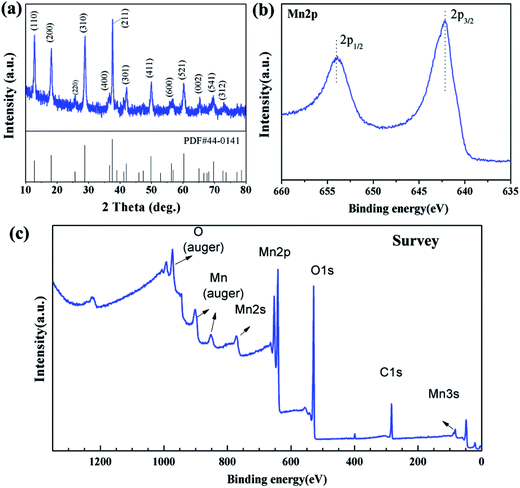
The pine tree-like manganese oxide was synthesized by a one-step solution route. The crystal structure of the as-prepared products (54 h) was investigated by X-ray powder diffraction (XRD). As shown in Fig. 1a, all the peaks can be indexed to pure α-MnO2 (JCPDS 44-0141) without any impurity. The product was further characterized by XPS to test the element composition and chemical state. The survey and Mn 2p spectra are displayed in Fig. 1c and b, respectively. The survey spectrum shows characteristic peaks for Mn, O and C, which is similar to that reported.30 In Fig. 1b, we can see clearly two major XPS peaks of Mn 2p3/2 at 642.2 eV and Mn 2p1/2 at 654.0 eV, which confirmed the presence of MnO2.31,32The morphology of the final product was studied by SEM and TEM as displayed in Fig. 2. The SEM images in Fig. 2a show that the α-MnO2 displays perfect pine tree-like nanostructures with high dispersity. On closer inspection (Fig. 2b and c), the hierarchical branched α-MnO2 structure is composed by a long nanowire as backbone with homogenously covered short nanorods having a uniform length of ca. 322 nm (ESI, Fig. S1,† based on 50 branched pedals selected randomly in 5 SEM images). The length of pine tree-like nanostructures extends to several micrometers, resulting in exceptionally large aspect ratio. The detailed structure of the branched α-MnO2 was further investigated using TEM (Fig. 2d) and HRTEM (Fig. 2e and f). As displayed in Fig. 2d, the core nanowire is wrapped by numerous nanorods interconnected with each other. The diameter of the core nanowire is about 200 nm. The HRTEM of the branch nanorod (Fig. 2f) demonstrates a interplanar spacing of 0.24 nm, corresponding to the (211) planes of α-MnO2. The HRTEM image of the interface of the core and the branch is shown in Fig. 2e. Across the interface, we can detect that two kind of lattices fringes of 0.31 nm and 0.24 nm, which belong to the (310) plane of α-MnO2 core nanowire and the (211) planes of α-MnO2 branch nanorod, respectively.33,34
The N2 adsorption–desorption isotherms (Fig. 3) of the sample can be assigned to the isothermals of type II according to the IUPAC classification, indicating that the pine tree-like α-MnO2 nanostructures belong to typical mesoporous material. The measured BET surface area of the α-MnO2 is 77.5 m2 g−1. Based on the pore size distribution, we can find that the hierarchical branched α-MnO2 has rich pore structures, locating at around three narrow ranges of 3.7, 7.6, and 12.4 nm, and a wide range from 15 to 100 nm.
 | ||
| Fig. 3 The N2 adsorption–desorption isotherms and pore size distribution of the hierarchical branched α-MnO2. | ||
To explore the formation process of the pine tree-like nanostructure, time-dependent experiment was carried out and the corresponding SEM images are exhibited in Fig. 4. At the first hour, we can see that nanorod or wire-like MnOOH is formed, however, the chemical source MnCO3 still remains in the reaction system as marked by the red arrow in Fig. 4a (the morphology of the MnCO3 is provide in the ESI, Fig. S2,† showing plate packed structure). When the reaction time is 4 h, the obtained product demonstrates the typical shape of nanowires of several micrometers with smooth surface (Fig. 4b). As the time increased to 15 h, very tiny nanorods are formed over the surfaces of the nanowires (Fig. 4c). The tiny nanorods are continuously to grow richer and bigger with reaction time rising to 26 h (Fig. 4d). From the inset figure in Fig. 4d, the smooth surface of the nanowire becomes rough, and even with small hole formed. When the reaction time is 37 h, the short nanorods are continuously deposited over the β-MnOOH nanowires surface forming branched structure (Fig. 4e). The inset figure in Fig. 4e demonstrates that the branched petals are becoming condensed. Further elongating the reaction time to 48 h, the hierarchical branched product turns to more complex and the size become even bigger (Fig. 4f). The pine tree-like morphology began to form. Finally, the pure phase of α-MnO2 with fine hierarchical architecture is obtained when the reaction time is 56 h (Fig. 2).
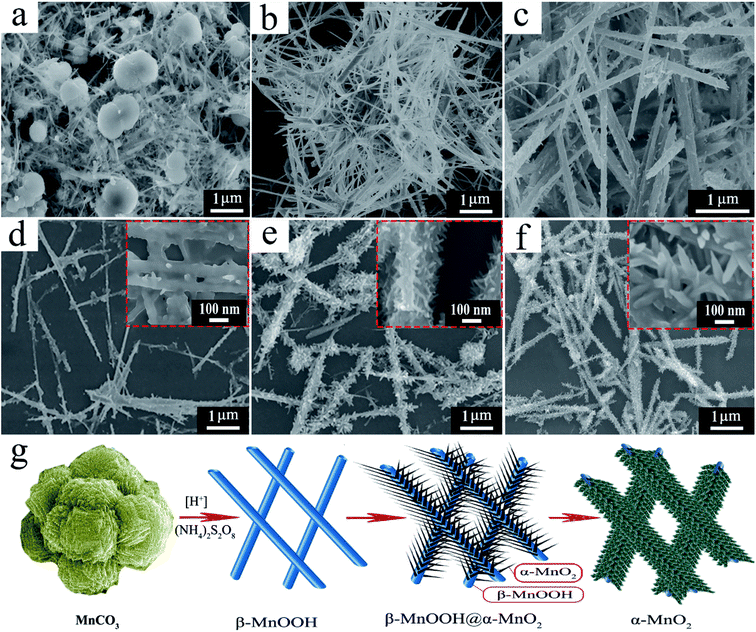 | ||
| Fig. 4 SEM images of the sample at different stage: (a) 1 h, (b) 4 h, (c) 15 h, (d) 26 h, (e) 37 h (f) 48 h; and (g) schematic illustration of the synthetic process of the α-MnO2. | ||
The crystal structures of the intermediate product at certain stage are studied by XRD, and the results are exhibited in Fig. 5. At a time of 4 h, the nanowire product shows a pure β-MnOOH phase (JCPDS 88-0649) as depicted in Fig. 5a. The sharp and intense diffraction peaks indicate that the product has good crystallinity. As the time rise to 26 h (Fig. 5b), the intensity of the β-MnOOH peaks become weaken, while new peaks of (200), (310), (211), (301), (411) and (541) are forming, and they are belonging to the α-MnO2 (JCPDS 44-0141). According to the corresponding SEM image in Fig. 4d, the tiny nanorod around the surface of the MnOOH has a crystal structure of α-MnO2. When the reaction time rises to 48 h, the dominate structure evolves into α-MnO2 with minority phase of β-MnOOH nanowire as proved by the XRD analysis (Fig. 5c). Finally, at 56 h, pure phase of α-MnO2 are synthesized without any impurity (Fig. 1a).
In the formation of the pine tree-like hierarchical branched α-MnO2, the role of MnCO3 is vital important. When we change MnCO3 into other source of Mn2+, we can not obtain the pine-tree like α-MnO2. Fig. S3† lists the SEM images by using Mn(NO3)2 and MnSO4 instead of MnCO3 at the same reaction conditions. We can clearly see that they show the shape of 3D urchin sphere composed by short nanorod, which is commonly observed in the reported literatures.17–19,35
In our reaction system, the MnCO3 is in solid state, and this is different from other commonly used Mn2+ source, which can be dissolved in the solvent. With condensed H2SO4 present in the reaction system, the MnCO3 will erode by the acid gradually and produce soluble MnSO4 (many bubbles of CO2 are observed at the first stage of the reaction; under the reaction conditions, the MnCO3 could be observed in the beginning 1 h). At the same time, the newly formed MnSO4 would react with S2O82− to obtain MnOOH firstly (eqn (1)), as proved by the SEM image is Fig. 4a. This is because that the dissolve of MnCO3 consuming large amount of acid, and less acid reduce the oxidation ability of the reaction system, the MnOOH will form preferentially.32 The formation of MnOOH is very quick (less in a hour) with extra H+ forming. Having enough H+ in the reaction system, the Mn2+ can be further oxided to MnO2 under the assistance of NH4+. Then two parallel reaction might happen: (a) the MnOOH could transform to MnO2 under the work of acid through a dissolution–recrystallization process (eqn (3)).32,36,37 The dissolution of MnOOH is clearly demonstrated by the SEM image in Fig. 4d; (b) the remaining Mn2+ could react with S2O82− producing MnO2 (eqn (2)). To reduce the system energy, the newly formed MnO2 nano-crystal prefer to grow over the surface of MnOOH, and to use MnOOH as the seed to grow via oriented attachment.
| 2Mn2+ + S2O82− + 4H2O = 2MnOOH + 2SO42− + 6H+ | (1) |
| Mn2+ + S2O82− + 2H2O = MnO2 + 2SO42− + 4H+ | (2) |
| 2MnOOH + 2H+ = MnO2 + Mn2+ + 2H2O | (3) |
The branched α-MnO2 was examined as a catalyst for the catalytic combustion of DME. The commercial MnO2 was also evaluated for comparison, and the result is shown in Fig. 6a. Apparently, the branched α-MnO2 outperformed commercial MnO2, with a light-off temperature (T10) at 169 °C and a complete conversion temperature (T90) at 235 °C. For the commercial MnO2, the T10 is 282 °C and T90 is more than 430 °C. Considering the complete conversion temperature, the branched α-MnO2 has the higher catalytic activity than the commercial MnO2. The apparent activation energy (Ea) of the DME combustion over the two MnO2 catalysts was calculated. From Fig. 6b, the Ea for commercial MnO2 and hierarchical α-MnO2 is 87.24 and 64.98 KJ mol−1, meaning that the catalytic combustion of DME could operate more easily over the branched α-MnO2 than commercial one.
 | ||
| Fig. 6 DEM combustion performance (a), Arrhenius plots (b), H2-TPR profiles (c) and O 1s XPS spectra (d) of the two MnO2. | ||
To explain the difference in their catalytic performance, H2-TPR and XPS experiments were carried on. The reduction profile of the commercial MnO2 is similar to many of the reported MnO2, of which only one wide peak is observed.17,38 The overlapped peak of the commercial MnO2 is centered at 515 °C, which contains the reduction process from Mn4+ to Mn3+ then to Mn2+ (Fig. 6c). Whereas, the TPR profile of the hierarchical α-MnO2 is different from the commercial and the conventional α-MnO2. We can detect one sharp peak at 265 °C and another continuous peak. This is a reflection of both the reduction of Mn4+ to Mn2+ and reduction from the branched part to the backbone part of MnO2. Careful comparison of the temperature of the reduction peak, the reducibility of the two MnO2 dropped in the order of pine tree-like α-MnO2 > commercial MnO2, and this agrees well with their catalytic performance. The mobility of oxygen species has great effect on the combustion activity. The higher reducibility indicates that the branched α-MnO2 has higher mobility of the oxygen species, which would lead to a better catalytic activity.39,40 XPS measurement was used to study the oxygen species. The O 1s spectral result of the branched α-MnO2 is shown in Fig. 6d. The binding energy at 529.7 eV belongs to the lattice oxygen species and the binding energy at 531.3 eV is attributed to the surface defective oxygen.41 The Oads/Olatt molar ratios of the branched α-MnO2 is 0.84, which is larger than that of the commercial MnO2(0.27). Therefore, the pine-tree like α-MnO2 has a large amounts of surface oxygen vacancy, and this is helpful for the catalytic performance.42,43
4 Conclusions
In summary, we have developed a facile one-step strategy without any template to synthesize the hierarchical pine tree-like α-MnO2 nanostructures, which is easily scaled up for mass production. The source of Mn (MnCO3) is considered to play an important role in the formation of α-MnO2 branched architectures. Our findings not only show that the pine tree-like α-MnO2 has an outstanding catalytic activity for DME combustion, but also provide a method to synthesize novel hierarchical materials, which may possess potential applications in other fields.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (21306026, 21576054), the Scientific Program of Guangdong Province (2014A010106030, 2016A010104017, 2016B020241003, 2016B090930004), the Foundation of Higher Education of Guangdong Province (2015KTSCX027).References
- M. H. Sun, S. Z. Huang, L. H. Chen, Y. Li, X. Y. Yang, Z. Y. Yuan and B. L. Su, Chem. Soc. Rev., 2016, 45, 3479–3563 RSC.
- Z. Chen, Z. Jiao, D. Pan, Z. Li, M. Wu, C.-H. Shek, C. M. L. Wu and J. K. L. Lai, Chem. Rev., 2012, 112, 3833–3855 CrossRef CAS PubMed.
- M. M. Najafpour, M. Hołyńska and S. Salimi, Coord. Chem. Rev., 2015, 285, 65–75 CrossRef CAS.
- H. Sun, Y. Shang, K. Xu, Y. Tang, J. Li and Z. Liu, RSC Adv., 2017, 7, 30283–30288 RSC.
- W. Xiao, P. Zhou, X. H. Mao and D. H. Wang, J. Mater. Chem. A, 2015, 3, 8676–8682 CAS.
- P. Pal, S. K. Pahari, A. K. Giri, S. Pal, H. C. Bajaj and A. B. Panda, J. Mater. Chem. A, 2013, 1, 10251–10258 CAS.
- B. Zhang, G. Cheng, W. Ye, X. Zheng, H. Liu, M. Sun, L. Yu, Y. Zheng and X. Cheng, Dalton Trans., 2016, 45, 8 Search PubMed.
- D. Sun, J. Chen, J. Yang and X. Yan, CrystEngComm, 2014, 16, 10476–10484 RSC.
- M. Du, Y. Bu, Y. Zhou, Y. Zhao, S. Wang and H. Xu, RSC Adv., 2017, 7, 12711–12718 RSC.
- J. Ma, Q. Cheng, V. Pavlinek, P. Saha and C. Li, New J. Chem., 2013, 37, 722–728 RSC.
- B. Z. Yu, X. Dan Zhao, J. Luo, H. G. Zhang, Y. W. Zhu, G. Y. Jing, P. Ma, Z. Y. Ren and H. M. Fan, Adv. Mater. Interfaces, 2016, 3, 1500761 CrossRef.
- D. Y. Li, X. F. Wu and Y. F. Chen, J. Phys. Chem. C, 2013, 117, 11040–11046 CAS.
- B. Li, G. Rong, Y. Xie, L. Huang and C. Feng, Inorg. Chem., 2006, 45, 6404–6410 CrossRef CAS PubMed.
- X. Ge, J. Liu, X. Song, G. Wang, H. Zhang, Y. Zhang and H. Zhao, Chem. Eng. J., 2016, 301, 139–148 CrossRef CAS.
- P. Yu, X. Zhang, D. Wang, L. Wang and Y. Ma, Cryst. Growth Des., 2008, 9, 528–533 Search PubMed.
- Z. Li, Y. Ding, Y. Xiong, Q. Yang and Y. Xie, Chem. Commun., 2005, 918–920 Search PubMed.
- T. Lin, L. Yu, M. Sun, G. Cheng, B. Lan and Z. Fu, Chem. Eng. J., 2016, 286, 114–121 CrossRef CAS.
- D. Li, J. Yang, W. Tang, X. Wu, L. Wei and Y. Chen, RSC Adv., 2014, 4, 26796–26803 RSC.
- R. Chen, J. Yu and W. Xiao, J. Mater. Chem. A, 2013, 1, 11682 CAS.
- H. Jiang, T. Sun, C. Li and J. Ma, J. Mater. Chem., 2012, 22, 2751–2756 RSC.
- S. Bag and C. R. Raj, J. Mater. Chem. A, 2016, 4, 8384–8394 CAS.
- H. J. Cui, J. W. Shi, F. Liu and M. L. Fu, J. Mater. Chem., 2011, 21, 18527–18529 RSC.
- D. Han, X. Jing, P. Xu, Y. Ding and J. Liu, J. Solid State Chem., 2014, 218, 178–183 CrossRef CAS.
- G. Cheng, S. Xie, B. Lan, X. Zheng, F. Ye, M. Sun, X. Lu and L. Yu, J. Mater. Chem. A, 2016, 4, 16462–16468 CAS.
- X. Zhang, B. Li, X. Li, Q. Chu, M. Yang, X. Wang, H. Chen, L. Peng and X. Liu, J. Mater. Sci.: Mater. Electron., 2014, 25, 906–913 CrossRef CAS.
- X. Su, X. Yang, L. Yu, G. Cheng, H. Zhang, T. Lin and F.-H. Zhao, CrystEngComm, 2015, 17, 5970–5977 RSC.
- G. Cheng, L. Yu, T. Lin, R. Yang, M. Sun, B. Lan, L. Yang and F. Deng, J. Solid State Chem., 2014, 217, 57–63 CrossRef CAS.
- Y. S. Ding, X. F. Shen, S. Gomez, H. Luo, M. Aindow and S. L. Suib, Adv. Funct. Mater., 2006, 16, 549–555 CrossRef CAS.
- J. Zhang, Y. Luan, Z. Lyu, L. Wang, L. Xu, K. Yuan, F. Pan, M. Lai, Z. Liu and W. Chen, Nanoscale, 2015, 7, 14881–14888 RSC.
- J. W. Long, J. M. Wallace, G. W. Peterson and K. Huynh, ACS Appl. Mater. Interfaces, 2016, 8, 1184–1193 CAS.
- Y. Li, Y. Li, Y. Wan, S. Zhan, Q. Guan and Y. Tian, RSC Adv., 2016, 6, 54926–54937 RSC.
- M. Sun, B. Lan, T. Lin, G. Cheng, F. Ye, L. Yu, X. Cheng and X. Zheng, CrystEngComm, 2013, 15, 7010 RSC.
- B. Zhang, G. Cheng, B. Lan, X. Zheng, M. Sun, F. Ye, L. Yu and X. Cheng, CrystEngComm, 2016, 18, 6895–6902 RSC.
- H. Li, W.-l. Wang, F. Pan, X. Xin, Q. Chang and X. Liu, Mater. Sci. Eng., B, 2011, 176, 1054–1057 CrossRef CAS.
- Y. Wang, M. Liu, K. Li, A. Zhang and X. Guo, CrystEngComm, 2015, 17, 3636–3644 RSC.
- M. Ramstedt and S. Sjöberg, Aquat. Geochem., 2005, 11, 413–431 CrossRef CAS.
- D. Portehault, S. Cassaignon, E. Baudrin and J.-P. Jolivet, Chem. Mater., 2007, 19, 5410–5417 CrossRef CAS.
- G. Cheng, L. Yu, B. He, M. Sun, B. Zhang, W. Ye and B. Lan, Appl. Surf. Sci., 2017, 409, 223–231 CrossRef CAS.
- H. Sun, Z. Liu, S. Chen and X. Quan, Chem. Eng. J., 2015, 270, 58–65 CrossRef CAS.
- Q. Ye, H. Lu, J. Zhao, S. Cheng, T. Kang, D. Wang and H. Dai, Appl. Surf. Sci., 2014, 317, 892–901 CrossRef CAS.
- V. P. Santos, M. F. R. Pereira, J. J. M. Orfao and J. L. Figueiredo, Appl. Catal., B, 2010, 99, 353–363 CrossRef CAS.
- S. H. Xie, J. G. Deng, S. M. Zang, H. G. Yang, G. S. Guo, H. Arandiyan and H. X. Dai, J. Catal., 2015, 322, 38–48 CrossRef CAS.
- M. Sun, L. Yu, F. Ye, G. Q. Diao, Q. Yu, Z. F. Hao, Y. Y. Zheng and L. X. Yuan, Chem. Eng. J., 2013, 220, 320–327 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra08567b |
| This journal is © The Royal Society of Chemistry 2017 |