Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A facile method to prepare energetic materials (EMs)†
Qian-You Wanga,
Shan Wangb,
Xiao Feng *b,
Le Wua,
Sheng-Han Zhangb,
Nan Dingb,
Wen-Chao Tonga,
Ming-Rui Zhouc,
Bo Wang
*b,
Le Wua,
Sheng-Han Zhangb,
Nan Dingb,
Wen-Chao Tonga,
Ming-Rui Zhouc,
Bo Wang b and
Li Yang
b and
Li Yang *a
*a
aState Key Laboratory of Explosion Science and Technology, Beijing Institute of Technology, Beijing 100081, P. R. China. E-mail: yanglibit@bit.edu.cn
bMinistry of Education of China, School of Chemistry, Beijing Institute of Technology, 5 South Zhongguancun Street, Beijing 100081, P. R. China. E-mail: fengxiao86@bit.edu.cn
cLogistics Center, China Academy of Launch Vehicle Technology, Beijing 100076, P. R. China
First published on 13th October 2017
Abstract
Two powerful yet safe energetic materials (EMs) have been synthesized with high yield via a facile method. We used low-cost industrial products as starting materials and water as a solvent rather than organic solvents without generating harmful by-products during the two-step synthetic procedure. Purification of the target materials could be obtained through a simple separation process with low energy consumption. Moreover, the thermal stability, insensitivity, and explosive performance of these two EMs are better than that of commonly used energetic materials such as TNT and TATB (2,4,6-triamino-1,3,5-trinitrobenzene).
Energetic materials (EMs) have a long application history and play an indispensable role in both military and civilian fields, such as aerospace propellants, mining engineering, and pyrotechnics.1–4 Over the past few years, researchers have devoted their efforts to balance the power and safety of EMs through designing novel molecular structures or developing innovative synthetic methods. Recently, EMs have attracted tremendous attention for the sake of global sustainability, improving cost-effectiveness and developing simple synthetic procedures.
Often times, EMs may contain extremely toxic substances such as lead cations or perchlorate anions. Nitrogen-rich ionic energetic salts are promising candidates for green EMs, since the main composition of their explosion products will be dinitrogen.5–7 These energetic materials show remarkable thermal stability, low sensitivity to physical stimuli, and excellent detonation performance.8,9 Up to now, some pioneering work on ionic energetic materials, such as 3,3′-dinitroamino-4,4′-azoxyfurazan,10 3,6,7-triamino-[1,2,4]-triazolo-[4,3b][1,2,4]-triazole,11 have been reported by T. M. Klapötke,12–14 J. n. M. Shreeve15–18 and other groups.19–22 Despite their remarkable high energy and low sensitivity, these nitrogen-rich ionic materials have some disadvantages: raw materials are usually expensive; harsh condition and long reaction time are needed; high boiling-point solvents are employed that raises the difficulty of the following purification; reaction procedures are usually complicated yet the yields are often low.
In this research, we reported a facile two-step synthetic strategy to prepare two nitrogen-rich energetic salts (EM-1 and EM-2) with high yield and purity under mild conditions by using low-cost industrial products as raw materials through a hydrothermal approach without the production of environmentally harmful by-products. Comprehensive studies of the reaction conditions and the effects of the substituent groups in the starting materials allowed for the synthesis of energetic salts. Their crystal structures, thermal decomposition behaviors, non-isothermal kinetics and sensitivities and calculated their detonation velocities and detonation pressures were investigated. Both of these salts possess high enthalpy of formation and good explosive performance with high thermal stability, insensitivity towards impact, friction, and electrostatic discharge. This strategy presents an alternative synthetic procedure that suitable for industrial preparation and applications.
Based on the following considerations, we designed and synthesized 1,2-bis(hydrazinocarbonyl)hydrazine (BHCH) as the cation component of the energetic salts through the reaction of commercial available dialkyl azodicarboxylates and hydrazine hydrate in high yield. Firstly, BHCH has high nitrogen content and multiple energetic N–N and N–C bonds, which give rise to high enthalpy of formation and good explosive performance. Secondly, the carbonyl group and hydrazine group of BHCH can form hydrogen bonds with other energetic anions, which may improve the thermal stability and safety of the energetic materials.23–25 Moreover, the flexible chain structure of BHCH is beneficial for the formation of more hydrogen bonds compared to normal N-heterocyclic compounds with rigid structure. Thirdly, it is worth noting that the starting materials, dialkyl azodicarboxylates, are cost effective and widely used in industry as foaming agents and plasticizers. Fourthly, the by-products of the reaction are N2, H2O and alcohols. Finally, the BHCH is able to react with picric acid (PA) or 2,4,6-trinitroresorcinol (TNR) to obtain halogen-free and metal-free BHCH-containing EMs in water at 60 °C minimizing pollution (Scheme 1).
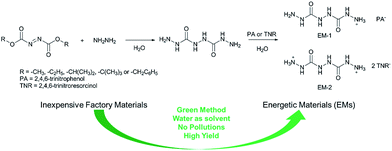
Initially, we focused our efforts on optimizing the reaction conditions for the synthesis of BHCH. Five industrially abundant and inexpensive dialkyl azodicarboxylates, dimethyl azodicarboxylate (DMAD), diethyl azodicarboxylate (DEAD), diisopropyl azodicarboxylate (DIAD), di-tert-butyl azodicarboxylate (DTAD) and dibenzyl azodicarboxylate (DBAD), were chosen to react with 60% hydrazine hydrate solution that acts as both nucleophilic reagent and reducing agent in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio. The reaction was carried out at 70 °C temperature overnight. Pure BHCH could be obtained via simple precipitating in ethanol without the need of recrystallization or column purification. The yields could reach 43%, 52%, 34%, 31%, and 39% for DMAD, DEAD, DIAD, DTAD, and DBAD as starting materials, respectively. Single-crystal X-ray analysis (SXRD), 1H-NMR, IR and MS spectra confirmed the structure and purity of the resultant product.
3 ratio. The reaction was carried out at 70 °C temperature overnight. Pure BHCH could be obtained via simple precipitating in ethanol without the need of recrystallization or column purification. The yields could reach 43%, 52%, 34%, 31%, and 39% for DMAD, DEAD, DIAD, DTAD, and DBAD as starting materials, respectively. Single-crystal X-ray analysis (SXRD), 1H-NMR, IR and MS spectra confirmed the structure and purity of the resultant product.
We chose DEAD as starting material and further investigated the effects of the ratio of the starting materials and reaction temperatures to optimize its reaction conditions. The yields were improved by increasing the amount of hydrazine hydrate and hit a plateau at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (Fig. 1c). It reached maximum yield of 92% with a reaction temperature of 70 °C (Fig. 1d), further increasing the temperature would lead to cyclization side reactions and formation of by-products.
5 (Fig. 1c). It reached maximum yield of 92% with a reaction temperature of 70 °C (Fig. 1d), further increasing the temperature would lead to cyclization side reactions and formation of by-products.
After optimizing the reaction conditions for BHCH, we prepared energetic salts by reacting BHCH with PA or TNR in water at 60 °C for 1 h. Then, the mixtures were cooled to room temperature, and EM-1 (PA− as counter ion) or EM-2 (TNR− as counter ion) were precipitated out of solution and recovered through filtration with up to 87% and 82% yield, respectively. The structures and purities of EM-1 and EM-2 were confirmed by IR, elemental analyses and SXRD. The synthesis of these two EMs undergo an acid–base reaction in water avoiding both the use of organic solvents and the formation of toxic by-products to produce two metal-free and halogen-free materials. Compared with commonly used decomposition reaction for the syntheses of energetic salts, the acid–base reaction is more eco-friendly and simple.
SXRD analyses showed that EM-1 is composed of the univalent cation of BHCH and PA− with the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and EM-2 is composed of the divalent cation of BHCH and TNR− with the ratio of 1
1 and EM-2 is composed of the divalent cation of BHCH and TNR− with the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. As shown in Table S1 (ESI†), these two EM crystals belong to the monoclinic system with space group P2(1)/c. The energetic N–N bond lengths in the EMs range from 1.378(4) to 1.421(4) Å. Due to intramolecular hydrogen bonding and distortion of BHCH, the N–N single bonds between carbon of EM-1 (1.3812 Å) and EM-2 (1.378 Å) are much shorter than normal N–N single bonds (1.454 Å) and even shorter than N
2. As shown in Table S1 (ESI†), these two EM crystals belong to the monoclinic system with space group P2(1)/c. The energetic N–N bond lengths in the EMs range from 1.378(4) to 1.421(4) Å. Due to intramolecular hydrogen bonding and distortion of BHCH, the N–N single bonds between carbon of EM-1 (1.3812 Å) and EM-2 (1.378 Å) are much shorter than normal N–N single bonds (1.454 Å) and even shorter than N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bonds (1.245 Å). The energetic N–C bond lengths in the EMs range from 1.348(4) to 1.467(2) Å. As the different group connected to N–C bond, the N–C bond connected to hydrazine group (1.424 to 1.467 Å) are longer than N–C bond connected to nitro group (1.348 to 1.385 Å). There exists significant hydrogen bonding between ion and π–π action between the benzene rings, which may contribute to their densities and stabilities. For example, EM-1 has three types of hydrogen bonds, which are an intramolecular hydrogen bond of BHCH (N2–H2⋯O2), an intermolecular hydrogen bond of BHCH (N5–H5⋯O2) and an intermolecular hydrogen bond between BHCH+ and PA− (N1–H1A⋯O3) (Fig. 2).
N double bonds (1.245 Å). The energetic N–C bond lengths in the EMs range from 1.348(4) to 1.467(2) Å. As the different group connected to N–C bond, the N–C bond connected to hydrazine group (1.424 to 1.467 Å) are longer than N–C bond connected to nitro group (1.348 to 1.385 Å). There exists significant hydrogen bonding between ion and π–π action between the benzene rings, which may contribute to their densities and stabilities. For example, EM-1 has three types of hydrogen bonds, which are an intramolecular hydrogen bond of BHCH (N2–H2⋯O2), an intermolecular hydrogen bond of BHCH (N5–H5⋯O2) and an intermolecular hydrogen bond between BHCH+ and PA− (N1–H1A⋯O3) (Fig. 2).
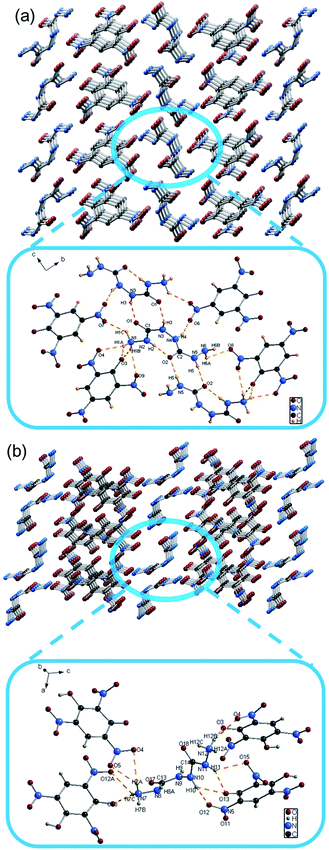 | ||
| Fig. 2 (a) Packing diagram of EM-1 and hydrogen bonds of EM-1 in the crystal structure; (b) packing diagram of EM-2 and hydrogen bonds of EM-2 in the crystal structure. | ||
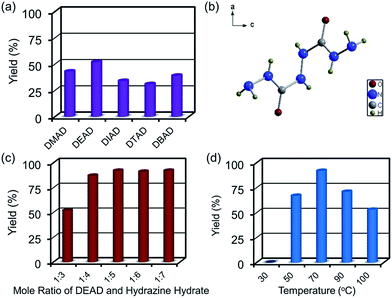
Thermal decomposition temperature of explosives is a crucial parameter in terms of performance.26 The thermal behaviors of EM-1 and EM-2 were investigated by differential scanning calorimetry (DSC) measurements with a linear heating rate of 5, 10, 15, 20 °C min−1 (Fig. 3). At the heating rate is 5 °C min−1, the DSC curve of EM-1 has an endothermic peak at 199.11 °C and an exothermic peak at 211.05 °C, corresponding to the melting process and the energy release process, respectively. EM-2 demonstrated one exothermic processes at 215.51 °C under the same heating rate, revealing its capability in heat generating. Typically, energetic materials with higher than 200 °C decomposition temperature imply good thermal stability.
Apparent activation energies (Ea) can be used to estimate the rate constants of the initial thermal decomposition process. We utilized both Kissinger's method and Ozawa's method to calculate the activation energies of these two EMs based on the following equations,27–29
Kissinger's method
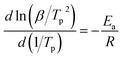 | (1) |
Ozawa's method
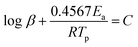 | (2) |
According to the exothermic peak temperatures determined at four different heating rates of 5, 10, 15 and 25 °C min−1, the thermo–kinetic parameters of exothermal processes for EM-1 and EM-2 are calculated and shown in Table 1, including the apparent activation energies EO and EK, pre-exponential factor AK and linear correlation coefficients RK and RO. The Ea values of both EM-1 (EK, 225.7 kJ mol−1; EO, 222.4 kJ mol−1) and EM-2 (EK, 240.7 kJ mol−1; EO, 236.7 kJ mol−1) are much higher than RDX (144 kJ mol−1).
| EM-1 | EM-2 | ||
|---|---|---|---|
| Tp (°C) | 5 | 211.05 | 215.51 |
| 10 | 217.32 | 220.32 | |
| 15 | 220.81 | 224.17 | |
| 20 | 222.68 | 226.74 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Kissinger's method | |||
| EK [kJ mol−1] | 225.7 | 240.7 | |
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AK AK |
22.33 | 23.75 | |
| RK | −0.998 | −0.9972 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Ozawa's method | |||
| EO [kJ mol−1] | 222.4 | 236.7 | |
| RO | −0.9981 | −0.9974 | |
The safety of these EMs to mechanical stimulation, including impact, friction and electrostatic discharge, were tested according to the corresponding standard methods and corresponding data are summarized in Table 2. The impact sensitivities (IS) of EM-1 and EM-2 are higher than 40 J, indicating that the compounds are insensitive to the impact. These IS values are lower than that of TNT (15 J) and even lower than 3D energetic metal organic frameworks famous for their insensitivies.31,34 No friction sensitivities are observed up to 360 N for these two EMs, while the value of which for RDX is 120 N. In addition, the two EMs are insensitive to electrostatic discharge (Table S5†), which are safer than TNT, RDX and HMX. The significantly high safety of these two EMs may be attributed to the multiple intramolecular and intermolecular interactions in the stacking structures, including hydrogen bonds, ionic bonds and π–π interactions as shown in literature.24,25
| Salts | Da | ΔHLb | ΔHfc cation | ΔHfd anion | ΔHfe | Pf | Dg | ISh | FSi | EDSj |
|---|---|---|---|---|---|---|---|---|---|---|
| a Densities obtained from X-ray measurements (g cm−3).b Lattice energy (kJ mol−1).c Molar enthalpy of the formation of cation (kJ mol−1).d Molar enthalpy of the formation of anion (kJ mol−1).e Molar enthalpy of the formation of salt (kJ mol−1).f Detonation pressure (GPa).g Detonation velocity (m s−1).h Impact sensitivity (J).i Friction sensitivity (J).j Electrostatic sensitivity (J).k Detonation velocity calculated by Explo 5 (m s−1).l Detonation pressure calculated by Explo 5 (GPa). | ||||||||||
| EM-1 | 1.817 | 440.3 | 643.2 | −108.5 | 94.4 | 28.5 (27.2k) | 8046 (8124l) | >40 | >360 | >24.75 |
| EM-2 | 1.719 | 967.0 | 2088.1 | −318.6 | 483.9 | 29.0 (30.7k) | 8197 (8330l) | >40 | >360 | >24.75 |
| TNT30,31 | 1.65 | — | — | — | 95.3 | 19.5 | 6881 | 15 | 353 | 0.57 |
| TATB32 | 1.93 | — | — | — | −139.7 | 30.5 | 8179 | 30–40 | — | — |
| RDX33 | 1.82 | — | — | — | 83.8 | 34.9 | 8748 | 7.4 | 120 | 0.15 |
The thermal stabilities of these EMs were characterized after 48 h heat treatment at 75 °C. Both EM-1 and EM-2 easily withstood a temperature of 75 °C after two days. Even higher temperatures of 90 °C did not lead to decomposition for one week. The results show that both EM-1 and EM-2 have a good thermal stability.
The enthalpy of formation is an important parameter for evaluating the performance of energetic salts, which is directly related to the number of nitrogen–nitrogen bonds in ionic species. The enthalpy of formation of EMs was calculated by quantum chemical calculations (ESI,† Section 6) and the results were summarized in Table 2. Both EM-1 and EM-2 exhibit positive heats of formation. In particular, the value of EM-2 (483.9 kJ mol−1) is much higher than TNT (95.3 kJ mol−1), TATB (−139.7 kJ mol−1), RDX (80.0 kJ mol−1) and HMX (104.8 kJ mol−1).
Based on the calculated values of the heats of formation and the densities of the EMs, the detonation velocities (D) and detonation pressures (P) were calculated by classic Kamlet–Jacobs equations35 and Explo 5. As shown in Table 2, the calculated detonation pressures of EM-1 (28.5 GPa by K–J equations and 27.2 GPa by Explo 5) and EM-2 (30.3 GPa by K–J equations and 30.7 GPa by Explo 5) are superior to TNT (19.5 GPa). The calculated detonation velocities of EM-1 are 8046 m s−1 by K–J equations and 8124 m s−1 by Explo 5 and the calculated detonation velocities of EM-2 are 8197 m s−1 by K–J equations and 8330 m s−1 by Explo 5, all of which outperform TNT (6881 m s−1).
Conclusions
In conclusion, two energetic materials have been synthesized with high yield and low energy-consumption separation process. The synthetic procedure utilized cost-effective simple chemicals as the starting materials, water as solvent and did not generate any toxic by-products. The two EMs are metal-free and halogen-free and exhibit high thermal stability. The apparent activation energies are much higher than those of the normal explosives such as TNT and RDX. Both EM-1 and EM-2 are insensitive to impact, friction, and electrostatic discharge, which indicates enhanced material safety for practical usage. The heats of formation, calculated detonation velocity and calculated detonation pressures of these two EMs demonstrate that they have high explosive power. This facile strategy offers a versatile method to synthesize safe energetic materials.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge financial support from: the State Key Laboratory of Explosion Science and Technology (No. YB2016-17); 973 Program (No. 2013CB834704); Provincial Key Project of China (No. 7131253); the National Natural Science Foundation of China (No. 11672040, 21471018, 21404010, 21490574); 1000 Plan (Youth).Notes and references
- D. M. Badgujar, M. B. Talawar, S. N. Asthana and P. P. Mahulikar, J. Hazard. Mater., 2008, 151, 289–305 CrossRef CAS PubMed.
- A. K. Sikder and N. Sikder, J. Hazard. Mater., 2004, 112, 1–15 CrossRef CAS PubMed.
- X. Liu, W. Gao, P. Sun, Z. Su, S. Chen, Q. Wei, G. Xie and S. Gao, Green Chem., 2015, 17, 831–836 RSC.
- Q. Y. Wang, W. C. Tong, C. Ma, T. L. Zhang and L. Yang, Z. Anorg. Allg. Chem., 2015, 641, 1550–1555 CrossRef CAS.
- G.-H. Tao, Y. Guo, Y.-H. Joo, B. Twamley and J. n. M. Shreeve, J. Mater. Chem., 2008, 18, 5524–5530 RSC.
- G. Drake, T. Hawkins, A. Brand, L. Hall, M. McKay, A. Vij and I. Ismail, Propellants, Explos., Pyrotech., 2003, 28, 174–180 CrossRef CAS.
- D. E. Chavez, M. A. Hiskey and R. D. Gilardi, Angew. Chem., Int. Ed., 2000, 112, 1861–1863 CrossRef.
- R. Wang, H. Xu, Y. Guo, R. Sa and J. n. M. Shreeve, J. Am. Chem. Soc., 2010, 132, 11904–11905 CrossRef CAS PubMed.
- C. B. Aakeröy, K. R. Seddon and M. Leslie, Struct. Chem., 1992, 3, 63–65 CrossRef.
- J. Zhang and J. n. M. Shreeve, J. Am. Chem. Soc., 2014, 136, 4437–4445 CrossRef CAS PubMed.
- T. M. Klapötke, P. C. Schmid, S. Schnell and J. Stierstorfer, Chem.–Eur. J., 2015, 21, 9219–9228 CrossRef PubMed.
- T. M. Klapötke, P. Mayer, C. Miró Sabaté, J. M. Welch and N. Wiegand, Inorg. Chem., 2008, 47, 6014–6027 CrossRef PubMed.
- T. M. Klapötke, J. R. Stierstorfer and A. U. Wallek, Chem. Mater., 2008, 20, 4519–4530 CrossRef.
- T. M. Klapötke and C. M. Sabaté, Dalton Trans., 2009, 1835–1841 RSC.
- R. P. Singh, R. D. Verma, D. T. Meshri and J. n. M. Shreeve, Angew. Chem., Int. Ed., 2006, 45, 3584–3601 CrossRef CAS PubMed.
- H. Gao and J. n. M. Shreeve, Chem. Rev., 2011, 111, 7377–7436 CrossRef CAS PubMed.
- Y. Tang, H. Gao, L. A. Mitchell, D. A. Parrish and J. n. M. Shreeve, Angew. Chem., Int. Ed., 2016, 128, 3252–3255 CrossRef.
- Y. Guo, G. H. Tao, Z. Zeng, H. Gao, D. A. Parrish and J. n. M. Shreeve, Chem.–Eur. J., 2010, 16, 3753–3762 CrossRef CAS PubMed.
- J.-T. Wu, J.-G. Zhang, X. Yin, Z.-Y. Cheng and C.-X. Xu, New J. Chem., 2015, 39, 5265–5271 RSC.
- C. Bian, M. Zhang, C. Li and Z. Zhou, J. Mater. Chem. A, 2015, 3, 163–169 CAS.
- Y.-T. Gao, L.-M. Zhao, F.-Q. Pang, X.-J. Qi, J.-L. Huang and F.-X. Chen, Chin. Chem. Lett., 2015, 27, 433–436 CrossRef.
- L. Liang, K. Wang, C. Bian, L. Ling and Z. Zhou, Chem.–Eur. J., 2013, 19, 14902–14910 CrossRef CAS PubMed.
- T. M. Klapötke and C. M. Sabaté, Chem. Mater., 2008, 20, 3629–3637 CrossRef.
- O. Bolton and A. J. Matzger, Angew. Chem., Int. Ed., 2011, 50, 8960–8963 CrossRef CAS PubMed.
- J. Zhang, Q. Zhang, T. T. Vo, D. A. Parris and J. M. Shreeve, J. Am. Chem. Soc., 2015, 137, 1697–1704 CrossRef CAS PubMed.
- T. B. Brill and K. J. James, Chem. Rev., 1993, 93, 2667–2692 CrossRef CAS.
- H. E. Kissinger, Anal. Chem., 1957, 29, 1702–1706 CrossRef CAS.
- T. Ozawa, Bull. Chem. Soc. Jpn., 1965, 38, 1881–1886 CrossRef CAS.
- C. D. Doyle, J. Appl. Polym. Sci., 1961, 5, 285–292 CrossRef CAS.
- Y. Zhang, Y. Guo, Y. H. Joo, D. A. Parrish and J. n. M. Shreeve, Chem.–Eur. J., 2010, 16, 10778–10784 CrossRef CAS PubMed.
- X. Y. Liu, Q. Yang, Z. Y. Su, S. P. Chen, G. Xie, Q. Wei and S. L. Gao, RSC Adv., 2014, 4, 16087–16093 RSC.
- T. M. Klapötke and C. M. Sabaté, New J. Chem., 2009, 33, 1605–1617 RSC.
- R. Meyer, J. Köhler and A. Homburg, Explosives. Sixth Edition, Wiley VCH Verlag GmbH & Co. KGaA, Weinheim, 2007 Search PubMed.
- S. Li, Y. Wang, C. Qi, X. Zhao, J. Zhang, S. Zhang and S. Pang, Angew. Chem., Int. Ed., 2013, 52, 14031–14035 CrossRef CAS PubMed.
- M. J. Kamlet and S. Jacobs, J. Chem. Phys., 2003, 48, 23–35 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1483054–1483056. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7ra08115d |
| This journal is © The Royal Society of Chemistry 2017 |