Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A phosphine-free, atom-efficient cross-coupling reaction of triorganoindiums with acyl chlorides catalyzed by immobilization of palladium(0) in MCM-41†
Jiankang Miao,
Bin Huang,
Haiyi Liu and
Mingzhong Cai *
*
Key Laboratory of Functional Small Organic Molecules, Ministry of Education, College of Chemistry & Chemical Engineering, Jiangxi Normal University, Nanchang 330022, P. R. China. E-mail: mzcai@jxnu.edu.cn
First published on 4th September 2017
Abstract
The first phosphine-free heterogeneous palladium(0)-catalyzed cross-coupling of triorganoindiums with acyl chlorides has been developed that proceeds smoothly in THF at 68 °C and provides a general and powerful tool for the synthesis of various valuable aryl ketones and α,β-acetylenic ketones with high atom-economy and high yield. This phosphine-free heterogeneous palladium(0) catalyst can be easily prepared from commercially available reagents and recovered by a simple filtration of the reaction solution and used for at least 10 consecutive trials without any decreases in activity. Our system not only avoids the use of phosphine ligands, but also solves the basic problem of palladium catalyst recovery and reuse.
1. Introduction
Both aryl ketones1 and α,β-acetylenic ketones2 are important building blocks for a large number of natural products and pharmaceutical compounds. The development of various approaches to these unsymmetrical ketones is of great interest and many methods for their preparation have been reported. One general and straightforward route to aryl ketones is the Friedel–Crafts acylation of aryl cycles with acid halides or acid anhydrides.3 However, this methodology suffers from some drawbacks such as the use of more than a stoichiometric amount of aluminum trichloride, which is incompatible with many functional groups, production of a large amount of highly toxic and corrosive waste, and narrow scope of substrates. Traditionally, α,β-acetylenic ketones are usually prepared via the acylation of alkynylmetal reagents with acid chlorides4 or palladium-catalyzed coupling of acyl chlorides with terminal alkynes.5 Generally, the preparation of unsymmetrical ketones by transition-metal-catalyzed cross-coupling reaction is severely limited, since the organometallic partners often react with the product ketone.The development of novel transition-metal-catalyzed cross-coupling reactions with high degrees of conversion of the organometallic species, high chemoselectivity, and the ability to form carbon–carbon bonds between all of the different carbon types (sp3, sp2, and sp) is still of considerable interest. Besides, the minimization of byproduct formation6 and the choice of chemical processes with little or no risk associated to humans or the environment are major contemporary concerns in the chemistry community.7 Some organometallic reagents used in cross-coupling reactions have shown limitations concerning the use of alkyl (sp3) organometallics as coupling partners, the toxicity associated with the metal, and undesirable side reactions. During the past two decades, the applications of indium in organic synthesis have increased steadily due to the apparent low toxicity8 associated with indium as well as other interesting chemical properties, such as low nucleophilicity and heterophilicity or similarities with magnesium, zinc, and tin.9 Triorganoindium compounds (R3In, R = alkyl, alkenyl, alkynyl, aryl) are increasingly gaining attention as efficient coupling partners for transition-metal-catalyzed cross-couplings with a variety of organic electrophiles such as aryl (or alkenyl) halides (or pseudohalides) and acid chlorides10 and the carbonylative cross-couplings11 because of their high efficiency, versatility, atom-economy, and chemoselectivity. However, in most cases, homogeneous palladium complexes such as Pd(PPh3)4, PdCl2(PPh3)2, and Pd(dppf)Cl2 are usually used as catalysts for these cross-coupling reactions. The problem with homogeneous catalysis is the difficulty to separate the expensive palladium catalyst from the reaction mixture and the impossibility to reuse it in consecutive reactions. In addition, homogeneous catalysis might result in unacceptable heavy metal contamination of the desired isolated product due to the leaching of the metal. These problems may have a very serious negative impact on their possible industrial applications, especially the pharmaceutical industry. In contrast, heterogeneous catalysts can be easily separated from the reaction mixture by a simple filtration of the reaction solution and reused in successive reactions provided that the active sites have not become deactivated. Heterogeneous catalysis also helps to reduce wastes derived from reaction workup, contributing to the development of green and sustainable chemical processes.12 From the viewpoints of economical and environmental concern, the development of immobilized transition-metal catalysts is challenging and important. So far, supported palladium catalysts have successfully been used in many carbon–carbon and carbon–heteroatom bond formation reactions,13 however, to the best of our knowledge, no example of a heterogeneous palladium-catalyzed cross-coupling reaction of triorganoindiums with organic electrophiles has been described until now.
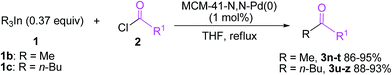
The discovery of mesoporous MCM-41 materials has provided a new possible candidate for an ideal solid support to immobilize homogeneous catalysts and given an enormous stimulus to research in heterogeneous metal catalysis.14 The hexagonally-ordered MCM-41 material possesses large and uniform pore size, ultrahigh surface area, big pore volume and rich silanol groups in the inner walls.15 To date, some functionalized MCM-41-supported palladium,16 rhodium,17 molybdenum,18 gold19 and copper20 complexes have been successfully used as potentially green and sustainable catalysts in organic reactions. Recently, we have reported the first preparation of an MCM-41-immobilized palladium(II)–Schiff base complex [MCM-41-N,N-Pd(OAc)2] and its successful application to the atom-efficient cross-coupling of triarylbismuths with aryl iodides.21 In continuing our efforts to develop greener synthetic pathways for organic transformations,16d–f,19d,20 herein we wish to report the first phosphine-free heterogeneous palladium(0)-catalyzed cross-coupling reaction of triorganoindiums with acid chlorides by using an MCM-41-immobilized palladium(0)–Schiff base complex [MCM-41-N,N-Pd(0)] as a recyclable catalyst. The reactions proceeded in THF smoothly under mild conditions, yielding a variety of aryl ketones and α,β-acetylenic ketones in good to excellent yields with high atom-economy and recyclability of the catalyst (Scheme 1).
 | ||
| Scheme 1 Synthesis of aryl ketones and α,β-acetylenic ketones via heterogeneous Pd(0)-catalyzed cross-coupling of triorganoindiums with acid chlorides. | ||
2. Results and discussion
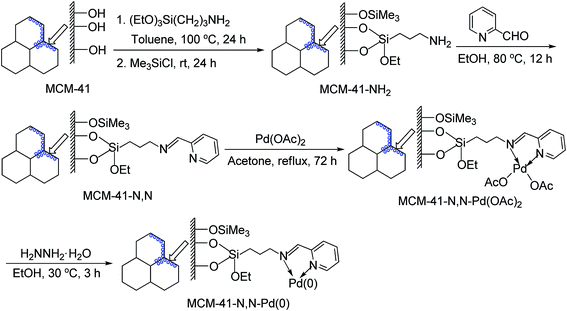
Although phosphine ligands can stabilize palladium and influence its catalytic activity, undoubtedly, the simplest and cheapest palladium catalysts are still the phosphine-free systems. Furthermore, the procedure for the synthesis of immobilized phosphine palladium complexes is rather complicated since the preparation of the heterogeneous phosphine ligands requires multi-step sequences. Therefore, the development of phosphine-free supported palladium complex catalysts having a high activity and good stability is a topic of enormous importance. The MCM-41-immobilized palladium(II)–Schiff base complex [MCM-41-N,N-Pd(OAc)2] could easily be prepared starting from mesoporous material MCM-41 and commercially available 3-aminopropyltriethoxysilane, pyridine-2-carboxaldehyde, and Pd(OAc)2 as shown in Scheme 2 according to our previous procedure.21 Firstly, the mesoporous MCM-41 was condensed with 3-aminopropyltriethoxysilane in toluene at 100 °C for 24 h, followed by the silylation with Me3SiCl in toluene at room temperature for 24 h to give 3-aminopropyl-functionalized MCM-41 material (MCM-41-NH2). The latter was subsequently treated with pyridine-2-carboxaldehyde in dry EtOH at 80 °C for 12 h to afford the Schiff base-functionalized MCM-41 (MCM-41-N,N), which was then reacted with Pd(OAc)2 in acetone under reflux for 72 h to generate the MCM-41-immobilized palladium(II)–Schiff base complex [MCM-41-N,N-Pd(OAc)2] as a light yellow powder. Finally, the treatment of MCM-41-N,N-Pd(OAc)2 with hydrazine hydrate in EtOH at 30 °C for 3 h afforded the MCM-41-immobilized palladium(0)–Schiff base complex [MCM-41-N,N-Pd(0)] as a gray powder.Elemental analyses and X-ray photoelectron spectroscopy (XPS) were used to characterize the MCM-41-immobilized palladium(0)–Schiff base complex. The N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pd mole ratio of the MCM-41-N,N-Pd(OAc)2 was determined to be 5.2. The XPS data for MCM-41-N,N-Pd(0) (fresh), MCM-41-N,N-Pd(OAc)2, MCM-41-N,N, Pd(OAc)2, metal Pd, and MCM-41-N,N-Pd(0) (used) are listed in Table 1. It can be seen that the binding energies of Si2p and O1s of MCM-41-N,N-Pd(OAc)2 were similar to those of MCM-41-N,N. However the difference of N1s binding energies between MCM-41-N,N-Pd(OAc)2 and MCM-41-N,N was 1.1 eV. The binding energy of Pd3d5/2 in MCM-41-N,N-Pd(OAc)2 was 0.9 eV less than that in Pd(OAc)2, but 1.9 eV larger than that in metal Pd. These results showed that a coordination bond between N and Pd was formed in MCM-41-N,N-Pd(OAc)2 and the oxidation state of palladium in MCM-41-N,N-Pd(OAc)2 was Pd(II).22 The binding energy (336.2 eV) of Pd3d5/2 of the freshly prepared MCM-41-N,N-Pd(0) was lower than the binding energy (337.4 eV) of Pd3d5/2 of MCM-41-N,N-Pd(OAc)2. The Pd3d5/2 binding energy depends strongly on the nature of the ligands. Consequently, it is impossible to identify the reduced complex as a zerovalent one on the basis of its Pd3d5/2 binding energy only. However, the shift (lower) of Pd3d5/2 binding energy together with the change in color from light yellow to gray suggests that the reduction of the starting palladium(II) complex to the zerovalent state has taken place. The binding energy of Pd3d5/2 of the used MCM-41-N,N-Pd(0) catalyst was determined to be 336.3 eV, which indicating that the oxidation state of palladium in used MCM-41-N,N-Pd(0) catalyst after reaction was still Pd(0).
Pd mole ratio of the MCM-41-N,N-Pd(OAc)2 was determined to be 5.2. The XPS data for MCM-41-N,N-Pd(0) (fresh), MCM-41-N,N-Pd(OAc)2, MCM-41-N,N, Pd(OAc)2, metal Pd, and MCM-41-N,N-Pd(0) (used) are listed in Table 1. It can be seen that the binding energies of Si2p and O1s of MCM-41-N,N-Pd(OAc)2 were similar to those of MCM-41-N,N. However the difference of N1s binding energies between MCM-41-N,N-Pd(OAc)2 and MCM-41-N,N was 1.1 eV. The binding energy of Pd3d5/2 in MCM-41-N,N-Pd(OAc)2 was 0.9 eV less than that in Pd(OAc)2, but 1.9 eV larger than that in metal Pd. These results showed that a coordination bond between N and Pd was formed in MCM-41-N,N-Pd(OAc)2 and the oxidation state of palladium in MCM-41-N,N-Pd(OAc)2 was Pd(II).22 The binding energy (336.2 eV) of Pd3d5/2 of the freshly prepared MCM-41-N,N-Pd(0) was lower than the binding energy (337.4 eV) of Pd3d5/2 of MCM-41-N,N-Pd(OAc)2. The Pd3d5/2 binding energy depends strongly on the nature of the ligands. Consequently, it is impossible to identify the reduced complex as a zerovalent one on the basis of its Pd3d5/2 binding energy only. However, the shift (lower) of Pd3d5/2 binding energy together with the change in color from light yellow to gray suggests that the reduction of the starting palladium(II) complex to the zerovalent state has taken place. The binding energy of Pd3d5/2 of the used MCM-41-N,N-Pd(0) catalyst was determined to be 336.3 eV, which indicating that the oxidation state of palladium in used MCM-41-N,N-Pd(0) catalyst after reaction was still Pd(0).
| Sample | Pd3d5/2 | N1s | Si2p | O1s |
|---|---|---|---|---|
| a The binding energies are referenced to C1s (284.6 eV) and the energy differences were determined with an accuracy of ±0.2 eV. | ||||
| MCM-41-N,N-Pd(0) (fresh) | 336.2 | 400.9 | 103.3 | 533.2 |
| MCM-41-N,N-Pd(OAc)2 | 337.4 | 400.8 | 103.4 | 533.2 |
| MCM-41-N,N | 399.7 | 103.3 | 533.1 | |
| Pd(OAc)2 | 338.3 | |||
| Metal Pd | 335.5 | |||
| MCM-41-N,N-Pd(0) (used) | 336.3 | 400.7 | 103.4 | 533.3 |
The MCM-41-immobilized palladium(0)–Schiff base complex [MCM-41-N,N-Pd(0)] was then used as the catalyst for the cross-coupling reaction of triorganoindiums with acid chlorides. In our initial screening experiments, the cross-coupling of benzoyl chloride with Ph3In (0.37 equiv.) in THF was chosen as the model reaction to optimize the reaction conditions and the results are summarized in Table 2. First, the effect of various supported palladium complexes on the model reaction was examined in THF at 68 °C (Table 2, entries 1–3). When the MCM-41-immobilized palladium(II)–Schiff base complex [MCM-41-N,N-Pd(OAc)2] was used as catalyst, benzophenone 3a was isolated in 71% yield (entry 1). To our delight, the yield of 3a could be improved to 88% by replacing the MCM-41-N,N-Pd(OAc)2 complex with the MCM-41-immobilized palladium(0)–Schiff base complex [MCM-41-N,N-Pd(0)] (entry 2). We also tested the catalytic activity of the MCM-41-supported thioether palladium(0) complex [MCM-41-S-Pd(0)],23 which is an efficient and phosphine-free heterogeneous palladium catalyst for Suzuki coupling reaction reported by our group, but a lower yield of 3a was obtained (entry 3), so the MCM-41-N,N-Pd(0) complex was finally selected as the catalyst for the reaction. The result means that all three of phenyl groups attached to the indium were transferred to benzoyl chloride in the heterogeneous coupling reaction. Control experiments showed that palladium catalyst is essential for this ketone formation reaction (entry 4). When Pd(PPh3)4 was used as the catalyst,10a the desired product 3a was also isolated in 89% yield (entry 5), which indicating that catalytic activity of the phosphine-free heterogeneous palladium(0) complex [MCM-41-N,N-Pd(0)] was comparable to that of Pd(PPh3)4. The heterogeneous cross-coupling reaction did not occur at 25 °C (entry 6). Lowering the reaction temperature to 50 °C resulted in a decreased yield (entry 7). Finally, the amount of the immobilized palladium catalyst was screened, and 1 mol% loading of palladium was found to be optimal, a lower yield was observed and a longer reaction time was required when the amount of the catalyst was decreased to 0.5 mol% (entry 8). Increasing the amount of the palladium catalyst could shorten the reaction time, but did not improve the yield of 3a obviously (entry 9). Therefore, the optimal catalytic system involved the use of MCM-41-N,N-Pd(0) (1 mol%) in THF at 68 °C under Ar for 2 h (Table 2, entry 2).
| Entry | Palladium catalyst | Temp. (°C) | Time (h) | Isolated yield (%) |
|---|---|---|---|---|
| a All reactions were performed using 1.0 mmol of benzoyl chloride, 0.37 mmol of Ph3In, 1 mol% of palladium catalyst in 5.0 mL of THF under Ar.b 0.5 mol% of MCM-41-N,N-Pd(0) was used.c 2 mol% of MCM-41-N,N-Pd(0) was used. | ||||
| 1 | MCM-41-N,N-Pd(II) | 68 | 2 | 71 |
| 2 | MCM-41-N,N-Pd(0) | 68 | 2 | 88 |
| 3 | MCM-41-S-Pd(0) | 68 | 2 | 65 |
| 4 | — | 68 | 12 | 0 |
| 5 | Pd(PPh3)4 | 68 | 2 | 89 |
| 6 | MCM-41-N,N-Pd(0) | 25 | 12 | 0 |
| 7 | MCM-41-N,N-Pd(0) | 50 | 8 | 67 |
| 8b | MCM-41-N,N-Pd(0) | 68 | 5 | 76 |
| 9c | MCM-41-N,N-Pd(0) | 68 | 1 | 89 |
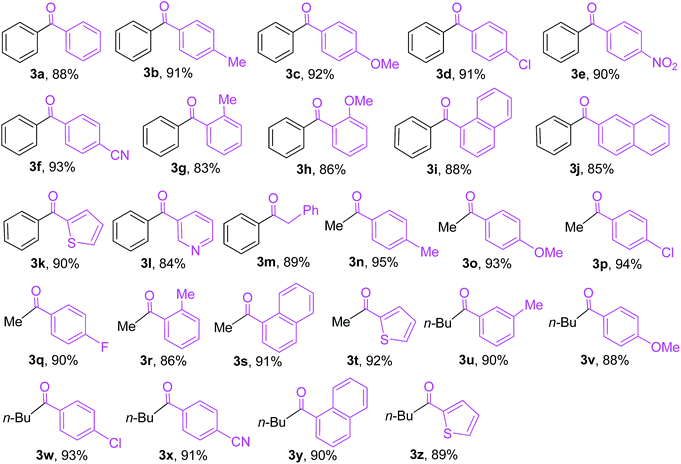
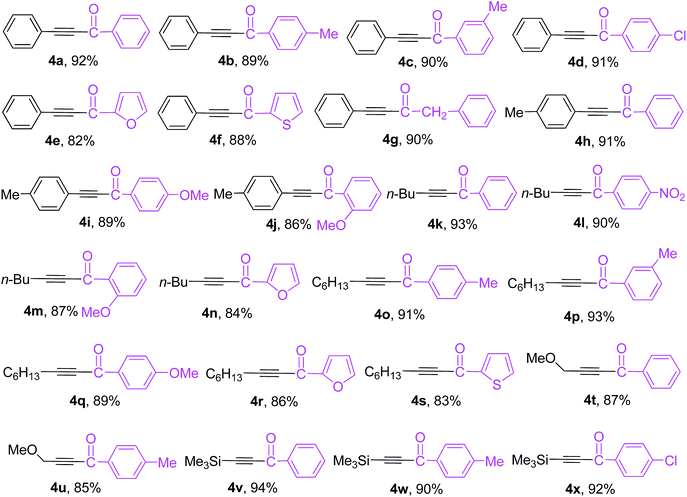
With the optimal reaction conditions established, we tried to investigate the scope and limitations of this heterogeneous palladium(0)-catalyzed cross-coupling reaction of triorganoindiums with acid chlorides and the results are summarized in Table 3. As shown in Table 3, the coupling reactions of Ph3In (1a) with a variety of aromatic acyl chlorides bearing either electron-donating or electron-withdrawing groups proceeded smoothly under the optimized conditions, generating the corresponding substituted benzophenones 3b–3f in excellent yields. The results indicated that the electronic properties of the substituents on benzene ring have limited influence on this heterogeneous palladium-catalyzed cross-coupling reaction. Reactions of sterically hindered 2-methylbenzoyl chloride and 2-methoxybenzoyl chloride also proceeded effectively to produce the desired 2-methylbenzophenone 3g and 2-methoxybenzophenone 3h in 83% and 86% yield, respectively. Bulky 1-naphthoyl chloride and 2-naphthoyl chloride could afford the expected products 3i and 3j in high yields. It is noteworthy that the reactions of heteroaroyl chlorides such as thiophene-2-carbonyl chloride and pyridine-3-carbonyl chloride with Ph3In also gave the desired phenyl heteroaryl ketones 3k and 3l in 90% and 84% yield, respectively. In addition to aroyl chlorides, phenylacetyl chloride proved to be also suitable coupling partner and could afford the expected 1,2-diphenylethanone 3m in 89% yield. However, other aliphatic acyl chlorides such as butyryl chloride and hexanoyl chloride were not reactive under the conditions optimized for aromatic acyl chlorides and no desired cross-coupling products were detected. The similar observation was made by our group in heterogeneous palladium-catalyzed cross-coupling of acyl chlorides with triarylbismuths.24 Under the reaction conditions optimized for Ph3In (1a), Me3In (1b) and n-Bu3In (1c) also exhibited high reactivity in cross-coupling reactions with acid chlorides (Scheme 3). For example, the reactions of Me3In (1b) with aroyl chlorides bearing various substituents, regardless of their electronic properties and substitution positions, produced the corresponding substituted acetophenones 3n–3r in 86–95% yields. The reactions of bulky 1-naphthoyl chloride and thiophene-2-carbonyl chloride with Me3In (1b) also proceeded smoothly to furnish the desired coupling products 3s and 3t in high yields. In addition, a variety of butyl aryl ketones 3u–3z could be obtained in high yields by the cross-coupling of n-Bu3In (1c) with various aroyl chlorides under the optimized conditions. In all these reactions, the tertiary alcohol resulting from double addition was not detected due to the high chemoselectivity of triorganoindiums. The present method provides a quite general and practical route for the synthesis of a variety of biaryl ketones, substituted acetophenones and butyl aryl ketones. A range of electron-donating and electron-withdrawing groups such as methyl, methoxy, chloro, fluoro, nitro, and cyano on aroyl chlorides were well tolerated.
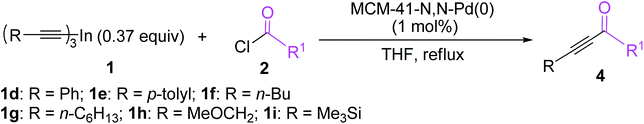
Encouraged by the above results, the heterogeneous cross-coupling reactions of various trialkynylindiums 1d–1i with a range of acid chlorides were then examined under optimized reaction conditions and the results are listed in Table 4. The coupling reactions of tri(phenylethynyl)indium (1d) with a range of electron-neutral, electron-rich and electron-deficient aroyl chlorides proceeded smoothly in THF at 68 °C to afford the corresponding α,β-acetylenic ketones 4a–4d in 89–92% yields. Heteroatoms turned out to be compatible with the employed reaction conditions. The reactions of heteroaroyl chlorides such as furan-2-carbonyl chloride and thiophene-2-carbonyl chloride with 1d produced the expected coupling products 4e and 4f in good yields. Besides aroyl and heteroaroyl chlorides, an aliphatic phenylacetyl chloride was also good coupling partner and gave the desired 1,4-diphenylbut-3-yn-2-one 4g in 90% yield. The coupling reaction worked equally well with tri(p-tolylethynyl)indium (1e), providing the desired coupling products 4h–4j in 86–91% yields. In addition to aromatic trialkynylindiums 1d and 1e, three aliphatic trialkynylindiums 1f, 1g and 1h also showed high reactivity in the cross-couplings with acid chlorides. The reactions of trialkynylindiums 1f–1h with a wide range of electron-neutral, electron-rich and electron-deficient aroyl chlorides or heteroaroyl chlorides proceeded effectively under the optimized reaction conditions to afford the corresponding α,β-acetylenic ketones 4k–4u in good to excellent yields. Interestingly, a trimethylsilyl-functionalized trialkynylindium reagent 1i also underwent the cross-coupling with various aroyl chlorides smoothly to afford the desired trimethylsilyl-functionalized alkynyl ketones 4v–4x in excellent yields. The developed methodology provides a quite general and practical route for the preparation of α,β-acetylenic ketones having various functionalities.
To verify whether the observed catalysis was due to the heterogeneous MCM-41-N,N-Pd(0) complex or to a leached palladium species in solution, we performed the hot filtration test.25 We focused on the coupling reaction of Ph3In with benzoyl chloride. We filtered off the MCM-41-N,N-Pd(0) complex after 0.5 h of reaction time and allowed the filtrate to react further. The catalyst filtration was performed at the reaction temperature (68 °C) in order to avoid possible recoordination or precipitation of soluble palladium upon cooling. We found that, after this hot filtration, no further reaction was observed. We also determined the palladium content in the filtrate by ICP analysis, and only 0.3 ppm of palladium was found in the clear solution. These results rule out any contribution to the observed catalysis from a homogeneous palladium species indicating that the catalyst was stable during the reaction and the observed catalysis was intrinsically heterogeneous.
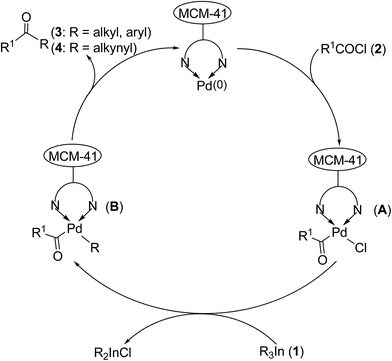
A plausible mechanism for this heterogeneous palladium(0)-catalyzed cross-coupling reaction of triorganoindiums with acid chlorides is illustrated in Scheme 4. First, oxidative addition of R1COCl (2) to the MCM-41-N,N-Pd(0) complex provides an MCM-41-bound acylpalladium(II) complex (A), which should be the rate-limiting step.10a Subsequent transmetalation between intermediate A and triorganoindium reagent (1) produces intermediate B and R2InCl, which can re-enter in a new catalytic cycle and effectively transfer its organic groups. Finally, reductive elimination of intermediate B affords the desired ketone (3 or 4) and regenerates the MCM-41-N,N-Pd(0) complex to complete the catalytic cycle.
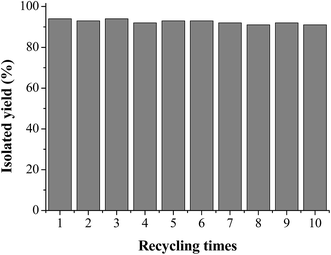
For the practical application of a heterogeneous catalyst system its stability and reusability are important factors. The MCM-41-N,N-Pd(0) complex could be easily separated and recovered by a simple filtration of the reaction solution. We next investigated the recyclability of the catalyst by using the cross-coupling reaction of benzoyl chloride with (Me3SiC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)3In (1i). After carrying out the reaction, the catalyst was recovered by simple filtration and washed with DMF and diethyl ether. After being air-dried, it can be reused directly without further purification. The recovered palladium catalyst was used in the next run, and almost the same yield of 4v was obtained for ten consecutive cycles (Fig. 1). In addition, palladium leaching in the heterogeneous catalyst was also determined by ICP-AES analysis on the recovered catalyst after ten consecutive runs, which revealing almost the same palladium content as the fresh one. The high catalytic performance and excellent recyclability of the MCM-41-N,N-Pd(0) catalyst may relate to the efficient site isolation, to the optimal dispersion of the active sites on the inner channel walls and to the relatively strong interaction between bidentate Schiff base ligand and the palladium centre anchored on the MCM-41. The result is important from the standpoint of green and sustainable chemistry.
C)3In (1i). After carrying out the reaction, the catalyst was recovered by simple filtration and washed with DMF and diethyl ether. After being air-dried, it can be reused directly without further purification. The recovered palladium catalyst was used in the next run, and almost the same yield of 4v was obtained for ten consecutive cycles (Fig. 1). In addition, palladium leaching in the heterogeneous catalyst was also determined by ICP-AES analysis on the recovered catalyst after ten consecutive runs, which revealing almost the same palladium content as the fresh one. The high catalytic performance and excellent recyclability of the MCM-41-N,N-Pd(0) catalyst may relate to the efficient site isolation, to the optimal dispersion of the active sites on the inner channel walls and to the relatively strong interaction between bidentate Schiff base ligand and the palladium centre anchored on the MCM-41. The result is important from the standpoint of green and sustainable chemistry.
3. Conclusions
In summary, we have successfully developed a novel, atom-efficient, practical and environmentally friendly method for the synthesis of aryl ketones and α,β-acetylenic ketones through the cross-coupling of triorganoindiums with acid chlorides by using an MCM-41-immobilized palladium(0)–Schiff base complex [MCM-41-N,N-Pd(0)] as catalyst. This heterogeneous phosphine-free palladium(0) catalyst could be easily prepared by a simple procedure from commercially readily available reagents and exhibited the same catalytic activity as Pd(PPh3)4. The reactions generated a variety of valuable aryl ketones and α,β-acetylenic ketones in good to excellent yields and were applicable to various triorganoindiums and a wide range of acid chlorides. In addition, this methodology offers the competitiveness of recyclability of the palladium catalyst without significant loss of activity, and the palladium catalyst can be easily recovered by a simple filtration of the reaction solution and reused at least 10 cycles, thus making this procedure economically and environmentally more acceptable.4. Experimental
All chemicals were reagent grade and used as purchased. All solvents were dried and distilled before use. The MCM-41-N,N-Pd(OAc)2 catalyst was prepared according to our previous procedure,21 the palladium content was determined to be 0.42 mmol g−1. The products were purified by flash chromatography on silica gel. Mixture of EtOAc and hexane was generally used as eluent. All coupling products were characterized by comparison of their spectra and physical data with authentic samples. 1H NMR spectra were recorded on a Bruker Avance 400 MHz spectrometer with TMS as an internal standard in CDCl3 as solvent. 13C NMR spectra (100 MHz) were recorded on a Bruker Avance 400 MHz spectrometer in CDCl3 as solvent. HRMS spectra were recorded on a Q-Tof spectrometer with micromass MS software using electrospray ionization (ESI). Melting points are uncorrected. X-ray photoelectron spectra was recorded on XSAM 800 (Kratos). Palladium content was determined with inductively coupled plasma atom emission Atomscan16 (ICP-AES, TJA Corporation).Preparation of the MCM-41-N,N-Pd(0) complex
A mixture of MCM-41-N,N-Pd(OAc)2 (2.5 g) and hydrazine hydrate (1.5 g) in EtOH (30 mL) was stirred at 30 °C for 3 h under Ar. The resulting product was filtered, washed with EtOH (3 × 20 mL) and Et2O (2 × 20 mL) and dried under vacuum at 60 °C for 3 h to afford 2.38 g of the MCM-41-N,N-Pd(0) complex as a gray powder. The nitrogen content was found to be 2.24 mmol g−1 by elemental analysis. The palladium content was determined to be 0.43 mmol g−1 by ICP-AES.General procedure for preparation of triorganoindium reagents
A 25 mL round-bottomed flask containing a stirrer bar was charged with InCl3 (0.37 mmol) and dried under vacuum with a heat gun. The flask was cooled, a positive argon pressure was established and anhydrous THF (2 mL) was added. The resulting solution was cooled to −78 °C and a solution of RLi or RMgBr (1.1 mmol, 1.0–2.5 M in hexane, THF, or Et2O) was slowly added (15–30 min). The reaction mixture was stirred for 30 min, the cooling bath was removed, and the mixture was warmed to room temperature over 30 min.General procedure for the heterogeneous palladium(0)-catalyzed cross-coupling of acid chlorides with triorganoindiums
A solution of R3In (0.37 mmol, ca. 0.18 M in dry THF) was added to a mixture of MCM-41-N,N-Pd(0) (24 mg, 1 mol%) and acid chloride (1 mmol) in dry THF (3 mL) under Ar. The resulting mixture was refluxed under Ar until the starting material had been consumed (TLC). After being cooled to room temperature, the mixture was diluted with Et2O (30 mL) and filtered. The palladium catalyst was washed with DMF (2 × 5 mL), Et2O (2 × 5 mL) and reused in the next run. The filtrate was washed with sat. aq NaHCO3 (5 mL), water (3 × 10 mL) and dried over MgSO4, filtered, and concentrated under vacuum. The residue was purified by flash chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane = 1
hexane = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25) to give the desired cross-coupling product.
25) to give the desired cross-coupling product.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
We thank the National Natural Science Foundation of China (No. 21462021) and Key Laboratory of Functional Small Organic Molecule, Ministry of Education (No. KLFS-KF-201409) for financial support.References
- (a) N. De Kimpe, M. Keppens and G. Froncg, J. Chem. Soc., Chem. Commun., 1996, 635–636 RSC; (b) R. K. Dieter, Tetrahedron, 1999, 55, 4177–4236 CrossRef CAS; (c) X.-J. Wang, L. Zhang, X. Sun, Y. Xu, D. Krishnamurthy and C. H. Senanayake, Org. Lett., 2005, 7, 5593–5595 CrossRef CAS PubMed; (d) B. Hatano, J. I. Kadokawa and H. Tagaya, Tetrahedron Lett., 2002, 43, 5859–5861 CrossRef CAS; (e) F. W. Patureau, T. Besset, N. Kuhl and F. Glorius, J. Am. Chem. Soc., 2011, 133, 2154–2156 CrossRef CAS PubMed; (f) L. Zheng, J. Ju, Y. Bin and R. Hua, J. Org. Chem., 2012, 77, 5794–5800 CrossRef CAS PubMed.
- (a) V. I. Dodero, L. C. Koll, M. B. Faraoni, T. N. Mitchell and J. C. Podesta, J. Org. Chem., 2003, 68, 10087–10091 CrossRef CAS PubMed; (b) B. G. Vong, S. H. Kim, S. Abraham and E. A. Theodorakis, Angew. Chem., Int. Ed., 2004, 43, 3947–3951 CrossRef CAS PubMed; (c) B. M. Trost and Z. T. Ball, J. Am. Chem. Soc., 2004, 126, 13942–13944 CrossRef CAS PubMed; (d) B. M. Trost and T. Schmidt, J. Am. Chem. Soc., 1988, 110, 2301–2303 CrossRef CAS; (e) A. V. Kel'in, A. W. Sromek and V. Gevorgyan, J. Am. Chem. Soc., 2001, 123, 2074–2075 CrossRef; (f) A. V. Kel'in and V. Gevorgyan, J. Org. Chem., 2002, 67, 95–98 CrossRef; (g) B. M. Trost and C.-I. Huang, J. Am. Chem. Soc., 2015, 137, 15940–15946 CrossRef CAS PubMed; (h) T. Wang, X. Duan, H. Zhao, S. Zhai, C. Tao, H. Wang, Y. Li, B. Cheng and H. Zhai, Org. Lett., 2017, 19, 1650–1653 CrossRef CAS PubMed.
- (a) A. Furstner, D. Voigtlander, W. Schrader, D. Giebel and M. T. Reetz, Org. Lett., 2001, 3, 417–420 CrossRef CAS PubMed; (b) C. E. Song, W. H. Shim, E. J. Roh and J. H. Choi, Chem. Commun., 2000, 1695–1696 RSC; (c) J. Ross and J. Xiao, Green Chem., 2002, 4, 129–133 RSC; (d) S. Gmouh, H. Yang and M. Vaultier, Org. Lett., 2003, 5, 2219–2222 CrossRef CAS PubMed; (e) E. Fillion, D. Fishlock, A. Wilsily and J. M. Goll, J. Org. Chem., 2005, 70, 1316–1327 CrossRef CAS PubMed.
- (a) R. B. Davis and D. H. Scheiber, J. Am. Chem. Soc., 1956, 78, 1675–1678 CrossRef CAS; (b) M. W. Logue and G. L. Moore, J. Org. Chem., 1975, 40, 131–132 CrossRef CAS; (c) U. Schmit and M. Schwochau, Chem. Ber., 1964, 97, 1649–1655 CrossRef; (d) L. I. Vereshchagin, O. G. Yashina and T. V. Zarva, Zh. Org. Khim., 1966, 2, 1895–1901 CAS; (e) D. R. M. Walton and F. Waugh, J. Organomet. Chem., 1972, 37, 45–56 CrossRef CAS; (f) M. W. Logue and K. Teng, J. Org. Chem., 1982, 47, 2549–2553 CrossRef CAS.
- (a) D. A. Alonso, C. Najera and M. C. Pacheco, J. Org. Chem., 2004, 69, 1615–1619 CrossRef CAS PubMed; (b) J. Yin, X.-J. Wang, Y. Liang, X. Wu, B. Chen and Y. Ma, Synthesis, 2004, 331–336 CAS; (c) L. Chen and C.-J. Li, Org. Lett., 2004, 6, 3151–3154 CrossRef CAS PubMed; (d) R. J. Cox, D. J. Ritson, T. A. Dane, J. Berge, J. P. H. Charmant and A. Kantacha, Chem. Commun., 2005, 1037–1038 RSC; (e) S. S. Palimkar, R. J. Lahoti and K. V. Srinivasan, Green Chem., 2007, 9, 146–152 RSC; (f) H.-L. Liu, H.-F. Jiang, M. Zhang, W.-J. Yao, Q.-H. Zhu and Z. Tang, Tetrahedron Lett., 2008, 49, 3805–3808 CrossRef CAS; (g) Y. Nishihara, E. Inoue, Y. Okada and K. Takagi, Synlett, 2008, 3041–3045 CrossRef CAS.
- B. M. Trost, Angew. Chem., Int. Ed., 1995, 34, 259–281 CrossRef CAS.
- (a) P. T. Anastas and J. C. Warner, Green Chemistry, Oxford University Press, Oxford, 1998, pp. 29–55 Search PubMed; (b) P. T. Anastas and M. M. Kirchhoff, Acc. Chem. Res., 2002, 35, 686–694 CrossRef CAS PubMed.
- I. J. Worrall and J. D. Smith, in Organometallic Compounds of Aluminum, Gallium, Indium and Thallium, ed. A. McKillop, J. D. Smith and I. J. Worrall, Chapman and Hall, London, U.K., 1985, p. 137 Search PubMed.
- (a) R. Jana, T. P. Pathak and M. S. Sigman, Chem. Rev., 2011, 111, 1417–1492 CrossRef CAS PubMed; (b) Z.-L. Shen, S.-Y. Wang, Y.-K. Chok, Y.-H. Xu and T.-P. Loh, Chem. Rev., 2013, 113, 271–401 CrossRef CAS PubMed.
- For selected examples, see: (a) L. Perez, J. Perez Sestelo and L. A. Sarandeses, J. Am. Chem. Soc., 2001, 123, 4155–4160 CrossRef PubMed; (b) M. A. Pena, J. Perez Sestelo and L. A. Sarandeses, Synthesis, 2005, 485–492 CAS; (c) M. A. Pena, J. Perez Sestelo and L. A. Sarandeses, J. Org. Chem., 2007, 72, 1271–1275 CrossRef CAS PubMed; (d) S. Bernhardt, Z.-L. Shen and P. Knochel, Chem.–Eur. J., 2013, 19, 828–833 CrossRef CAS PubMed; (e) C. Perez-Caaveiro, J. Perez Sestelo and L. A. Sarandeses, J. Org. Chem., 2014, 79, 9586–9593 CrossRef CAS PubMed; (f) A. Mosquera, M. I. Fernandez, M. Canle Lopez, J. Perez Sestelo and L. A. Sarandeses, Chem.–Eur. J., 2014, 20, 14524–14530 CrossRef CAS PubMed; (g) S. Thapa, S. K. Gurung, D. A. Dickie and R. Giri, Angew. Chem., Int. Ed., 2014, 53, 11620–11624 CrossRef CAS PubMed.
- (a) P. H. Lee, S. W. Lee and K. Lee, Org. Lett., 2003, 5, 1103–1106 CrossRef CAS PubMed; (b) M. A. Pena, J. Perez Sestelo and L. A. Sarandeses, Synthesis, 2003, 780–784 CAS; (c) S. K. Lee, K. Lee, D. Seomoon, S. Kim, H. Kim, H. Kim, E. Shim, M. Lee, S. Lee, M. Kim and P. H. Lee, J. Org. Chem., 2004, 69, 4852–4855 CrossRef CAS PubMed.
- (a) M. Poliakoff, J. M. Fitzpatrick, T. R. Farren and P. T. Anastas, Science, 2002, 297, 807–810 CrossRef CAS PubMed; (b) D. J. Cole-Hamilton, Science, 2003, 299, 1702–1706 CrossRef CAS PubMed.
- (a) N. E. Leadbeater and M. Marco, Chem. Rev., 2002, 102, 3217–3274 CrossRef CAS PubMed; (b) L. Yin and J. Liebscher, Chem. Rev., 2007, 107, 133–173 CrossRef CAS PubMed; (c) J. Liu and P. H. Toy, Chem. Rev., 2009, 109, 815–838 CrossRef PubMed; (d) A. Molnar, Chem. Rev., 2011, 111, 2251–2320 CrossRef CAS PubMed.
- (a) C. T. Kresge, M. E. Leonowicz, W. J. Roth, J. C. Vartuli and J. S. Beck, Nature, 1992, 359, 710–712 CrossRef CAS; (b) T. Maschmeyer, F. Rey, G. Sankar and J. M. Thomas, Nature, 1995, 378, 159–162 CrossRef CAS; (c) W. Zhou, J. M. Thomas, D. S. Shephard, B. F. G. Johnson, D. Ozkaya, T. Maschmeyer, R. G. Bell and Q. Ge, Science, 1998, 280, 705–708 CrossRef CAS PubMed; (d) A. Taguchi and F. Schuth, Microporous Mesoporous Mater., 2005, 77, 1–45 CrossRef CAS; (e) R. M. Martin-Aranda and J. Cejka, Top. Catal., 2010, 53, 141–153 CrossRef CAS.
- J. S. Beck, J. C. Vartuli, W. J. Roth, M. E. Leonowicz, C. T. Kresge, K. D. Schmitt, C. T.-W. Chu, D. H. Olson, E. W. Sheppard, S. B. McCullen, J. B. Higgins and J. L. Schlenker, J. Am. Chem. Soc., 1992, 114, 10834–10843 CrossRef CAS.
- For selected examples, see: (a) P. C. Mehnert, D. W. Weaver and J. Y. Ying, J. Am. Chem. Soc., 1998, 120, 12289–12296 CrossRef; (b) K. Mukhopadhyay, B. R. Sarkar and R. V. Chaudhari, J. Am. Chem. Soc., 2002, 124, 9692–9693 CrossRef CAS PubMed; (c) J. Y. Ying, C. P. Mehnert and M. S. Wong, Angew. Chem., Int. Ed., 1999, 38, 56–77 CrossRef CAS; (d) M. Cai, G. Zheng and G. Ding, Green Chem., 2009, 11, 1687–1693 RSC; (e) M. Cai, J. Peng, W. Hao and G. Ding, Green Chem., 2011, 13, 190–196 RSC; (f) W. Hao, H. Liu, L. Yin and M. Cai, J. Org. Chem., 2016, 81, 4244–4251 CrossRef CAS PubMed; (g) F. Havasi, A. Ghorbani-Choghamarani and F. Nikpour, New J. Chem., 2015, 39, 6504–6512 RSC.
- (a) S.-G. Shyu, S.-W. Cheng and D.-L. Tzou, Chem. Commun., 1999, 2337–2338 RSC; (b) Y. Yang and R. M. Rioux, Chem. Commun., 2011, 47, 6557–6559 RSC.
- (a) C. D. Nunes, A. A. Valente, M. Pillinger, A. C. Fernandes, C. C. Romao, J. Rocha and I. S. Goncalves, J. Mater. Chem., 2002, 12, 1735–1742 RSC; (b) M. Jia, A. Seifert and W. R. Thiel, Chem. Mater., 2003, 15, 2174–2180 CrossRef CAS.
- (a) A. Corma, E. Gutierrez-Puebla, M. Iglesias, A. Monge, S. Perez-Ferreras and F. Sanchez, Adv. Synth. Catal., 2006, 348, 1899–1907 CrossRef CAS; (b) A. Corma, C. Gonzalez-Arellano, M. Iglesias and F. Sanchez, Angew. Chem., Int. Ed., 2007, 46, 7820–7822 CrossRef CAS PubMed; (c) G. Villaverde, A. Corma, M. Iglesias and F. Sanchez, ACS Catal., 2012, 2, 399–406 CrossRef CAS; (d) W. Yang, R. Zhang, F. Yi and M. Cai, J. Org. Chem., 2017, 82, 5204–5211 CrossRef CAS PubMed.
- (a) R. Xiao, H. Zhao and M. Cai, Tetrahedron, 2013, 69, 5444–5450 CrossRef CAS; (b) H. Zhao, W. He, R. Yao and M. Cai, Adv. Synth. Catal., 2014, 356, 3092–3098 CrossRef CAS; (c) M. Cai, R. Yao, L. Chen and H. Zhao, J. Mol. Catal. A: Chem., 2014, 395, 349–354 CrossRef CAS; (d) H. Zhao, Y. Jiang, Q. Chen and M. Cai, New J. Chem., 2015, 39, 2106–2111 RSC; (e) H. Zhao, W. He, L. Wei and M. Cai, Catal. Sci. Technol., 2016, 6, 1488–1495 RSC.
- C. Xu, L. Yin, B. Huang, H. Liu and M. Cai, Tetrahedron, 2016, 72, 2065–2071 CrossRef CAS.
- L. Yu, Y. Huang, Z. Wei, Y. Ding, C. Su and Q. Xu, J. Org. Chem., 2015, 80, 8677–8683 CrossRef CAS PubMed.
- M. Cai, Q. Xu and Y. Huang, J. Mol. Catal. A: Chem., 2007, 271, 93–97 CrossRef CAS.
- H. Zhao, L. Yin and M. Cai, Eur. J. Org. Chem., 2013, 1337–1345 CrossRef CAS.
- H. E. B. Lempers and R. A. Sheldon, J. Catal., 1998, 175, 62–69 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra08355f |
| This journal is © The Royal Society of Chemistry 2017 |