Open Access Article
Open Access ArticleAcid-promoted oxidative methylenation of 1,3-dicarbonyl compounds with DMSO: application to the three-component synthesis of Hantzsch-type pyridines†
LuLu Xueb,
Guolin Cheng*a,
Ruifeng Zhub and
Xiuling Cui *b
*b
aCollege of Materials Science & Engineering, Huaqiao University, Xiamen 361021, China. E-mail: glcheng@hqu.edu.cn
bEngineering Research Center of Molecular Medicine, Ministry of Education, Key Laboratory of Molecular Medicine of Fujian Province, Key Laboratory of Xiamen Marine and Gene Drugs, Institutes of Molecular Medicine and School of Biomedical Sciences, Huaqiao University, Xiamen, 361021, China. E-mail: cuixl@hqu.edu.cn
First published on 11th September 2017
Abstract
A highly convergent one-pot synthesis of Hantzsch-type pyridines has been developed based on a three-component annulation of 1,3-dicarbonyl compounds, DMSO, and ammonium salt. A transition-metal-free oxidative methylenation reaction/Hantzsch pyridine synthesis cascade reaction was involved in this process. This intermolecular annulation reaction proceeds under mild reaction conditions, wherein DMSO serves as solvent, carbon source, and oxidant. A series of polysubstituted pyridines and methylene-bridged bis-1,3-dicarbonyl compounds were prepared in high yields.
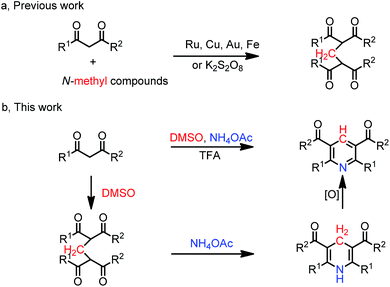
Methylene-bridged bis-1,3-dicarbonyl compounds are useful building blocks or intermediates in organic synthesis.1 Traditionally, these compounds are synthesized from bis-1,3-dicarbonyl compounds by using CH2Br2 (ref. 2) or formaldehyde3 as one-carbon sources with low efficiency. Meanwhile, these compounds were constructed by using N-methyl compounds as one-carbon sources through transition-metal catalysed methylenation reactions, such as Ru,4 Cu/Au,5 and Fe,6 as well as through K2S2O8 (ref. 7) oxidative methylenation reactions (Scheme 1a). However external oxidants are always required.
Over the past decades, the solvent-participated reaction is considered as an important strategy for the developing economic chemical methodologies. As a less toxic and inexpensive solvent, dimethyl sulfoxide (DMSO) is commonly employed as an effective oxidant8 and the source of –SMe,9 –SOMe,10 –SO2Me,11 –CH2SMe,12 –CN,13 –CHO,14 ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2,15 –CH2–,16 and –Me.17 We have recently developed a method to 1,3,5-triarylbenzenes18a and bis(1H-indol-yl)methanes,18b in which DMSO served as a precursor of methine and methylene unit, respectively. Despite those advantages, the using of DMSO as methine source is still rare.19 We hypothesisd that DMSO could be used as methylenation reagent instead of N-methyl compounds to form methylene-bridged bis-1,3-dicarbonyl compounds, which could be further trapped with ammonium salt leading to Hantzsch-type pyridines. Recently, multicomponent reactions have emerged as attractive processes for the assembly of complex molecules.20 During our ongoing investigations in constructing of heterocycle compounds,21 herein, we wish to report an efficient protocol for the synthesis of Hantzsch-type pyridines via a three-component cascade reaction of 1,3-dicarbonyl compounds, DMSO, and ammonium salt, in which two C–C bonds and two C–N bonds were formed in one-pot manner (Scheme 1b).
CH2,15 –CH2–,16 and –Me.17 We have recently developed a method to 1,3,5-triarylbenzenes18a and bis(1H-indol-yl)methanes,18b in which DMSO served as a precursor of methine and methylene unit, respectively. Despite those advantages, the using of DMSO as methine source is still rare.19 We hypothesisd that DMSO could be used as methylenation reagent instead of N-methyl compounds to form methylene-bridged bis-1,3-dicarbonyl compounds, which could be further trapped with ammonium salt leading to Hantzsch-type pyridines. Recently, multicomponent reactions have emerged as attractive processes for the assembly of complex molecules.20 During our ongoing investigations in constructing of heterocycle compounds,21 herein, we wish to report an efficient protocol for the synthesis of Hantzsch-type pyridines via a three-component cascade reaction of 1,3-dicarbonyl compounds, DMSO, and ammonium salt, in which two C–C bonds and two C–N bonds were formed in one-pot manner (Scheme 1b).
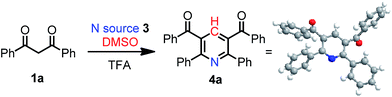
Initially, 1,3-dipheylpropane-1,3-dione 1a was selected as a model substrate with NH4Cl as nitrogen source in DMSO to explore the reaction efficiency (Table 1). To our delight, the desired pyridine product 4a was afforded in 16% yield in the presence of TFA as an additive (entry 1). The configuration of 4a was determined by single crystal X-ray diffraction analysis. With this preliminary result in hand, we continued to optimize the reaction conditions. Gratifyingly, increasing the reaction temperature resulted in a positive effect on the reaction (entry 2). However, higher temperature (140 °C) gave slight poorer result (entry 3). When the loading of TFA was decreased to 6 equivalents, the corresponding yield sharply enhanced from 30% to 71%. Further decreasing the amount of TFA gave a reduced yield of 31% (entries 4, 5). The formation of product was also affected by the loading of 3a (entries 6, 7). After a brief screening of different ammonium salts, such as NH4OH, NH4HCO3, and NH4OAc, we found that NH4OAc was the most effective nitrogen source (entries 8–10). The yield increased with the extension of the reaction time from 24 h to 48 h (entry 11). The reaction did not proceed in the absence of acid (entry 12). When the reaction was performed under N2 atmosphere, the yield was lower than that under air atmosphere (entry 13). This result clearly revealed that O2 as an oxidant sharply increased the yield and DMSO may serve as an oxidant in the absence of O2. A control experiment showed that the yield was decreased to 81% when AcOH was used instead of TFA (entry 14). These studies indicate that the optimal one-pot system for this annulation reaction was 1a (0.5 mmol), 3b (1.0 mmol), TFA (3.0 mmol) in DMSO (2 mL) at 120 °C under air for 48 h.
| Entry | N source | TFA (eq.) | Temp. (°C) | T (h) | Yieldb (%) |
|---|---|---|---|---|---|
| a General conditions: 1a (0.5 mmol) and 3a (1.0 mmol) with TFA in 2 mL DMSO under air.b Isolated yield.c 3.0 equiv. of 3a.d 1.0 equiv. of 3a.e The reaction was carried out under N2.f AcOH was used instead of TFA. | |||||
| 1 | NH4Cl | 12 | 100 | 24 | 16 |
| 2 | NH4Cl | 12 | 120 | 24 | 37 |
| 3 | NH4Cl | 12 | 140 | 24 | 30 |
| 4 | NH4Cl | 6 | 120 | 24 | 71 |
| 5 | NH4Cl | 3 | 120 | 24 | 31 |
| 6 | NH4Cl | 6 | 120 | 24 | 73c |
| 7 | NH4Cl | 6 | 120 | 24 | 56d |
| 8 | NH4OH | 6 | 120 | 24 | 78 |
| 9 | NH4HCO3 | 6 | 120 | 24 | 62 |
| 10 | NH4OAc | 6 | 120 | 24 | 83 |
| 11 | NH4OAc | 6 | 120 | 48 | 97 |
| 12 | NH4OAc | 0 | 120 | 24 | np |
| 13 | NH4OAc | 6 | 120 | 48 | 56e |
| 14 | NH4OAc | 6 | 120 | 48 | 81f |
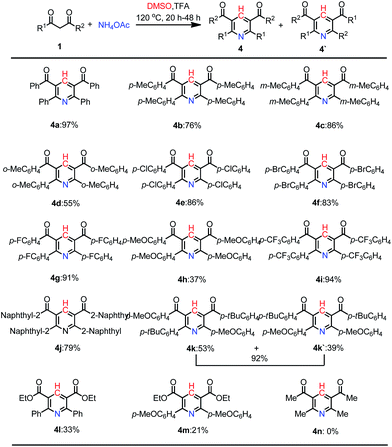
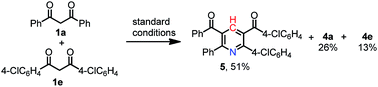
Having identified the reaction conditions for the synthesis of pyridine derivatives, a wide variety of substituted 1,3-dicarbonyl compounds were submitted to investigate the substrate scope and generality. As displayed in Scheme 2, the reaction exhibited satisfactory tolerance of the substrates containing substitutions of distinct properties, such as Me, MeO, F, Cl, Br, CF3, and CO2Me. The steric hindrance influenced this reaction obviously. The substrates with ortho-substituent gave lower yield than those with para- or meta-substituent (4d vs. 4c, 4b). On the other hand, 1,3-diketones with p-halogen-substituents reacted smoothly to provide the desired products in good yields (4e, 4f and 4g). In addition, substitution with strong electron-withdrawing groups such as 4-CF3 was also tolerated under the reaction conditions, giving pyridine 4i in 94% yield. However, strong electron-donating substituent such as methoxyl gave the desired product only in 37% yield (4h). A naphthalene derivative (1j) reacted in the same condition, producing the corresponding 4j in 79% yield. From comparison with symmetrical 1,3-dione substrates, unsymmetric substrate tended to give two major products (4k and 4k′). Moreover, β-ketone esters reacted smoothly with NH4OAc and give the desired products in 33% and 21%, respectively (4l and 4m). An aliphatic 1,3-dione failed to give the corresponding product (4n).
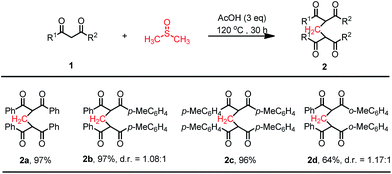
As mentioned above, methylene-bridged bis-1,3-dicarbonyl compounds are synthetically important chemicals. Thus, we began to synthesize these compounds by using our strategy. However, in the absence of NH4OAc, the desired methylene-bridged bis-1,3-dicarbonyl compound 2a was obtained in only 43% yield. NH4OAc is a more suitable ammonium salt than NH4Cl or NH4HCO3, in the abovementioned three-component annulation reaction (Table 1). We thus presume that acetate anion may play a significant role in this transformation. Then, AcOH was used instead of TFA. To our delight, the use of 3 equivalent of AcOH in DMSO at 120 °C could produce 2a in 97% yield. These conditions were subsequently employed when we examined the substrate scope of this reaction. Gratifyingly, a variety of 1,3-diketones 1a–d successfully reacted under the aforesaid conditions to afford the desired methylene-bridged bis-1,3-dicarbonyl compounds 2a–d in good to excellent yields (Scheme 3). Two diastereomers were obtained in ratios between 1.2 and 1, when unsymmetric substrates were used.
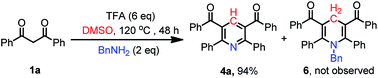
Moreover, the novel pyridine compound 5 containing four different substituents could be generated from the cross-over reaction in 51% yield from 1a and 1e (eqn (1)). When benzyl amine was used as nitrogen source, the aromatic product 4a was obtained in 94% and the desired N-benzyl dihydropyridine 6 was not observed. This result suggests that the methylenation reaction/Hantzsch pyridine synthesis cascade reaction is substantially slower than the final oxidation reaction under the optimized conditions (eqn (2)).
 | (1) |
 | (2) |
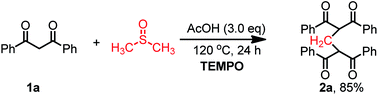
To gain more insight into the reaction mechanism, control reactions were conducted. DMSO-d6 was used as solvent instead of DMSO under the standard reaction conditions. D-labeled product 5 was obtained in 76% yield with 87% incorporation of deuterium, confirming that DMSO is the methylene source (eqn (3)). The radical scavengers, such as TEMPO (2,2,6,6-tetramethylpiperidinooxy) did not influence this reaction obviously (eqn (4)), which indicated that a radical pathway may not be involved in this reaction.
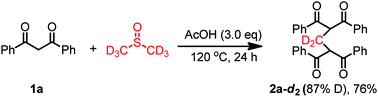 | (3) |
 | (4) |
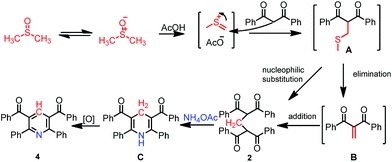
Subsequently, the proposed reaction mechanism was shown as Scheme 4. The acetate acid-promoted reaction of 1 and DMSO affords the oxidative coupling product A (Pummerer-type reaction). 2 is formed by either nucleophilic substitution reaction or the tandem reaction of elimination and Michael addition via an intermediate B.22 In addition, the substituted 1,4-dihydropyridine (1,4-DHP) C was obtained by the reaction of B with ammonium acetate, then via oxidation reaction to obtain the final pyridine products (Scheme 4).
Conclusions
In conclusion, we have developed a general and efficient method for the synthesis of substituted pyridines from readily available 1,3-dicarbonyl compounds. In this system, DMSO could serve as a simple, cheap solvent, and easy-to-handle one carbon source, and directly reacted with 1,3-dicarbonyl compounds to give the corresponding methylene-bridged bis-1,3-dicarbonyl compounds, which could be further transformed to the Hantzsch-type pyridines in the presence of NH4OAc.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was NSF of China (21672075), Science and Technology Bureau of Xiamen City (3502Z20150054), Xiamen Southern Oceanographic Center (15PYY052SF01), Outstanding Youth Scientific Research Cultivation Plan of Colleges and Universities of Fujian Province (JA14012), and Promotion Program for Young and Middle-aged Teacher in Science and Technology Research of Huaqiao University (ZQN-PY120).Notes and references
- (a) G. R. Newkome, G. R. Baker, S. Arai, M. J. Saunders, P. S. Russo, K. J. Theriot, C. N. Moorefield, L. E. Rogers and J. E. Miller, J. Am. Chem. Soc., 1990, 112, 8458 CrossRef CAS; (b) K. Maruyama, K. Kubo, Y. Toda, K. Kawase, T. Mashino and A. Nishinaga, Tetrahedron, 1995, 36, 5609 CrossRef CAS; (c) G. Kaupp, M. R. Naimi-Jamal and J. Schmeyers, Tetrahedron, 2003, 59, 3753 CrossRef CAS; (d) S. Xue, Q.-F. Zhou, L.-Z. Li and Q.-X. Guo, Synlett, 2005, 2990 CrossRef CAS; (e) A. Sachar, P. Gupta, S. Gupta and R. Sharma, Can. J. Chem., 2010, 88, 478 CrossRef CAS.
- Y.-S. Hon, T.-R. Hsu, C.-Y. Chen, Y.-H. Lin, F.-J. Chang, C.-H. Hsieh and P.-H. Szu, Tetrahedron, 2003, 59, 1509 CrossRef CAS.
- P. R. Blakemore, C. Kilner, N. R. Norcross and P. C. Astles, Org. Lett., 2005, 7, 4721 CrossRef CAS PubMed.
- W. J. Yoo, A. Tanoue and S. Kobayashi, Chem.–Asian J., 2012, 7, 2764 CrossRef CAS PubMed.
- R. Balamurugan and S. Manojveer, Chem. Commun., 2011, 47, 11143 RSC.
- H. Li, Z. He, X. Guo, W. Li, X. Zhao and Z. Li, Org. Lett., 2009, 11, 4176 CrossRef CAS PubMed.
- X. Wang, Y. Wang, Y. Yuan and C.-H. Xing, Tetrahedron, 2014, 70, 2195 CrossRef CAS.
- (a) A. J. Mancuso and D. Swern, Synthesis, 1981, 1981, 165 CrossRef; (b) R. Chebolu, A. Bahuguna, R. Sharma, V. K. Mishra and P. Ravikumar, Chem. Commun., 2015, 51, 15438 RSC; (c) N. R. Connor, P. Bolgar and B. M. Stoltz, Tetrahedron, 2016, 57, 849 CrossRef.
- (a) C. Dai, Z. P. Xu, F. Huang, Z. Yu and Y.-F. Gao, J. Org. Chem., 2012, 77, 4414 CrossRef CAS PubMed; (b) S. M. Patil, S. Kulkarni, M. Mascarenhas, R. Sharma, S. M. Roopan and A. Roychowdhury, Tetrahedron, 2013, 69, 8255 CrossRef CAS; (c) Z. An, Y. She, X. Yang, X. Pang and R. Yan, Org. Chem. Front., 2016, 3, 1746 RSC.
- M. M. D. Pramanik and N. Rastogi, Chem. Commun., 2016, 52, 8557 RSC.
- A. Shao, M. Gao, S. Chen, T. Wang and A. Lei, Chem. Sci., 2017, 8, 2175 RSC.
- (a) J. Liu, X. Wang, H. Guo, X. Shi, X. Ren and G. Huang, Tetrahedron, 2012, 68, 1560 CrossRef CAS; (b) T. Shen, X. Huang, Y.-F. Liang and N. Jiao, Org. Lett., 2015, 17, 6186 CrossRef CAS PubMed.
- X. Ren, J. Chen, F. Chen and J. Cheng, Chem. Commun., 2011, 47, 6725 RSC.
- (a) H. Fei, J. Yu, Y. Jiang, H. Guo and J. Cheng, Org. Biomol. Chem., 2013, 11, 7092 RSC; (b) Z. Zhang, Q. Tian, J. Qian, Q. Liu, T. Liu, L. Shi and G. Zhang, J. Org. Chem., 2014, 79, 8182 CrossRef CAS PubMed; (c) H. Cao, S. Lei, N. Li, L. Chen, J. Liu, H. Cai, S. Qiu and J. Tan, Chem. Commun., 2015, 51, 1823 RSC.
- (a) S. Xu, Y. Gao, R. Chen, K. Wang, Y. Zhang and J. Wang, Chem. Commun., 2016, 52, 4478 RSC; (b) S. M. A. H. Siddiki, A. S. Touchy, K. Kon and K.-i. Shimizu, Chem.–Eur. J., 2016, 22, 6111 CrossRef CAS PubMed.
- (a) K. Sun, X. Wang, Y. Jiang, Y. Lv, L. Zhang, B. Xiao, D. Li, Z. Zhu and L. Liu, Chem.–Asian J., 2015, 10, 536 CrossRef CAS PubMed; (b) P. Liu, Z. Shen, Y. Yuan and P. Sun, Org. Biomol. Chem., 2016, 14, 6523 RSC; (c) O. P. S. Patel, D. Anand, R. K. Maurya and P. P. Yadav, J. Org. Chem., 2016, 81, 7626 CrossRef CAS PubMed.
- (a) J. Jia, Q. Jiang, A. Zhao, B. Xu, Q. Liu, W.-P. Luo and C.-C. Guo, Synthesis, 2016, 48, 421 CAS; (b) R. Caporaso, S. Manna, S. Zinken, A. R. Kochnev, E. R. Lukyanenko, A. V. Kurkin and A. P. Antonchick, Chem. Commun., 2016, 52, 12486 RSC.
- (a) F. Wang, J. Shen, G. Cheng and X. Cui, RSC Adv., 2015, 5, 73180 RSC; (b) P. Li, Y. Weng, X. Xu and X. Cui, J. Org. Chem., 2016, 81, 3994 CrossRef CAS PubMed.
- T. Duan, T. Zhai, H. Liu, Z. Yan, Y. Zhao, L. Feng and C. Ma, Org. Biomol. Chem., 2016, 14, 6561 CAS.
- For the selected recently examples on multi-component cascade reaction, see: (a) W. Dai, X.-L. Jiang, J.-Y. Tao and F. Shi, J. Org. Chem., 2016, 81, 185 CrossRef CAS PubMed; (b) F. Shi, G.-J. Xing, R.-Y. Zhu, W. Tan and S. Tu, Org. Lett., 2013, 15, 128 CrossRef CAS PubMed; (c) F. Shi, Z.-L. Tao, S.-W. Luo, S.-J. Tu and L.-Z. Gong, Chem.–Eur. J., 2012, 18, 6885 CrossRef CAS PubMed; (d) F. Shi, W. Tan, R.-Y. Zhu, G.-J. Xing and S.-J. Tu, Adv. Synth. Catal., 2013, 355, 1605 CrossRef CAS.
- (a) R. Zhu, G. Cheng, C. Jia, L. Xue and X. Cui, J. Org. Chem., 2016, 81, 7539 CrossRef CAS PubMed; (b) J. Shen, L. Xue, X. Lin, G. Cheng and X. Cui, Chem. Commun., 2016, 52, 3292 RSC; (c) J. Shen, X. Wang, X. Lin, Z. Yang, G. Cheng and X. Cui, Org. Lett., 2016, 18, 1378 CrossRef CAS PubMed; (d) G. Cheng, Y. Weng, X. Yang and X. Cui, Org. Lett., 2015, 17, 3790 CrossRef CAS PubMed; (e) J. Shen, D. Cai, C. Kuai, Y. Liu, M. Wei, G. Cheng and X. Cui, J. Org. Chem., 2015, 80, 6584 CrossRef CAS PubMed; (f) X. Yang, G. Cheng, J. Shen, C. Kuai and X. Cui, Org. Chem. Front., 2015, 2, 366 RSC; (g) X. Wang, G. Cheng, J. Shen, X. Yang, M.-e. Wei, Y. Feng and X. Cui, Org. Chem. Front., 2014, 1, 1001 RSC; (h) G. Cheng, X. Zeng, J. Shen, X. Wang and X. Cui, Angew. Chem., Int. Ed., 2013, 52, 13265 CrossRef CAS PubMed; (i) G. Cheng and X. Cui, Org. Lett., 2013, 15, 1480 CrossRef CAS PubMed; (j) J. Shen, G. Cheng and X. Cui, Chem. Commun., 2013, 49, 10641 RSC.
- J.-N. Tan, H. Li and Y. Gu, Green Chem., 2010, 12, 1772 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1560452. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7ra07442e |
| This journal is © The Royal Society of Chemistry 2017 |