Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Solid-contact Ca2+-selective electrodes based on two-dimensional black phosphorus as ion-to-electron transducers†
Lijuan Kou*ab,
Minglang Fua and
Rongning Liang *b
*b
aSchool of Enology, Binzhou Medical University, Yantai, Shandong 264003, P. R. China. E-mail: koulijuan@bzmc.edu.cn
bKey Laboratory of Coastal Environmental Processes and Ecological Remediation, Yantai Institute of Coastal Zone Research (YIC), Chinese Academy of Sciences (CAS), Shandong Provincial Key Laboratory of Coastal Zone Environmental Processes, YICCAS, Yantai, Shandong 264003, P. R. China. E-mail: rnliang@yic.ac.cn
First published on 11th September 2017
Abstract
A new type of solid-contact polymeric membrane Ca2+-selective electrode has been developed by using black phosphorus as the transducing layer for the first time. The ion-to-electron transducing ability of black phosphorus was examined. The proposed electrode exhibits a good Nernstian slope with a detection limit of 4.0 × 10−7 M.
Ion-selective electrodes (ISEs) have become a routine analytical tool in clinical diagnostics, process control, environmental and agricultural relevance.1–5 With a view to miniaturization, mass fabrication and measurements in small volumes, solid-contact ion-selective electrodes (SC-ISEs) with a solid inner contact as the ion-to-electron transducer, have been receiving much attention.6–8
The first and most common SC-ISEs were constructed using polypyrrole (PPy), poly(3-octhylthiophene) (POT) and poly(3,4-ethylene-dioxythiophene) (PEDOT) as the ion-to-electron transducers.9–11 However, these sensors may still suffer from problems of sensitivity to light and gases, undesired side reactions with the solid contact and the water layers.12 It is therefore highly desired to develop alternative materials as solid contacts for SC-ISEs. In recent years, SC-ISEs have subsequently experienced rapid growth which was mainly based on the development of nanomaterials. A wide variety of approaches that used carbon-based nanomaterials such as graphene and carbon nanotubes, gold nanoclusters and MoS2 nanoparticles as the ion-to-electron transducers have been successfully explored.13–16 Due to their large double layer capacitances, remarkable charge-transfer capability and high hydrophobicity, these SC-ISEs possess high potential stability and excellent resistance to gases and light. Therefore, the superior performance of these materials has led to the exploration of alternative two-dimensional materials as the ion-to-electron transducers for the development of SC-ISEs.17
Black phosphorus (BP), a new member of two-dimensional (2D) materials, has recently attracted global interest for both its fundamental properties and its applications.18–21 Recently, it has been reported that black phosphorus displays high charge carrier mobility and electrical conductance.19,22 These properties, together with its better surface-to-volume ratio, make BP a promising candidate for chemical sensing applications. Several papers have appeared recently including field-effect transistors for nitrogen dioxide (NO2), electrochemical impedance spectroscopy for methanol and electrochemical sensing for humidity.23–25 However, without protection, black phosphorus flakes are reported to chemically degrade shortly with degradation of their electric properties and device performances.26 A recent theoretical paper demonstrated that black phosphorus flakes show improved stability for electrochemical sensing after being encapsulated by a hydrophobic polymeric membrane.27 Inspired by this, black phosphorus will be a very promising material for fabrication of SC-ISEs. As such, to the best of our knowledge, little is known about the performance of the black phosphorus as the ion-to-electron transducer of SC-ISEs.
Herein, we report here the first presenting of using black phosphorus for preparing the SC-ISEs. The Ca2+-selective electrode was chosen as a model system. The main performance characteristics of black phosphorus based SC-ISEs were estimated. The fabricated sensors exhibit excellent Nernstian responses and potential stability. No water layer or interferences by light, O2, or CO2 are observed.
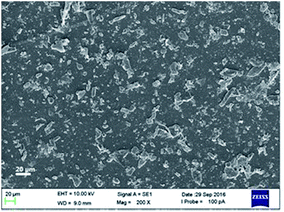
The conducting film of SC-ISEs was prepared by drop-casting black phosphorus ethanol solution on electrode surface (see details in the ESI†). The morphology of black phosphorus powder on the electrode was characterized with a scanning electron microscope (SEM) on a ZEISS EVO LS15 instrument at an accelerating voltage of 10 kV. A panoramic picture of the black phosphorus conducting polymer is illustrated in Fig. 1. It can be seen that obtained black phosphorus dispersion material shows high dispersity from each other. In addition, black phosphorus has an irregular shape with an average size of approximately 10 μm. When used in a SC-ISE, these materials are bound together by the poly(vinyl chloride) (PVC) binder as well as the plasticized polymeric membrane.
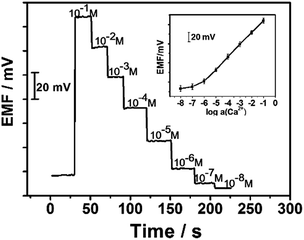
To further characterize the quality of black phosphorus-based solid contact, electrochemical impedance spectroscopy (EIS) was performed. EIS was performed with a three-electrode setup (see details in the ESI†), where the glassy carbon electrode (GCE) coated with or without black phosphorus was connected as the working electrode, while an Au electrode as the auxiliary electrode and the Ag/AgCl electrode as the reference electrode. Impedance measurements of GCE/black phosphorus/Ca2+-ISE and GCE/Ca2+-ISE recorded in 10−3 M CaCl2 are shown in Fig. 2. The diameter of the high-frequency semicircle is related to the bulk membrane coupled to the contact resistance between GC or solid contact and the ion-selective PVC membrane. The resistance of the GC/black phosphorus/Ca2+-ISE (0.64 MΩ) is smaller than that of the GC/Ca2+-ISE (1.13 MΩ), which indicates that the charge transfer across the interfaces is facilitated due to the presence of black phosphorus.
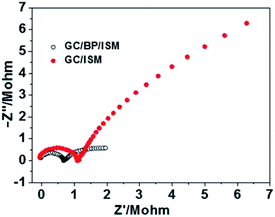 | ||
| Fig. 2 Impedance spectra for the GC/black phosphorus/Ca2+-ISE (hollow circle) and GC/Ca2+-ISE (solid circle) in 10−3 M CaCl2. Frequency range, 0.3 Hz to 10 kHz; Edc, 0.2 V; ΔEdc, 10 mV. | ||
Current-reversal chronopotentiometry was used to evaluate the short-term potential stability of the black phosphorus-based Ca2+-ISE by recording potentials of the ISE under currents of ± 1 nA in 1 mM CaCl2 solution. Fig. 3 shows that the potential drift of the GC/black phosphorus/Ca2+-ISE, derived from the ratio ΔE/Δt, is 72 μV s−1, which is much lower than that of the GC/Ca2+-ISE under the same conditions (500 μV s−1). These results indicate that the potential stability of the electrode can be obviously improved by using the black phosphorus film as the ion-to-electron transducer.
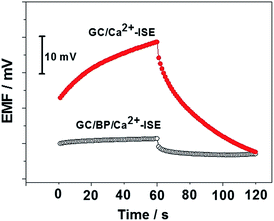 | ||
| Fig. 3 Chronopotentiograms for the GC/black phosphorus/Ca2+-ISE and GC/Ca2+-ISE recorded in 10−3 M CaCl2. | ||
The potentiometric performance characteristics such as the working concentration range, the limit of detection, the slope of the linear curve and response time were determined. The results of these measurements are summarized in Fig. 4. As can be seen, a linear range of the developed GC-BP-Ca2+-ISE from 1.0 × 10−1 to 1.0 × 10−6 M is observed with a Nernst slope of 28.3 ± 0.7 mV decade−1 (R2 = 0.99). The detection limit calculated as the intersection of the slope line is 4.0 × 10−7 M. The response time of the electrode was measured after successive immersion of the electrode in a series of Ca2+ solutions. A response time of about 10 s was required to achieve a steady potential within 0.7 mV for measuring Ca2+ at concentrations ranging from 1.0 × 10−1 to 1.0 × 10−6 M. In addition, experiments also show that the potential response of the proposed electrode can be fully reversible with a relative standard deviation of 3.8% (n = 5). The comparison of the analytical performance of the developed sensor with some of other reported solid-contact ISEs is summarized in Table S1 in the ESI.† As illustrated, the response characteristics of the proposed electrode are comparable with those of other solid-contact ISEs.
The potentiometric selectivity coefficients were obtained in an alkali metal or alkaline earth metal chloride solutions using the separate solution method. The results are presented in Table S1.† As can be seen, GC-black phosphorus-Ca2+-ISEs exhibited a high selectivity over interfering ions, which are all very close to the values reported in the literature. These phenomena indicate that the selectivity of the developed Ca2+-ISEs is not affected by the ion-to-electron transducing layer, but is dependent on the ionic sensing polymeric membrane itself. This selectivity satisfied the requirements for calcium assay in the wine samples.
A potentiometric water layer test was conducted with all electrodes to evaluate the water layer formation between the polymeric ion-selective membrane and its solid contact. The electrode was first immersed in 0.1 mol L−1 of CaCl2 solution. Then the solution was changed to 0.1 mol L−1 NaCl solution. After 2 h the NaCl solution was replaced by 0.1 mol L−1 of CaCl2 solution. The potential responses of the tested electrodes are shown in Fig. 5. As can be seen, a negative EMF change is observed for both electrodes upon replacing the CaCl2 solution with the NaCl solution. Such EMF change reflects the change in the outer phase boundary potential as a consequence of the selectivity behavior of the Ca2+-selective membrane. In this process, the electrode based on black phosphorus exhibits a stable potential response while an obvious positive potential drift is observed for the electrode without solid contact. These results demonstrate that no undesirable water layer is formed for the GC/black phosphorus-Ca2+-ISE while there was a water layer at the sensing polymer/electrode interface in case of absence of the ion-to-electron transducer layer.
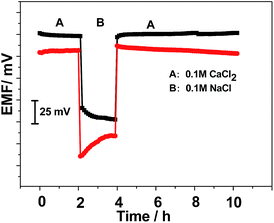 | ||
| Fig. 5 Water layer tests for the GC/black phosphorus/Ca2+-ISE and GC/Ca2+-ISE in 0.1 M CaCl2 (A) and 0.1 M NaCl (B). | ||
It has been reported that light, oxygen, and carbon dioxide may cause interference to several SC-ISEs. Oxygen or carbon dioxide can permeate plasticized PVC membranes which affect the boundary potential or alter the local pH. In addition, SC-ISE can be photosensitive if the solid contact is an organic semiconductor. In this study, the effect of O2 and CO2 was tested by bubbling both gases into 10−3 M CaCl2 solution for 25 min respectively followed by purging with N2 to remove O2 or CO2 for 25 min. In addition, the effect of light on the GC/BP-Ca2+-ISE was investigated by recording the potential response in a 10−3 M CaCl2 solution while turning on/off the ambient light. As clearly illustrated in Fig. 6, no significant potential changes are observed during the measurements, indicating that the black phosphorus film-based solid contact has no light, O2 or CO2 sensitivity.
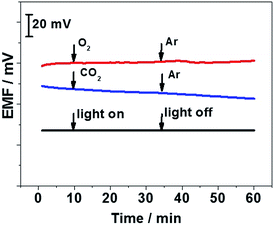 | ||
| Fig. 6 Effects of light, O2, and CO2 on the potential stability of the GC/black phosphorus/Ca2+-ISE in 10−3 M CaCl2 solution. | ||
The proposed GC/black phosphorus/Ca2+-ISE was finally applied to determination of Ca2+ in wine samples that are widely consumed by the local public. It should be noted that alcohol was removed from wine samples using rotary evaporators and the samples were diluted 10 times with deionized water before potentiometric detection. In order to illustrate its accuracy, the comparison between the proposed sensor and the atomic absorption spectrophotometry (AAS) method was performed.28 The results are given in Table S2.† It can be seen the data obtained by the proposed electrode agree well with those obtained by the AAS method, indicating that the proposed GC/black phosphorus/Ca2+-ISE has a promising potential for real sample analysis.
Conclusions
In summary, we have for the first time used a few-layer black phosphorus film as the ion-to-electron transducer in solid-contact Ca2+-ISE. The presence of this layer significantly reduces the resistance of membrane compared to coated disk electrode. There are also many other important advantages, including long-term potential stability, no undesirable water layer and their insensitivity to O2 or CO2 and light. In addition, the electrodes demonstrate excellent sensing properties including fast response time, a wide dynamic response range, low detection limit and good selectivity. Moreover, this robust sensor was successfully applied for Ca2+ determination in the wine samples with similar results to AAS.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the Natural Science Foundation of Shandong province (BS2014NY004), the National Natural Science Foundation of China (41576106), the Science and Technology Project of Yantai (2014ZH086), the Youth Innovation Promotion Association of CAS (2014190). Many thanks to Prof. Wei Qin's support for the whole experiment.Notes and references
- E. Bakker, Anal. Chem., 2016, 88, 395–413 CrossRef CAS PubMed.
- T. Guinovart, D. Hernandez-Alonso, L. Adriaenssens, P. Blondeau, F. X. Rius, P. Ballester and F. J. Andrade, Biosens. Bioelectron., 2017, 87, 587–592 CrossRef CAS PubMed.
- V. K. Gupta, M. R. Ganjali, P. Norouzi, H. Khani, A. Nayak and S. Agarwal, Crit. Rev. Anal. Chem., 2011, 41, 282–313 CrossRef CAS PubMed.
- C. Z. Lai, M. A. Fierke, R. C. da Costa, J. A. Gladysz, A. Stein and P. Bühlmann, Anal. Chem., 2010, 82, 7634–7640 CrossRef CAS PubMed.
- S. T. Mensah, Y. Gonzalez, P. Calvo-Marzal and K. Y. Chumbimuni-Torres, Anal. Chem., 2014, 86, 7269–7273 CrossRef CAS PubMed.
- G. A. Crespo, S. Macho and F. X. Rius, Anal. Chem., 2008, 80, 1316–1322 CrossRef CAS PubMed.
- M. A. Fierke, C. Z. Lai, P. Bühlmann and A. Stein, Anal. Chem., 2010, 82, 680–688 CrossRef CAS PubMed.
- J. P. Veder, R. De Marco, G. Clarke, R. Chester, A. Nelson, K. Prince, E. Pretsch and E. Bakker, Anal. Chem., 2008, 80, 6731–6740 CrossRef CAS PubMed.
- J. Sutter and E. Pretsch, Electroanalysis, 2006, 18, 19–25 CrossRef CAS.
- P. Sjoberg-Eerola, J. Nylund, J. Bobacka, A. Lewenstam and A. Ivaska, Sens. Actuators, B, 2008, 134, 878–886 CrossRef.
- M. Wagner, G. Lisak, A. Ivaska and J. Bobacka, Sens. Actuators, B, 2013, 181, 694–701 CrossRef CAS.
- J. Sutter, E. Lindner, R. E. Gyurcsányi and E. Pretsch, Anal. Bioanal. Chem., 2004, 380, 7–14 CrossRef CAS PubMed.
- G. A. Crespo, S. Macho and F. X. Rius, Anal. Chem., 2008, 80, 1316–1322 CrossRef CAS PubMed.
- Z. A. Boeva and T. Lindfors, Sens. Actuators, B, 2016, 224, 624–631 CrossRef CAS.
- J. A. Xu, F. Jia, F. H. Li, Q. B. An, S. Y. Gan, Q. X. Zhang, A. Ivaska and L. Niu, Electrochim. Acta, 2016, 222, 1007–1012 CrossRef CAS.
- X. Z. Zeng, S. Y. Yu, Q. Yuan and W. Qin, Sens. Actuators, B, 2016, 234, 80–83 CrossRef CAS.
- C. Bieg, K. Fuchsberger and M. Stelzle, Anal. Bioanal. Chem., 2017, 409, 45–61 CrossRef CAS PubMed.
- A. Castellanos-Gomez, J. Phys. Chem. Lett., 2015, 6, 4280–4291 CrossRef CAS PubMed.
- C. C. Mayorga-Martinez, Z. Sofer and M. Pumera, Angew. Chem., Int. Ed., 2015, 54, 14317–14320 CrossRef CAS PubMed.
- H. U. Lee, S. Y. Park, S. C. Lee, S. Choi, S. Seo, H. Kim, J. Won, K. Choi, K. S. Kang, H. G. Park, H.-S. Kim, H. R. An, K.-H. Jeong, Y.-C. Lee and J. Lee, Small, 2016, 12, 214–219 CrossRef CAS PubMed.
- Z. Sofer, D. Bousa, J. Luxa, V. Mazanek and M. Pumera, Chem. Commun., 2016, 52, 1563–1566 RSC.
- Z. Sofer, D. Sedmidubsky, S. Huber, J. Luxa, D. Bousa, C. Boothroyd and M. Pumera, Angew. Chem., Int. Ed., 2016, 55, 3382–3386 CrossRef CAS PubMed.
- A. N. Abbas, B. Liu, L. Chen, Y. Ma, S. Cong, N. Aroonyadet, M. Koepf, T. Nilges and C. Zhou, ACS Nano, 2015, 9, 5618–5624 CrossRef CAS PubMed.
- P. Yasaei, A. Behranginia, T. Foroozan, M. Asadi, K. Kim, F. Khalili-Araghi and A. Salehi-Khojin, ACS Nano, 2015, 9, 9898–9905 CrossRef CAS PubMed.
- Y. Du, H. Liu, Y. Deng and P. D. Ye, ACS Nano, 2014, 8, 10035–10042 CrossRef CAS PubMed.
- S. Gamage, Z. Li, V. S. Yakovlev, C. Lewis, H. Wang, S. B. Cronin and Y. Abate, Adv. Mater. Interfaces, 2016, 3, 1600121 CrossRef.
- P. Li, D. Z. Zhang, J. J. Liu, H. Y. Chang, Y. Sun and N. L. Yin, ACS Appl. Mater. Interfaces, 2015, 7, 24396–24402 CAS.
- R. Sowmya, K. P. Indumathi, S. Arora, V. Sharma and A. K. Singh, J. Food Sci. Technol., 2015, 52, 1188–1193 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra07743b |
| This journal is © The Royal Society of Chemistry 2017 |