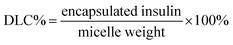Open Access Article
Open Access ArticleNovel amphiphilic glucose-responsive modified starch micelles for insulin delivery†
Na Wen ,
Chunmei Gao,
Shaoyu Lü
,
Chunmei Gao,
Shaoyu Lü *,
Xiubin Xu,
Xiao Bai
*,
Xiubin Xu,
Xiao Bai ,
Can Wu,
Piao Ning,
Shaofei Zhang and
Mingzhu Liu*
,
Can Wu,
Piao Ning,
Shaofei Zhang and
Mingzhu Liu*
State Key Laboratory of Applied Organic Chemistry, Key Laboratory of Nonferrous Metal Chemistry and Resources Utilization of Gansu Province, Department of Chemistry, Lanzhou University, Lanzhou, 730000, People's Republic of China. E-mail: lshy@lzu.edu.cn; mzliu@lzu.edu.cn
First published on 27th September 2017
Abstract
The high pKa (8.26 to 8.6) of PBA has restricted its glucose-responsiveness in physiological conditions, and the high cytotoxicity of polymers is also a limiting problem in their potential application for insulin delivery. Novel amphiphilic glucose-sensitive dialdehyde starch polymers containing 3-aminophenylboronic acid (APBA) as a glucose-responsive group and mPEGylated dialdehyde starch (mPEG-DAS) with hydrophobic 7-hydroxycoumarin-4-acetic acid (Cou) were synthesized. This dialdehyde starch derivative can self-assemble into mPEG-DAS–APBA–Cou micelles with “shell–core” structures in phosphate-buffered saline solution (PBS). In addition, the drug-loaded micelles can release insulin rapidly in response to hyperglycemia in a physiological environment. The results demonstrated that the mPEG-DAS–APBA–Cou micelles showed notable glucose responsive behavior near the physiological range. The insulin release from the nanocarriers is sensitive to different concentrations of glucose, releasing insulin rapidly under the conditions of 3 mg mL−1 glucose while demonstrating comparatively inert release at 1 mg mL−1 glucose (pH 7.4). MTT assays and hemolysis studies both confirmed that the mPEG-DAS–APBA–Cou micelles have low cytotoxic activity to A549 cells and low blood toxicity. These results suggest that the glucose-sensitive dialdehyde starch micelles (mPEG-DAS–APBA–Cou) have potential applications as a glucose-responsive material for insulin delivery.
Introduction
Glucose-responsive materials based on phenylboronic acid (PBA) have attracted great interest due to their self-regulated insulin delivery capacity, which can be applied in current clinical diabetes treatment.1–4 Various forms of delivery systems based on PBA materials have been constructed, including gels,5,6 micelles7 and vesicles.8 For PBA-containing gel systems, the addition of glucose can induce deswelling instead of swelling. Asher et al.9 first reported PBA-containing hydrogels in which polyethylene glycol (PEG) or crown ether functional groups were incorporated. In these hydrogels, glucose could simultaneously bind with two boronates of the hydrogel. In addition, with increasing crosslinking density, the swelling degree of the hydrogels gradually decreased. Therefore, much effort has been made to design polymers with glucose-responsiveness behavior. In drug delivery systems, gels with glucose-responsiveness maintain structural integrity as drug carriers; however, they encounter the dilemma of possible burst release of the drug in the blood, leading to hypoglycemia. In addition, the gels are large in size, up to micron dimensions, and are easily removed by the permeability and retention effect (EPR), which is undesirable for long circulation.10 Particularly, self-assembled glucose-responsive micelles7,11–13 and vesicles8,14 with PBA-containing polymers have shown uncommon and notable properties.Compared to gels, which lack protection from a corona layer,15 self-assembled micelles are formed with the hydrophilic groups of PEG as an outer shell that is resistant to the approach of proteins to the surfaces of the nanocarriers; this shields the nanocarriers from recognition by the body's immune system16,17 and increases blood circulation time in vivo by the protection of PEG, providing potential applications as drug carriers for insulin delivery.15,18–20 Kim et al.21,22 synthesized a new class of polymers possessing styreneboroxole and N-functionalized maleimide with sugar-responsive behavior. The boroxole-based monomer bound to oligo (ethylene glycol) groups as a chain-transfer agent adjusted to changes in glucose solubility and demonstrated sensitive behavior at reduced glucose concentrations close to physiological conditions. Shi et al.7 developed drug-loading micelles, which were self-assembled by covalent complexation of phenylboronic acid and glycosyl. The sensitive behavior of the complex micelles required the protection of PEG. Shi et al.23 also reported micelles with favourable responsiveness based on polyethylene glycol-co-poly(acrylic acid) with modified APBA (PEG-PAA-PBA); the lack of degradation behavior of PAA and PBA limited their potential for degradation behavior in vivo. It is necessary to adopt biodegradable and biocompatible materials, such as natural polysaccharides, in place of refractory fragments.
The medical treatment for diabetes is a persistent process; hence, it requires baseline drugs that are non-toxic, show sustained release, are biodegradable, and are non-toxic to the body for in vivo applications. However, the compatibility between favourable glucose-responsiveness and high biodegradability and biocompatibility is a limitation for drug delivery in current studies. Therefore, designing glucose-sensitive materials is imminently and greatly needed to achieve high biodegradability and biocompatibility in insulin delivery systems.24 Starch, a major natural source of polysaccharides, exhibits nontoxicity, compatibility, and nonimmunogenicity. Thus, it has been widely used in the pharmaceutical field as an excipient for drug delivery systems.25,26 Starch has superior advantages of low cost and biodegradability due to hydrolysis and human enzymes in comparison to other polysaccharides.27 In addition, various functional materials can be attained via modification of starch due to the large numbers of functional hydroxyl groups in the chains.28,29 The use of dialdehydes has been explored to obtain more biocompatible materials;30 aldehyde-functionalized starch has aroused considerable interest due to its minimal toxicity.31 The preparation of dialdehyde starches containing aldehyde groups has been achieved by reaction with sodium periodate (NaIO4).32,33 It is noteworthy that micelles can be prepared via self-assembly of amphiphilic starch derivatives.29,34,35 Lehr et al.27 prepared starch-graft-polyethylenimine copolymers for efficient biodegradable gene delivery. Zhang et al.36 synthesized grafted copolymers by a reaction between starch and poly(L-glutamic acid) with pH-responsive behavior for controlled release of insulin; this is a fascinating option for synthetic polymers in drug delivery systems.
For most reports focusing on glucose-responsiveness, the high pKa (8.26 to 8.6) of PBA has restricted its practical applications.37,38 However, researchers have attempted to decrease the pKa of PBA. For example, Yoon et al.39 reported a PBA-containing complex with polyol polymer, a stronger acid, and decreased the pKa of the PBA derivative. Wu et al.40 introduced amino groups into polymers to enhance the binding affinity of nitrogen and boron and to self-regulate insulin release, even responding positively to glucose in physiological conditions. A subtle relationship may exist in polymers between the structure of coumarins and the pKa of PBA. Therefore, PBA-based materials are susceptible to glucose-responsiveness at lower pH values than the pKa of PBA.
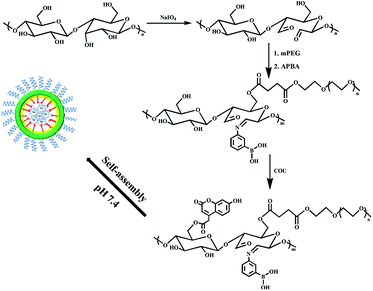
Herein, a series of dialdehyde starch derivative polymers (mPEG-DAS–APBA–Cou) were prepared by grafting PEG as the hydrophilic outer shell, Cou as the hydrophobic core and PBA as the glucose-responsive group to a dialdehyde starch backbone. Introducing Cou may decrease the pKa of a material, and the starch backbone mainly enhances its biodegradable behavior. The polymers were able to self-assemble into spherical micelles in PBS solution. The polymers showed rapid response to glucose due to the hydrophobic micelle core and the apparently low pKa of PBA in physiological conditions, as illustrated in Scheme 1. The micelles quickly disassembled to release insulin because of the sensitivity of the PBA-based polymer to different concentrations of glucose in the blood microenvironment. The safety and biocompatibility of the micelles as drug carriers were also confirmed by cytotoxicity and hemolysis tests.
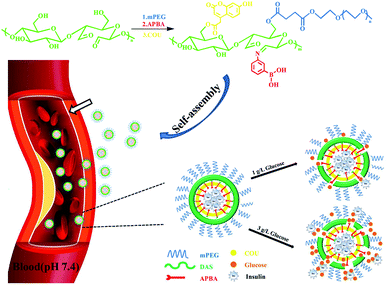 | ||
| Scheme 1 The formation of insulin-loaded core–shell mPEG-DAS–APBA–Cou micelles and their insulin release behavior under different concentrations of glucose in the blood microenvironment. | ||
Experimental
Materials
Soluble starch (Mw 8.8 kDa) was provided by the Zhejiang Linghu Chemical Reagent Factory. Polyethylene glycol monomethyl ether (mPEG, Mn 1.9 kDa), 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC·HCl), 7-hydroxycoumarin-4-acetic acid, 3-aminophenylboronic, insulin from porcine pancreas and pyrene were purchased from Aladdin Chemistry Co., Ltd. Succinic anhydride, N-hydroxysuccinimide, and 4-dimethylaminopyridine (DMAP) were purchased from J&K Reagent Company. All other chemicals were of analytical grade and were used without further purification.Synthesis and characterization of dialdehyde starch derivatives
mPEG-DAS (0.2 g) and APBA (0.1 g) were dissolved in DMSO and heated to 60 °C for 6 h under nitrogen atmosphere. Then, the mixture was purified in a dialysis bag (MWCO 3.5 kDa) for three days and lyophilized to obtain the product APBA-functional dialdehyde starch (mPEG-DAS–APBA).
Preparation and characterization of dialdehyde starch derivative micelles
In vitro stability study of micelles
The stabilities of the micelles were tested by dispersion in PBS solution (pH 7.4) and gentle shaking at 100 rpm min−1 at physiological temperature. The diameters of the micelles were measured at intervening times (4 h, 8 h, 24 h and 48 h) using DLS.Glucose-responsiveness of the complex micelles
The pKa values of the complex micelles were determined by UV-vis according to a reported method.45 The glucose-sensitivity behaviors of the mPEG-DAS–APBA and mPEG-DAS–APBA–Cou micelles in PBS were measured by DLS. The concentrations of glucose solution were 0, 1 and 3 mg mL−1, referring to the final diluted concentration after adding glucose to the micelle solutions. The micelle solutions were prepared for DLS analysis by filtering as mentioned.Hemolysis assay
The membrane disruption of red blood cells (RBCs) can be employed to evaluate the blood toxicity of polymers. Fresh human blood samples were obtained from Lanzhou University first affiliated hospital and were centrifuged at 1600 rpm min−1 for 5 min; all experiments were performed in compliance with the approval of the Institutional Authority for Laboratory Animal Care. A549 cells were derived from an assay on rabbit mesenchymal stem cells. Afterwards, the micelle groups were prepared by adding 0.2 mL RBC supernatant to 0.8 mL micelle solution (1 mg mL−1). Then, the micelle systems were incubated at 37 °C for 2 h. Furthermore, the micelle systems were centrifuged at 1600 rpm min−1 for 5 min. Finally, the supernatant (0.5 mL) was added to 5 mL PBS, and the percentages of hemolysis of the RBCs with various samples were calculated via the absorbance of released hemoglobin at 570 nm by UV-vis spectrophotometer (Perkin-Elmer Lambda 35, USA) based on the following equation:where Asample, Anegative, and Apositive represent the absorbances measured from the sample, PBS, and water groups, respectively.
Insulin loading and glucose-responsive release
Circular dichroism spectroscopy
The structure of the released insulin was determined by circular dichroism (CD) spectra, which were acquired using a DSM1000 CD spectropolarimeter (USA). A standard insulin solution of 1 mg mL−1 was prepared in PBS (pH 7.4) for CD measurements.Cytotoxicity test
The cell suspension in culture medium was plated at 5 × 104 cells per well in a 96-well plate and incubated in a humidified atmosphere with 5% CO2 for 24 h at 37 °C. Then, a methyl thiazolyl tetrazoliumviability assay (MTT) was carried out with A549 cancer cells from rabbit mesenchymal stem cells. The culture medium was replaced with 200 μL of the prepared culture medium containing blank micelles with concentrations of 1, 5, 10, 50, and 100 μg mL−1 for 24 h and 48 h. MTT solution was added to each well, and the wells were incubated for another 4 h. The optical density was measured using a microplate reader at 490 nm. The cell viability was calculated by means of the percent ratio of the absorbance of the mixtures and micelles to the control.Results
Synthesis and characterization of dialdehyde starch derivatives
In this work, we successfully synthetized amphiphilic dialdehyde starch derivatives containing hydrophilic mPEG, APBA functional groups and hydrophobic Cou. The dialdehyde starch derivatives of mPEG-DAS–APBA–Cou were synthesized via three steps (Scheme 2). Firstly, mPEG-COOH was introduced as a hydrophilic group to dialdehyde starch by an etherification reaction. Then, the conjugated polymer was readily synthesized by reacting the aldehyde groups of mPEG-DAS with PBA via Schiff bases (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–) due to its high stability. Finally, Cou as the hydrophobic group was attached by esterification reactions of the hydroxyl groups on the dialdehyde starch chains. Dialdehyde starch with different ODs was synthesized and determined by hydroxylamine hydrochloride titration and confirmed by FTIR spectra, as shown in Fig. S1 and Table S1.†
N–) due to its high stability. Finally, Cou as the hydrophobic group was attached by esterification reactions of the hydroxyl groups on the dialdehyde starch chains. Dialdehyde starch with different ODs was synthesized and determined by hydroxylamine hydrochloride titration and confirmed by FTIR spectra, as shown in Fig. S1 and Table S1.†
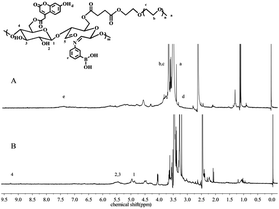
Typical 1H NMR spectra of the copolymers are presented in Fig. 1. Specifically, the glucose units and aldehyde groups of dialdehyde starch (4.99 to 5.51 and 9.26 ppm, respectively) are shown in Fig. 1B.46 The respective assigned peaks of both dialdehyde starch and mPEG could be found in their spectra. According to our published method,47 the degree of substitution (DS) of mPEG in mPEG-St polymer was calculated to be 0.25. The protons of the phenyl groups appeared in the range from 6.98 to 7.6 ppm in PBA in Fig. 1A (e, 7.46 ppm), while typical peaks of mPEG from the methylene (–CH2–CH2–, b and c) and terminal methoxyl (–O–CH3, a) groups were observed at 3.51 ppm and 3.34 ppm, respectively. Because Cou and PBA contain phenyl groups, the Cou-OH spectrum features absorption peaks at 3.24, as described previously.48 The DS of APBA was 0.15 according to the calculated copolymer compositions of the integrated area between the saccharide (5.11 ppm) and APBA (7.46 ppm) signals. Similarly, the DS of the Cou signals at 3.24 ppm was 0.1. In order to prepare mPEG-DAS–APBA polymers with different degrees of cross-linking, a series of experiments was designed with different ODs of mPEG-DAS and APBA. The degrees of cross-linking in the mPEG-DAS–APBA polymers are listed in Table 1. For different ODs of 20%, 40% and 60% of mPEG-DAS, the corresponding degrees of cross-linking were 0.08, 0.15, and 0.17, respectively.
| Sample | Feeding radio | Graft radio | Zeta (mV) | CMC (mg mL−1) | DLE (%) | DLC (%) | ||
|---|---|---|---|---|---|---|---|---|
| mPEG-DAS/APBA | mPEG-DAS/Cou | APBA | Cou | |||||
| 20% mPEG-DAS–APBA | 1/5 | — | 0.08 | — | −1.39 ± 0.12 | 1.32 × 10−3 | — | — |
| 40% mPEG-DAS–APBA | 1/5 | — | 0.15 | — | −3.39 ± 0.49 | 8.13 × 10−3 | — | — |
| 60% mPEG-DAS–APBA | 1/5 | — | 0.17 | — | −4.09 ± 0.42 | 3.02 × 10−2 | — | — |
| mPEG-DAS–APBA–Cou | 1/5 | 1/3 | 0.15 | 0.1 | −9.96 ± 0.87 (pH 7.4) | 3.57 × 10−3 | 30.4 | 9.4 |
| −6.34 ± 0.32 (pH 5.4) | ||||||||
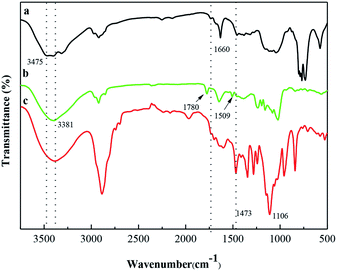
The FTIR spectra of St, DAS and mPEG-DAS–APBA–Cou are shown in Fig. 2. Fig. 2a shows the characteristic peaks of the St sample; a faint adsorption band can be found at 1660 cm−1 that corresponds to starch bone stretching vibrations. Compared with starch, the FTIR spectrum of Fig. 2c shows –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O– stretching vibrations and a new strong characteristic absorption band at 1106 cm−1 resulting from C–O asymmetric stretching vibrations; moreover, the –C
O– stretching vibrations and a new strong characteristic absorption band at 1106 cm−1 resulting from C–O asymmetric stretching vibrations; moreover, the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O– absorption peak was weaker than that in the spectrum of DAS (Fig. 2b).
O– absorption peak was weaker than that in the spectrum of DAS (Fig. 2b).
Characterization of dialdehyde starch derivative micelles
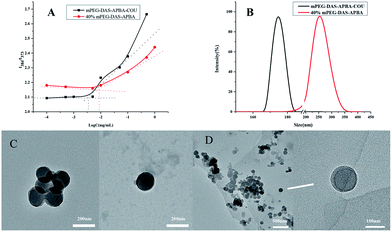
During the self-assembly process, CMC is a vital characteristic of polymers that not only indicates the self-aggregation behavior of micelles, but also evaluates their stability.49 The self-assembly behaviors of different ODs of mPEG-DAS–APBA micelles and mPEG-DAS–APBA–Cou micelles were investigated by fluorimetry. The changes in the intensity ratio of I384/I373, which are dependent upon high sensitivity to hydrophobicity, are plotted in Table 1 and Fig. 3A. It can be observed that the CMC increased with increasing value of OD.As shown in Fig. 3, TEM and DLS were performed to evaluate the aggregation behaviors of 40% mPEG-DAS–APBA and mPEG-DAS–APBA–Cou micelles as well as their sizes and morphologies. The morphologies of the 40% mPEG-DAS–APBA and mPEG-DAS–APBA–Cou polymeric self-assemblies were spherical, with diameters of about 175 ± 7.5 nm and 113.5 ± 3.5 nm, respectively. On the other hand, the “core–shell” structures of the micelles with different compositions were also very distinct in the TEM observations (Fig. 3C and D). Moreover, the DLS results indicated that the hydrodynamic diameters of the 40% mPEG-DAS–APBA (241.4 nm) and mPEG-DAS–APBA–Cou (177.9 nm) micelles decreased upon addition of hydrophobic Cou, where each of the groups exhibited a monomodal size distribution (Fig. 3B). The zeta potential values of the 40% mPEG-DAS–APBA and mPEG-DAS–APBA–Cou polymers with “core–shell” structures are shown in Table 1. The zeta value of 40% mPEG-DAS–APBA is −25.39 ± 0.49 mV (PBS 7.4), whereas slight changes in the zeta potential of the mPEG-DAS–APBA–Cou micelles were observed at −9.96 ± 0.87 mV (PBS 7.4) and −6.34 ± 0.32 mV (PBS 5.4).
In vitro stability study of dialdehyde starch derivative micelles
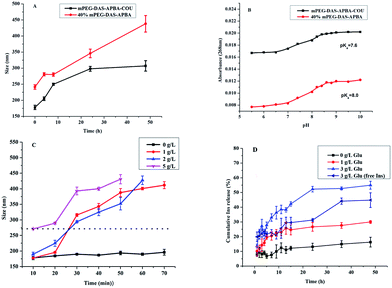
First, the stabilities of the dialdehyde starch derivative micelles were measured by DLS. As shown in Fig. 4A, the stabilities of the 40% mPEG-DAS–APBA and mPEG-DAS–APBA–Cou micelles were investigated at 1 mg mL−1 for 4 h, 8 h, 24 h and 48 h at 37 °C. The 40% mPEG-DAS–APBA and mPEG-DAS–APBA–Cou micelles changed distinctly from 241.4 nm to around 438.2 nm and from 177.9 nm to around 307 nm after 48 h, respectively. The 40% mPEG-DAS–APBA and mPEG-DAS–APBA–Cou micelles both showed slight increases in particle size after 8 h and considerable increases after 24 h.Glucose-responsiveness of dialdehyde starch derivative micelles
The UV absorbance (268 nm) is shown in Fig. 4B; the pKa values of 40% mPEG-DAS–APBA and mPEG-DAS–APBA–Cou were estimated to be 8.0 and 7.6. It can be clearly seen in Fig. 4C that the particle size of the mPEG-DAS–APBA–Cou micelles remained almost unchanged in 70 min in the presence of 0 g L−1 glucose. Notably, glucose-responsive behavior was observed at 1, 2 and 5 g L−1 in different concentrations of glucose solution; the micelles remained stable in size in the presence of 1 g L−1 glucose and changed slightly in the presence of 2 g L−1 glucose in 10 min. Then, the micelles completely disintegrated in 20 to 30 min in the presence of 1 and 2 g L−1 glucose. Finally, at a concentration of 5 g L−1 glucose, the complex micelles completely disintegrated at the outset.Hemolysis assay
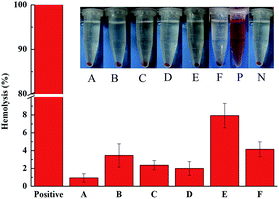
As shown in Fig. 5, compared with the positive control, the dialdehyde starch derivative micelles had lower hemolytic activity. We found that 60% mPEG-DAS–APBA showed a higher hemolytic activity value of 7.93%, whereas 40% mPEG-DAS–APBA (2.00%) exhibited the lowest hemolysis value. Compared to mPEG-DAS–APBA–Cou (4.15%), the hemolytic activity was slightly greater than that of 40% mPEG-DAS–APBA. In addition, a photograph showed that RBCs were released into the supernatant after treatment with dialdehyde starch derivative micelles. However, a slight red color was observed for the dialdehyde starch derivative micelles-treated cells.Insulin loading and glucose-responsive release
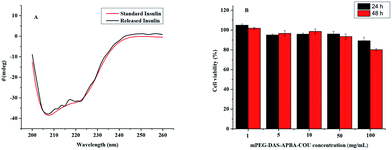
The results are shown in Table 1. The drug loading efficiency (DLE) and drug loading content (DLC) of the insulin-loaded micelles were found to be 30.4% and 9.4% with respect to the standard curve of insulin, respectively.Fig. 4D shows that the cumulative release of insulin-loaded mPEG-DAS–APBA–Cou polymers and free insulin upon exposure to different glucose media (0, 1 and 3 mg mL−1) at 37 °C (pH 7.4) increased with increasing glucose concentration. As indicated in Fig. 6A, little conformational change was detected for the insulin released from mPEG-DAS–APBA–Cou compared with standard insulin (pH 7.4); the [ϕ]208/[ϕ]223 ratios for standard insulin and released insulin were 1.24 and 1.17, respectively.
Cytotoxicity test
To evaluate the potential toxicity of the copolymers, an MTT assay was performed to measure the cell cytotoxicity of the micelles in A549 lung cancer cells for 24 h and 48 h (Fig. 6B). Fig. 6B shows that the cells were exposed to various concentrations of the blank mPEG-DAS–APBA–Cou and incubated for 24 and 48 h. More than 80% A549 lung cancer cell viability was retained at all concentrations from 1 to 100 mg mL−1 after 48 h cultivation as the nanoparticle concentration increased.Discussion
In the present study, we developed a novel amphiphilic glucose-sensitive dialdehyde starch polymer composed of APBA and Cou with an mPEG-DAS molecular skeleton for insulin release in diabetes treatment. On the basis of the 1H NMR and FTIR spectral analyses, we confirmed that the targeted dialdehyde starch derivatives were successfully synthesized. During the self-assembly process, the CMC value of 20% mPEG-DAS–APBA provided a lower value than 60% mPEG-DAS–APBA owing to the different ODs based on different grafting ratios of hydrophobic PBA. The CMC value of 40% mPEG-DAS–APBA–Cou was lower than that of 40% mPEG-DAS–APBA due to the presence of hydrophobic Cou groups; a lower CMC results in higher micelle stability.50The morphologies of the 40% mPEG-DAS–APBA and mPEG-DAS–APBA–Cou polymeric self-assemblies were spherical. The “core–shell” structures of the micelles with different compositions were also very distinct in the TEM observations. However, the micelles showed lower dispersibility in the dry state; this can be attributed to the lower ratio of hydrophilic groups grafted onto dialdehyde starch, whose chain segments tangle easily. Moreover, the DLS results indicated that the hydrodynamic diameters of the 40% mPEG-DAS–APBA and mPEG-DAS–APBA–Cou micelles were reduced upon addition of hydrophobic Cou, where each of the groups exhibited a monomodal size distribution. The interactions of electrostatic, hydrophobic and hydrogen bonds, as well as existing intermolecular forces, may lead to micellization in aqueous solution.
The stability of the dialdehyde starch derivative micelles was measured by DLS because the physiological stability of polymeric micelles is of importance for long-term storage, transportation, and scalable processing in drug carrier applications.51 Because the micellar structure is susceptible to exposure of the hydrophobic core, the 40% mPEG-DAS–APBA and mPEG-DAS–APBA–Cou micelles showed a slight increase in particle size after 8 h and 24 h due to the appearance of abundant aggregation of the cores. Compared with the 40% mPEG-DAS–APBA micelles, the mPEG-DAS–APBA–Cou micelles indicated preferable stability. These results confirmed that the mPEG-DAS–APBA–Cou micelles have extraordinarily enhanced stability, which is propitious for a drug carrier.
As prospective self-regulated materials for insulin delivery, PBA-based polymer materials have been widely studied. However, favourable response to different glucose concentrations is difficult under physiological conditions for PBA-based nanopolymers because of the high pKa of PBA.37,38 Dialdehyde starch derivative polymers were prepared to build a platform to attach Cou, which is more acidic than the phenylboronic acid derivative in the polymer. From the UV absorbance spectra (268 nm) shown in Fig. 4B, the pKa values of 40% mPEG-DAS–APBA and mPEG-DAS–APBA–Cou imply that the boronate ester would remain stable at pH values between 8.0 and 7.6. Particularly, the pKa value of mPEG-DAS–APBA–Cou was close to physiological conditions. As the glucose sensitivity in complexation with polyol compounds increased under physiological conditions, the pKa values of the PBA-based materials apparently decreased.52 The mPEG-DAS–APBA micelles with different ODs displayed diverse glucose-responsiveness behaviors, showing that the complex micelles with high contents of dialdehyde groups were more sensitive to glucose; this would lead to uncontrolled release, as briefly shown in Fig. S2.† In this study, the micelles with glucose attached indeed manifested water-solublility. These phenomena completely clarified the disintegrative process in the micelles “core–shell” structure and also indicated the high solubility of the amphipathic nanoparticles. The polymer was expected to form micelles with enhanced glucose-responsiveness for applications in self-regulated insulin delivery close to physiological range.
Determining the blood compatibility and cytotoxicity of dialdehyde starch derivative micelle systems is the primary screening for their in vivo applications.53 Because the hydrophobic polymers are combined with human blood, the possibility of absorptivity of plasma proteins will be enhanced. As a result, an embolism or thrombosis at the blood-contacting side of the polymer interface in the bloodstream introduces blood platelet activation.54–56 As shown in Fig. 5, compared with the positive control, the dialdehyde starch derivative micelles had lower hemolytic activity. Thus, these results also indicate that 40% mPEG-DAS–APBA and mPEG-DAS–APBA–Cou can prevent hemolytic activity by blocking the hydration layer around the polymer surfaces and electrostatic interactions with the RBC membrane, improving their blood compatibility. To evaluate the potential cytotoxicities of the copolymers, the cytotoxicities of the micelles toward A549 lung cancer cells were determined by MTT assays for 24 h and 48 h, respectively (Fig. 6B). The blank mPEG-DAS–APBA–Cou micelles demonstrated low toxicity and good cytocompatibility with the backbone of DAS. Compared with the PBA-based material reported by Yang et al.,11 blank mPEG-DAS–APBA–Cou had lower toxicity. The formation of hemocompatible and cytocompatible polymers has been recognized as an essential characteristic to maintain their protein resistance properties57 and low cytotoxicity. Therefore, mPEG-DAS–APBA–Cou has better cytocompatibility for drug delivery.
The practicability of the mPEG-DAS–APBA–Cou micelles as a novel nanocarrier showed sensitivity to glucose and achieved controlled release of insulin. Insulin was loaded into mPEG-DAS–APBA–Cou nanoparticles on the basis of hydrogen bonding, electrostatic and hydrophilic–hydrophobic interactions. The drug loading efficiency (DLE) and drug loading content (DLC) of the micelles are shown in Table 1 with respect to the standard curve of insulin. Fig. 4D exhibits the cumulative release of insulin-loaded polymers upon exposure to different glucose media (0, 1 and 3 mg mL−1) at 37 °C (pH 7.4). The insulin was released from drug-loaded mPEG-DAS–APBA–Cou nanoparticles in the glucose-free medium because the nanoparticle surface was covered with insulin. With increasing glucose concentration (0 to 3 g L−1), the amount of released insulin significantly increased because of the higher glucose concentration. The more APBA moieties dissociated from the mPEG-DAS–APBA–Cou, the more the moieties were associated with higher concentrations of glucose. Compared with glucose in 1 mg L−1 and 3 mg L−1 treatment, there was a close burst release phenomenon for mPEG-DAS–APBA–Cou within the first 5.0 h, which was ascribed to the adsorption of insulin on the surface of the nanoparticles. Then, the insulin release rate became slow; it finally reached a plateau phase in the same time within 13.0 h and approximately increased by 25.0%. At only 3 g L−1 glucose concentration, the structure of the nanoparticles was able to break and quickly release insulin. Compared with a report by Zhang et al.58 on the cumulative release of insulin-loaded polymers, mPEG-DAS–APBA–Cou showed a high amount of release in a short treatment time.
A convenient technique, CD spectroscopy is the best way to evaluate the conformational changes and self-association of insulin.59 The ratio of the band at 208 nm arising from the α-helix structure and that at 223 nm arising from the β-structure ([ϕ]208/[ϕ]223) can qualitatively measure the overall conformational structure of insulin (Fig. 6A). According to the spectral characteristics, the structure of the released insulin has not been distorted.
Conclusions
Novel amphiphilic glucose-sensitive dialdehyde starch polymer micelles with “shell–core” structures were synthesized by Schiff base bonds. Their DLC and DLE values were as high as 9.4% and 30.4%, respectively. Due to the carbohydrate moieties introduced into the polymers, the mPEG-DAS–APBA–Cou nanoparticles had low cytotoxic activity and hemolytic activity. The nanocarriers, whose pKa was reduced by the introduction of Cou, showed notable glucose responsive behaviour; they released insulin rapidly in 3 mg mL−1 glucose (pH 7.4), with comparatively inert release in 1 mg mL−1 glucose (pH 7.4). The glucose-sensitive insulin release could be adjusted by changing the glucose medium. Therefore, the amphiphilic mPEG-DAS–APBA–Cou nanoparticles have potential applicability as a glucose-responsive material for insulin delivery.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge the financial support from the National Natural Science Foundation of China (grant no. 51541304, 51273086, 51503091, 51603097), the Special Doctorial Program Fund from the Ministry of Education of China (grant no. 20130211110017), and the Fundamental Research Funds for the Central Universities (grant no. lzujbky-2016-41, lzujbky-2017-it43).Notes and references
- K. M. Bratlie, R. L. York, M. A. Invernale, R. Langer and D. G. Anderson, Adv. Healthcare Mater., 2012, 1, 267–284 CrossRef CAS PubMed.
- I. G. Véronique Lapeyre, S. Chevreux and V. Ravaine, Biomacromolecules, 2006, 7, 3356–3363 CrossRef PubMed.
- F. Cheng and F. Jäkle, Polym. Chem., 2011, 2, 2122 RSC.
- W. Chen, Y. Cheng and B. Wang, Angew. Chem., 2012, 51, 5293–5295 CrossRef CAS PubMed.
- V. L. Alexeev, S. A. Asher, A. V. Goponenko, A. C. Sharma, I. K. Lednev, C. S. Wilcox and D. N. Finegold, J. Am. Chem. Soc., 2003, 125, 3322–3329 CrossRef PubMed.
- Y. G. Yongjun Zhang and S. Zhou, Biomacromolecules, 2006, 7, 3196–3201 CrossRef PubMed.
- R. Ma, H. Yang, Z. Li, G. Liu, X. Sun, X. Liu, Y. An and L. Shi, Biomacromolecules, 2012, 13, 3409–3417 CrossRef CAS PubMed.
- H. Yang, C. Zhang, C. Li, Y. Liu, Y. An, R. Ma and L. Shi, Biomacromolecules, 2015, 16, 1372–1381 CrossRef CAS PubMed.
- A. C. Sharma, V. L. Alexeev, A. V. Goponenko, S. Das, I. K. Lednev, C. S. Wilcox, D. N. Finegold and S. A. Asher, Anal. Chem., 2003, 75(10), 2316–2323 CrossRef.
- Z. Wu, X. Zhang, H. Guo, C. Li and D. Yu, J. Mater. Chem., 2012, 22, 22788 RSC.
- H. Yang, X. Sun, G. Liu, R. Ma, Z. Li, Y. An and L. Shi, Soft Matter, 2013, 9, 8589 RSC.
- R. Ma, B. Wang, P. Sun and L. Shi, Chin. J. Chem., 2014, 32, 97–102 CrossRef CAS.
- J. Ren, Y. Zhang, J. Zhang, H. Gao, G. Liu, R. Ma, Y. An, D. Kong and L. Shi, Biomacromolecules, 2013, 14, 3434–3443 CrossRef CAS PubMed.
- H. Yang, R. Ma, J. Yue, C. Li, Y. Liu, Y. An and L. Shi, Polym. Chem., 2015, 6, 3837–3846 RSC.
- R. Ma and L. Shi, Polym. Chem., 2014, 5, 1503–1518 RSC.
- J. M. Harris and R. B. Chess, Nat. Rev. Drug Discovery, 2003, 2, 214–221 CrossRef CAS PubMed.
- C. Deng, Y. Jiang, R. Cheng, F. Meng and Z. Zhong, Nano Today, 2012, 7, 467–480 CrossRef CAS.
- J. N. Cambre, D. Roy, S. R. Gondi and B. S. Sumerlin, J. Am. Chem. Soc., 2007, 129(34), 10348–10349 Search PubMed.
- D. Roy and B. S. Sumerlin, ACS Macro Lett., 2012, 1, 529–532 CrossRef CAS.
- D. Roy, J. N. Cambre and B. S. Sumerlin, Chem. Commun., 2008, 2477–2479 RSC.
- H. Kim, Y. J. Kang, E. S. Jeong, S. Kang and K. T. Kim, ACS Macro Lett., 2012, 1, 1194–1198 CrossRef CAS.
- H. Kim, Y. J. Kang, S. Kang and K. T. Kim, J. Am. Chem. Soc., 2012, 134, 4030–4033 CrossRef CAS PubMed.
- B. Wang, R. Ma, G. Liu, X. Liu, Y. Gao, J. Shen, Y. An and L. Shi, Macromol. Rapid Commun., 2010, 31, 1628–1634 CrossRef CAS PubMed.
- L. Zhao, C. Xiao, L. Wang, G. Gai and J. Ding, Chem. Commun., 2016, 52, 7633–7652 RSC.
- A. Rodrigues and M. Emeje, Carbohydr. Polym., 2012, 87, 987–994 CrossRef CAS.
- G. H. Ahmed Besheer, J. Kressler and K. Mäder, Biomacromolecules, 2007, 8, 359–367 CrossRef PubMed.
- H. Yamada, B. Loretz and C. M. Lehr, Biomacromolecules, 2014, 15, 1753–1761 CrossRef CAS PubMed.
- M. J. Santander-Ortega, T. Stauner, B. Loretz, J. L. Ortega-Vinuesa, D. Bastos-Gonzalez, G. Wenz, U. F. Schaefer and C. M. Lehr, J. Controlled Release, 2010, 141, 85–92 CrossRef CAS PubMed.
- H. Horchani, M. Chaâbouni, Y. Gargouri and A. Sayari, Carbohydr. Polym., 2010, 79, 466–474 CrossRef CAS.
- L. Zhang, X. Gong, Y. Wang and H. Qu, J. Chem. Eng. Data, 2012, 57, 2018–2022 CrossRef CAS.
- S. H. Yoo, J. S. Lee, S. Y. Park, Y. S. Kim, P. S. Chang and H. G. Lee, Int. J. Biol. Macromol., 2005, 35, 27–31 CrossRef CAS PubMed.
- Q. Wang, C. X. Li, X. Fan, P. Wang and L. Cui, Biocatal. Biotransform., 2009, 26, 437–443 CrossRef.
- X. He, M. Du, H. Li and T. Zhou, Int. J. Biol. Macromol., 2016, 82, 174–181 CrossRef CAS PubMed.
- J.-Y. Kim and S.-T. Lim, Carbohydr. Polym., 2009, 76, 110–116 CrossRef CAS.
- V. Mazíková, I. Sroková and A. Ebringerová, Chem. Pap., 2009, 63(1), 71–76 Search PubMed.
- Z. Zhang, H. Shan, L. Chen, C. He, X. Zhuang and X. Chen, Eur. Polym. J., 2013, 49, 2082–2091 CrossRef CAS.
- Y. M. Daijiro Shiino, A. Kubo, Y. J. Kim, K. Kataoka, Y. Koyama, A. Kikuchi, M. Yokoyama, Y. Sakurai and T. Okano, J. Controlled Release, 1995, 37, 269–276 CrossRef.
- S. I. Akira Matsumoto, A. Harada and K. Kataoka, Biomacromolecules, 2003, 4, 1410–1416 CrossRef PubMed.
- J. Yoon and A. W. Czarnik, J. Am. Chem. Soc., 1992, 114, 5874–5875 CrossRef CAS.
- N. M. Weitai Wu, E. C. Y. Yan and S. Zhou, ACS Nano, 2010, 4, 4831–4839 CrossRef PubMed.
- Z. Yang, W. Zhang, J. Zou and W. Shi, Polymer, 2007, 48, 931–938 CrossRef CAS.
- A. Zhang, Z. Zhang, F. Shi, J. Ding, C. Xiao, X. Zhuang, C. He, L. Chen and X. Chen, Soft Matter, 2013, 9, 2224 RSC.
- N. L. Chun Zhang and D. E. Hirt, Langmuir, 2006, 22, 6851–6857 CrossRef PubMed.
- M. Chen, C. Gao, S. Lü, Y. Chen and M. Liu, RSC Adv., 2016, 6, 46159–46169 RSC.
- M. Badawi, S. Soundararajan, C. Montafio Kohlrust and J. H. Hagernan, Anal. Biochem., 1989, 178, 125–134 CrossRef.
- H. Liu, D. Chaudhary, S.-i. Yusa and M. O. Tadé, Carbohydr. Polym., 2011, 83, 1591–1597 CrossRef CAS.
- J. Yang, C. Gao, S. Lü, X. Wang, M. Chen and M. Liu, RSC Adv., 2014, 4, 55139–55149 RSC.
- M. G. Mohamed, K.-C. Hsu and S.-W. Kuo, Polym. Chem., 2015, 6, 2423–2433 RSC.
- W. Yu, H. Zhou, X. Guo, X. Liu, N. Li, Y. Zhang and X. Ma, Biomacromolecules, 2010, 11, 3480–3486 CrossRef PubMed.
- H. Zhang, X. Kong, Y. Tang and W. Lin, ACS Appl. Mater. Interfaces, 2016, 8, 16227–16239 CAS.
- H. Deng, X. Zhao, J. Liu, J. Zhang, L. Deng, J. Liu and A. Dong, Nanoscale, 2016, 8, 1437–1450 RSC.
- G. S. a. B. Wang, Tetrahedron, 2002, 58, 5291–5300 CrossRef.
- A. Yildirim, E. Ozgur and M. Bayindir, J. Mater. Chem. B, 2013, 1, 1909 RSC.
- M. S. W. Mingchao Shen, D. G. Castner, B. D. Ratner and T. A. Horbett, Langmuir, 2003, 19, 1692–1699 CrossRef.
- D. Kwak, Y. Wu and T. A. Horbett, J. Biomed. Mater. Res., Part A, 2005, 74, 69–83 CrossRef PubMed.
- D. Eglin, S. Grad, S. Gogolewski and M. Alini, J. Biomed. Mater. Res., Part A, 2010, 92, 393–408 CrossRef PubMed.
- Y. J. Shih, Y. Chang, A. Deratani and D. Quemener, Biomacromolecules, 2012, 13, 2849–2858 CrossRef CAS PubMed.
- G. Zhang, X. Zhang, H. Shen, J. Yang and J. Yang, RSC Adv., 2014, 4, 49964–49973 RSC.
- K. Kim, S. Lee, T. Suresh Kumar, J. Lee, S. Kyoon Kim, D. Yun Lee, Y.-k. Lee and Y. Byun, Bioconjugate Chem., 2005, 16, 615–620 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Characterization of dialdehyde starch, FTIR spectra of starch and dialdehyde starch derivatives and glucose-responsiveness of different ODs of mPEG-DAS–APBA micelles in the presence of glucose with 2 g L−1 Glu. See DOI: 10.1039/c7ra08291f |
| This journal is © The Royal Society of Chemistry 2017 |