Open Access Article
Open Access ArticleEffect of xylose on the structural and physicochemical properties of peanut isolated protein based films
Li Liu†
 ab,
Wei-Jing Lin†a,
Hong-Zhi Liua,
Ai-Min Shia,
Hui Hua,
Mehmet Nail Nasirb,
Magali Deleu*b and
Qiang Wang*a
ab,
Wei-Jing Lin†a,
Hong-Zhi Liua,
Ai-Min Shia,
Hui Hua,
Mehmet Nail Nasirb,
Magali Deleu*b and
Qiang Wang*a
aInstitute of Food Science and Technology, Chinese Academy of Agricultural Sciences, Key Laboratory of Agro-Products Processing, Ministry of Agriculture, P.O. Box 5109, Beijing 100193, China. E-mail: wangqiang06@caas.cn; liuli02@caas.cn; llwjing@foxmail.com; lhz0416@126.com; sam_0912@163.com; littleh@vip.sina.com; Fax: +86 10 62815837; Tel: +86 10 62815837
bAgricultureisLife Plateform, Laboratoire de Biophysique Moléculaire aux Interfaces, Gembloux Agro-Bio Tech – University of Liege, Passage des Déportés 2, 5030 Gembloux, Belgium. E-mail: magali.deleu@ulg.ac.be; mn.nasir@ulg.ac.be
First published on 10th November 2017
Abstract
Films based on xylose and peanut protein isolate (PPI) were prepared by solution casting. The effects of xylose content (1%, 2%, 5%, 10%, 20% PPI, w/w) on the structure and mechanical properties of PPI–xylose (PPI–X) film were systematically investigated. The xylose-modified PPI was firstly prepared by crosslinking and modifying of peanut isolated protein with xylose. The degree of glycosylation and surface hydrophobicity significantly increased from 5.6% to 10.9% and from 130.6 to 370.0, respectively, with the increase of the xylose content. Maillard reactions occurring between PPI and xylose were confirmed by Fourier transform infrared spectroscopy. Then the xylose-modified PPI was developed into films. PPI–X films showed markedly increased tensile strength (increased by 77%) and elongation (increased by 67%) and decreased solubility (from 96.6% to 43.4%) compared to PPI films, suggesting that the incorporation of xylose could markedly enhance the mechanical properties and the water resistance of films. This property improvement was correlated to the increased protein surface hydrophobicity and sulfhydryl group content with the addition of xylose. No significant differences were detected among L*, a*, b* colour values of all films (P > 0.05). The scanning electron microscope images of the PPI–X film showed that the addition of xylose up to 10% produced a more homogeneous and denser structure. Finally, the improvement mechanism of the PPI–X film was proposed.
1. Introduction
Biodegradable films have gained increasing research interest due to their potential use in food packaging. They are obtained from renewable and natural biopolymers such as proteins, polysaccharides and their combinations. Among them, protein-based materials such as soybean, wheat gluten, and peanut protein films are the most attractive candidates to replace non-biodegradable plastics because they are environmentally friendly, and have similar physical and mechanical properties compared to synthetic films.1–4Peanut meal is a byproduct generated during oil extraction and is mainly used as animal feed and fertilizer in China.36 Peanut protein isolate (PPI), extracted from peanut meal, has a low cost, presents good biodegradability and ability to polymerize, and could then be a good source to develop films for various applications.5
However, protein-based films have relatively low tensile strength, low elongation and water resistance compared to synthetic films, due to the hydrophilicity of plant proteins.5 The crosslinking or blending with polymers is one approach used to make the protein films suitable for practical use. Most protein-based films use glycerol as a plasticizer to improve the elongation of the film. Modification of peanut proteins by glycosylation or cross-linking with saccharides is a potential way to improve the mechanical strength and the barrier properties of the films by reinforcing the intermolecular bonds.6–8
In literature, several recent papers deal with the improvement of the mechanical and barrier properties of protein-based films by glycosylation reactions.4,9,10 Glycosylation with gum arabic11 or glucomannan9 increases the tensile strength, decreases the water vapor permeability of protein films, but decreases the elongation ability. Generally, plasticizers having a smaller molecular weight show an easier incorporation into the protein matrix than plasticizers with a larger molecular weight.12,13 They also exhibit a more efficient plasticizing effect and lead to a stronger and tighter protein networking with stronger intermolecular covalent bonds. Lin et al.4,7 reported that crosslinking with xylose increases the strength and elongation of peanut protein films and also decreases quite remarkably their solubility in water. Therefore, xylose could be a good choice for the formation of protein-polysaccharides films. A previous study was focused on the optimization of pH, temperature and time of reaction to obtain the xylose glycosylated protein (PPI–X) solutions.4 The effect of PPI–X dissolution temperature and glycerol concentration on mechanical properties and water solubility of glycosylated films were also investigated.7 However, only little information exists on the molecular structure and physicochemical properties of peanut protein films cross-linked with different xylose content.
In this context, one goal of this work was to provide an array of data to support comparative characterization of PPI film, at various levels of addition of xylose (1%, 2%, 5%, 10%, 20%, w/w). In this research, peanut protein isolate (PPI) was glycosylated with xylose, and the glycosylated PPI solution was converted into powder. The effect of xylose on the structure of xylose-modified PPI was analyzed in detail. In addition, the glycosylated PPI was used to form films. The effects of glycosylation on the tensile strength and elongation, solubility in water, color, sulfhydryl groups content and microstructure of PPI–X films were evaluated. Finally, the mechanism of PPI–X film formation was also proposed.
2. Materials and methods
2.1. Materials and chemicals
Peanut meal with a protein content of 47.81% was purchased from Gaotang Lanshan Co., Ltd. (Shandong Province, China). Xylose, NaOH and NaBr (Sinopharm Chemical Reagent Co., Ltd., Beijing, China) used in the experiments were analytical grade and used without further purification.2.2 Preparation of peanut protein isolated (PPI)
Peanut protein isolate (PPI) was extracted from the peanut meal according to previous study with slight modifications.4 Briefly, peanut meal was dissolved in (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, w/v) deionized water, the pH of dispersions was adjusted to 9.0. After stirring with 150 rpm for 2 h at 25 °C, the supernatant was obtained by centrifuging at 3500g for 10 min. The pH of the supernatant was adjusted to pH 4.5. After centrifuging at 3500g for 10 min, the precipitate (peanut protein isolated) was recovered and freeze dried. The protein content was 90.93 ± 1.36%.
10, w/v) deionized water, the pH of dispersions was adjusted to 9.0. After stirring with 150 rpm for 2 h at 25 °C, the supernatant was obtained by centrifuging at 3500g for 10 min. The pH of the supernatant was adjusted to pH 4.5. After centrifuging at 3500g for 10 min, the precipitate (peanut protein isolated) was recovered and freeze dried. The protein content was 90.93 ± 1.36%.
2.3 Preparation of xylose-modified PPI powder
PPI was modified with xylose by glycosylation, at five different ratios of xylose: 0.01 g, 0.02 g, 0.05 g, 0.10 g, 0.20 g g−1 PPI. The pH was adjusted to 9.0 using 1 mol L−1 NaOH and the solutions were heated at 90 °C for 90 min. The mixtures were then spray-dried to obtain the PPI–X powder.2.4 Film preparation
The film-forming solutions were prepared by dispersing PPI, or xylose-modified PPI in distilled water (5%, w/w). Glycerol was added at 25% of the total components. After degassing by filtrating through nylon filters, 15 mL of the film forming solutions were poured onto nonstick plates (15 cm diameter). Films were dried at 60 °C for 1 h and peeled entirely after cooling. The peeled films were conditioned at 58% relative humidity (RH) for at least 48 h prior to analysis.2.5. Structural characterization of the xylose-modified PPI powder
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 20 min. Protein concentration of the supernatant was determined with Folin phenol method. Fluorescence intensity was measured at 490 nm after excitation at 390 nm. The initial slope of fluorescence intensity versus protein concentration plot was used as an index of H0.
000g for 20 min. Protein concentration of the supernatant was determined with Folin phenol method. Fluorescence intensity was measured at 490 nm after excitation at 390 nm. The initial slope of fluorescence intensity versus protein concentration plot was used as an index of H0.2.6. Characterization of the mechanical, structural and physical properties of the films
2.6.2.1. Solubility in water. The total soluble matter (TSM) of the films was calculated by employing the method described by Lin et al.7 The initial dry matter of the preconditioned film pieces (2 × 2 cm) was determined by drying in an air-circulating oven at 105 °C for 24 h (Wi). The insoluble matter was separated carefully and dried at 105 °C for 24 h for determination of the final dry weight (Wf). All tests were carried out in triplicate, and the total soluble matter (%) in the film was calculated by using the following equation: TSM = (Wi − Wf)/Wi × 100%.
2.6.2.2. Color. The color of the films was determined with a colorimeter (CR-400, Konica Minolta co., LTD, Japan). L*, a*, and b* color values were measured in triplicate.
2.6.3.1. Sulfhydryl groups content. The contents of sulfhydryl groups (SH) were determined according to the methods of Beveridge et al.16 with slight modifications. Briefly, protein samples (60 mg) were dispersed in Ttris–glycine buffer (0.086 M Tris, 0.09 M glycine, 4 mM EDTA, pH 8.0) in a blender containing 8 M urea, and mixed by shaking for 24 h at 25 °C. The mixture was then centrifuged at 10
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 10 min to obtain the supernatant. Ellman's reagent (4 mg mL−1) was added to the supernatant. The absorbance of the mixture was determined at 412 nm. A solution without protein was used as blank control.
000g for 10 min to obtain the supernatant. Ellman's reagent (4 mg mL−1) was added to the supernatant. The absorbance of the mixture was determined at 412 nm. A solution without protein was used as blank control.
2.6.3.2. Microstructure. Film microstructure was observed using Hitachi S570 scanning electron microscope at a magnification of ×3000 with an accelerating voltage of 12 kV.
2.7. Statistical analysis
The data are presented as means ± standard deviation. All experimental data were the average of at least three independent tests. Differences between experimental groups were calculated using Tukey's multiple comparison test. A p-value less than 0.05 was considered statistically significant.3. Results and discussion
3.1. Structural characterization of the PPI and xylose-modified PPI powder
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–N groups,22 in the amide II 1600–1500 cm−1 region associated to the N–H bending,21,23 and in the range of 1000–1200 cm−1 corresponding to the stretching C–O groups.21,24,25
O and C–N groups,22 in the amide II 1600–1500 cm−1 region associated to the N–H bending,21,23 and in the range of 1000–1200 cm−1 corresponding to the stretching C–O groups.21,24,25The FTIR spectra of xylose and PPI glycosylated with different contents of xylose is shown in Fig. 3. It has been reported that the protein in the infrared region had several characteristics absorption band, including amide I (1600–1700 cm−1) and amide II (1600–1500 cm−1) which were the most valuable to study secondary structure.22,26 All samples exhibit strong IR absorbance at around 1655 cm−1, 1555 cm−1, corresponding to amide I and amide II bands respectively.
The spectra of the xylose and xylose-modified PPI have showed significant different in the 3600–3000 and 1200–1000 cm−1, as shown in Fig. 3a. In the 3600–3000 cm−1, free hydroxyl groups have stretching vibration absorption, and in the 1200–1000 cm−1 stretching vibrations of C–O bond have strong absorption peak. When protein is conjugated with sugar molecules by covalent bond, the increasing hydroxyl content is a typical characteristic. This is expected because the sugars contain OH groups which contribute to an enhanced absorption of the free hydroxyl band.19 In 3600–3000 and 1200–1000 cm−1, when analyzing the absorption peaks at 3445 cm−1 and 1117 cm−1 attributed to –OH and C–O groups, we observed that the absorption of xylose-modified PPI is enhanced with xylose increasing, with a particular emphasis at 10% and 20% xylose.
Therefore, a broad absorption band at about 3445 cm−1 was observed for composites at xylose level 1–5%, which can be attributed to the overlapping of free and bound O–H xylose and N–H groups coming from the protein. PPI–X composites showed a shift to high wavenumbers, and obviously lower width than PPI, with a particular emphasis at 10% and 20% xylose indicating the presence of O–H groups. This could come from a higher degree of crosslinking between protein and xylose.27
3.2 Mechanical, physical and structural properties of PPI–X films
3.2.2.1. Film solubility in water. Film solubility can be viewed as a measure of the water resistance and the integrity of a film, is usually required for potentially commercial films. A way to evaluate this resistance is to measure the water solubility which is an indicator of film's hydrophilicity.28 Addition of xylose significantly decreased PPI–X film solubility (p < 0.05),while the moisture content of PPI–X films was not changed significantly (in the range of 10.4 ± 0.9% to 10.86 ± 0.4%) as shown in Fig. 5. Compared to PPI film, a sharply decrease (from 96.6% to 52.6%) of the solubility of PPI–X film was observed with addition of 1% xylose. When the content of xylose varied from 1% to 10%, the solubility of the films continually decreased to 43.4%. When the xylose content reached 20%, the solubility decreased slightly although no significant difference (p > 0.05) was found. This decrease in solubility can be attributed to the interactions between protein side chains and exposure of the free sulfhydryl (SH) groups and hydrophobic side chains induced by cross-linking. This observation is in agreement with results presented above, which showed significantly higher values of H0 and higher degree of graft in the case of films with higher xylose content.
 | ||
| Fig. 5 Effect of xylose content on the solubility of PPI–X film different letters (a–e) indicate significant differences of the film (p < 0.05). | ||
The PPI film dissolves immediately (Fig. 6(1-a)) after immersion in water and is disintegrated into small pieces. Most of the films (xylose content > 2%) maintain their integrity after incubation in water for 12 h. However, only the 10% and 20% xylose containing films maintained their integrity after the film immersion for 24 h. This indicated that the protein polymer network in presence of 10% or 20% of xylose was highly stable and that probably only small molecules such as small peptides, and non protein material were soluble.29
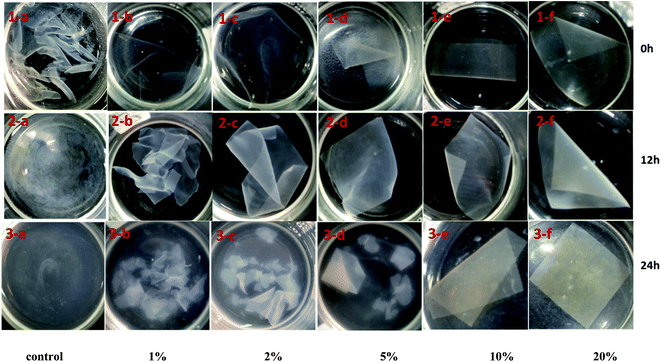 | ||
| Fig. 6 Pictorial view of dissolution (solubility) of PPI and PPI–X film. The first, second and third row show solubility of PPI–X film when immersed in water for 0 h, 12 h and 24 h, respectively. | ||
3.2.2.2. Colour. The colour of a food product makes a great contribution to the consumers' willingness to buy it.28 Thus, to better evaluate the acceptability of PPI–X films, colour parameters L*, a*, b* were analyzed, as well as the total colour difference (ΔE) due to xylose addition was determined. The effect of xylose content on the color of PPI–X films is shown in Table 1. PPI–X films show high lightness (L* parameter) values, which remains fairly constant, with a slightly decreasing tendency due to addition of xylose. However, an increasing content from 1% to 20% of xylose causes a slight decrease (p > 0.05) in the value of redness a* (2.4–2.7) and b* (11.8–12.7). The color change of the film is mainly caused by the products coming from Maillard reaction.30 The tendency of increasing values of a* and b* was obtained for other protein films containing saccharides.9 Taking into account statistical data, the total colour difference (ΔE) is not significantly affected by the addition of xylose, showing that incorporation of xylose in PPI film does not significantly affect the appearance of the food product.
| Value | Xylose concentration (%) | |||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 5 | 10 | 20 | |
| a Mean values in the same row with same letters are not significantly (p > 0.05) different, as determined by Tukey's multiple range test. | ||||||
| L* | 89.62 ± 0.27a | 89.54 ± 0.55a | 89.29 ± 1.24a | 89.04 ± 1.14a | 89.46 ± 0.78a | 89.12 ± 0.56a |
| a* | 2.39 ± 0.19a | 2.52 ± 0.20a | 2.41 ± 0.27a | 2.53 ± 0.29a | 2.54 ± 0.27a | 2.66 ± 0.22a |
| b* | 11.84 ± 0.63a | 11.92 ± 0.53a | 11.81 ± 1.08a | 12.32 ± 0.83a | 12.28 ± 0.59a | 12.67 ± 0.85a |
| ΔE | 12.68 ± 0.69a | 12.89 ± 0.17a | 12.92 ± 1.62a | 13.11 ± 1.14a | 13.21 ± 0.89a | 13.74 ± 1.01a |
3.2.3.1 Sulfhydryl group content. As it can be seen from Fig. 7, the SH content of all PPI–X films is significantly lower than the one corresponding to xylose-modified PPI powder. The higher SH contents of xylose-modified PPI powder correlates with the lower SH contents of PPI–X films. When the xylose level increases from 1% to 10%, a gradually decrease of SH contents of PPI–X films is observed, and reaches a minimum value of 1.8 μmol g−1 at 10%. Glycation-induced cross-linking through covalent bonding exposes the free sulfhydryl groups and favors the formation of new disulfide bonds due to the evaporation of solvent during drying step of film formation.31 However, when the xylose content is increased from 10% to 20%, SH content of PPI–X film increases (p < 0.05). That can be explained by the fact that the number of hydroxyl groups of xylose increases, which enhances the absorption of water molecules, and thus constrains the interaction of sulfhydryl groups. It is in agreement with results presented below, which show less compact structures of PPI–X film upon 20% xylose.
 | ||
| Fig. 7 Effect of xylose content on sulfhydryl group content of PPI–X and PPI–X films different letters (a–d) and (a′–e′) indicate significant differences between the film (p < 0.05). | ||
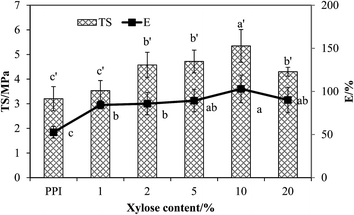
3.2.3.2 Microstructure. The mechanical and physical properties of protein films are closely related to their microstructural characteristics, which are highly influenced by the film formulation and the manufacturing process. SEM was used to visualize the surface of each film. Imaging of the film structure is useful to understand the film behavior and properties.32 The micrographs of the PPI and PPI–X films are shown in Fig. 8. PPI film displays ridges, valleys, pores and cracks in the cross-section of films. With 1% xylose content, the PPI–X film shows a more smooth structure, but loose lamellar structure, with less pores or cracks. When xylose content increases from 2% to 5%, less loose lamellar in PPI–X films is observed, but pores or cracks are observed. It can explain the gradual improvement of the tensile strength of PPI–X film with increase of xylose content (Fig. 4). When xylose content increased to 10%, the PPI–xylose films become relatively more dense and compact with a reduced film heterogeneity, but little pores can still be observed. It suggests that the dense structure is caused by intermolecular covalent and non-covalent interactions,9,11,33 indicating the effectiveness of the glycosylation between PPI and xylose. Anker32 reported that water evaporates resulting in a denser structure with smaller pores. However, when xylose content reaches 20%, a non-smooth structure with large clumps appears, which is probably due to the formation of more macromolecular polymers.9 Since increasing the concentration of reactants could increase the glycosylation yield, it could also result in greater protein denaturation and polymerization/aggregation.34,35 So this structure may result in a film with weaker mechanical properties.
4. Conclusion
The effect of xylose content on the structural and functional properties of PPI–X films was investigated. PPI–X films has some superior characteristics, e.g., much higher tensile strength and elongation, lower total soluble mass, as compared to PPI films. The results indicate that the addition of xylose improves the structural and mechanical properties of the film. Compared to PPI, DG and H0 of xylose-modified PPI increase with increasing xylose content. Different contents of xylose have significant effect on the physical and structural properties of the PPI–xylose films. Presence of xylose until a level of 10% increases the tensile strength and the elongation and decreases the solubility of PPI–X films. Overall results from this study indicate that the film properties improvement obtained by blending xylose with PPI can be attributed to covalent (Maillard reaction) and non-covalent interaction occurring between xylose and PPI. Moreover, glycosylated PPI–X solution could be converted into powder. Glycosylated PPI powder can then be used as material for film production, which enhances the convenience of transportation and storage, and provides more alternatives for peanut protein film production including solution casting, compression moulding and blow moulding. It is also more convenient to scale up to an industrial production. So the films developed in this work are suggested to be suitable for food inside packing, e.g. flavoring bag.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was financially supported by National Key R&D Program of China (2016YFD0400205), and the Agricultural Science and Technology Innovation Program. The authors also acknowledge the laboratory facility and technical assistance of the Key Laboratory of Agro-Products Processing, Ministry of Agriculture of China. We thank the University of Liège-Gembloux Agro-Bio Tech and more specifically the research platform AgricultureIsLife for the funding of the scientific stay in Belgium that made this paper possible. M. D. thanks the Fund for National Scientific Research in Belgium (FNRS) for her position has Senior Research Associate. MNN is working for the ARC-FIELD project 3/17–10.References
- H. Tian, G. Xu, B. Yang and G. Guo, Microstructure and mechanical properties of soy protein/agar blend films: Effect of composition and processing methods, J. Food Eng., 2011, 107(1), 21–26 CrossRef CAS.
- R. Kuktaite, T. S. Plivelic, Y. Cerenius, M. S. Hedenqvist, M. Gallstedt, S. Marttila, R. Ignell, Y. Popineau, O. Tranquet and P. R. Shewry, et al., Structure and morphology of wheat gluten films: From polymeric protein aggregates toward superstructure arrangements, Biomacromolecules, 2011, 12(5), 1438–1448 CrossRef CAS PubMed.
- N. Reddy, L. Chen and Y. Yang, Thermoplastic films from peanut proteins extracted from peanut meal, Ind. Crops Prod., 2013, 43(1), 159–164 CrossRef CAS.
- W. J. Lin, H. Z. Liu, A. M. Shi, L. Liu, B. Adhikari and Q. Wang, Preparation and characterisation of films from xylose-glycosylated peanut protein isolate powder, Int. J. Food Sci. Technol., 2015, 50(7), 1538–1544 CrossRef CAS.
- N. Reddy, Q. Jiang and Y. Yang, Preparation and properties of peanut protein films crosslinked with citric acid, Ind. Crops Prod., 2012, 39(1), 26–30 CrossRef CAS.
- F. Hammann and M. Schmid, Determination and quantification of molecular interactions in protein films: A review, Materials, 2014, 7(12), 7975–7996 CrossRef PubMed.
- W. J. Lin, H. Z. Liu, A. M. Shi, L. Liu, Q. Wang and B. Adhikari, Effect of glycosylation with xylose on the mechanical properties and water solubility of peanut protein films, J. Food Sci. Technol., 2015, 52(10), 6242–6253 CrossRef CAS PubMed.
- G. Rocha Plácido Moore, S. Maria Martelli, C. Gandolfo, P. José do Amaral Sobral and J. Borges Laurindo, Influence of the glycerol concentration on some physical properties of feather keratin films, Food Hydrocolloids, 2006, 20(7), 975–982 CrossRef.
- H. Zhang, S. Wang, X. Zheng, L. Jiang, X. Lv, Y. Shi and L. Li, Effect of glycosylation on the mechanical properties of edible soy protein packaging film, Eur. Food Res. Technol., 2014, 238(6), 1049–1055 CrossRef CAS.
- L. M. Pérez, G. N. Piccirilli, N. J. Delorenzi and R. A. Verdini, Effect of different combinations of glycerol and/or trehalose on physical and structural properties of whey protein concentrate-based edible films, Food Hydrocolloids, 2016, 56, 352–359 CrossRef.
- C. Li, W. Zhu, H. Xue, Z. Chen, Y. Chen and X. Wang, Physical and structural properties of peanut protein isolate-gum Arabic films prepared by various glycation time, Food Hydrocolloids, 2015, 43, 322–328 CrossRef CAS.
- M. Wihodo and C. I. Moraru, Physical and chemical methods used to enhance the structure and mechanical properties of protein films: A review, J. Food Eng., 2013, 114(3), 292–302 CrossRef CAS.
- R. Sothornvit and J. M. Krochta, Plasticizer effect on mechanical properties of β-lactoglobulin films, J. Food Eng., 2001, 50(3), 149–155 CrossRef.
- H. Zhang and Y. Chi, Modified soy protein isolate with improved gelling stability by glycosylation under the conditions of ocean shipping, Int. J. Food Sci. Technol., 2011, 46(1), 14–22 CrossRef CAS.
- X.-H. He, H.-Z. Liu, L. Liu, G.-L. Zhao, Q. Wang and Q.-L. Chen, Effects of high pressure on the physicochemical and functional properties of peanut protein isolates, Food Hydrocolloids, 2014, 36, 123–129 CrossRef CAS.
- T. Beveridge, S. J. Toma and S. Nakai, Determination of Sh- and Ss-Groups in Some Food Proteins Using Ellman’S Reagent, J. Food Sci., 1974, 39(1), 49–51 CrossRef CAS.
- C. Li, H. Xue, Z. Chen, Q. Ding and X. Wang, Comparative studies on the physicochemical properties of peanut protein isolate-polysaccharide conjugates prepared by ultrasonic treatment or classical heating, Food Res. Int., 2014, 57, 1–7 CrossRef CAS.
- C. H. Tang and Y. Jiang, Modulation of mechanical and surface hydrophobic properties of food protein films by transglutaminase treatment, Food Res. Int., 2007, 40(4), 504–509 CrossRef CAS.
- M. Khajehpour, J. L. Dashnau and J. M. Vanderkooi, Infrared spectroscopy used to evaluate glycosylation of proteins, Anal. Biochem., 2006, 348(1), 40–48 CrossRef CAS PubMed.
- J. F. Su, X. Y. Yuan, Z. Huang, X. Y. Wang, X. Z. Lu, L. D. Zhang and S. B. Wang, Physicochemical properties of soy protein isolate/carboxymethyl cellulose blend films crosslinked by Maillard reactions: Color, transparency and heat-sealing ability, Mater. Sci. Eng., C, 2012, 32(1), 40–46 CrossRef PubMed.
- Ó. L. Ramos, I. Reinas, S. I. Silva, J. C. Fernandes, M. A. Cerqueira, R. N. Pereira, A. A. Vicente, M. F. Poças, M. E. Pintado and F. X. Malcata, Effect of whey protein purity and glycerol content upon physical properties of edible films manufactured therefrom, Food Hydrocolloids, 2013, 30(1), 110–122 CrossRef.
- H. Yang, S. Yang, J. Kong, A. Dong and S. Yu, Obtaining information about protein secondary structures in aqueous solution using Fourier transform IR spectroscopy, Nat. Protoc., 2015, 10(3), 382–396 CrossRef CAS PubMed.
- E. Vass, M. Hollósi, F. Besson and R. Buchet, Vibrational spectroscopic detection of beta- and gamma-turns in synthetic and natural peptides and proteins, Chem. Rev., 2003, 103(5), 1917–1954 CrossRef CAS PubMed.
- P. Guerrero and K. De La Caba, Thermal and mechanical properties of soy protein films processed at different pH by compression, J. Food Eng., 2010, 100(2), 261–269 CrossRef CAS.
- X. Geng, B. Cui, Y. Li, W. Jin, Y. An, B. Zhou, T. Ye, L. He, H. Liang and L. Wang, et al., Preparation and characterization of ovalbumin and carboxymethyl cellulose conjugates via glycosylation, Food Hydrocolloids, 2014, 37, 86–92 CrossRef CAS.
- M. Deleu, J. M. Crowet, M. N. Nasir and L. Lins, Complementary biophysical tools to investigate lipid specificity in the interaction between bioactive molecules and the plasma membrane: A review, Biochim. Biophys. Acta, Biomembr., 2014, 1838(12), 3171–3190 CrossRef CAS PubMed.
- Y. Jiang, Y. Li, Z. Chai and X. Leng, Study of the physical properties of whey protein isolate and gelatin composite films, J. Agric. Food Chem., 2010, 58(8), 5100–5108 CrossRef CAS PubMed.
- S. Galus and J. Kadzińska, Whey protein edible films modified with almond and walnut oils, Food Hydrocolloids, 2016, 52, 78–86 CrossRef CAS.
- L. A. Kunte, M. A. Hanna and C. L. Weller, Cast Films from Soy Protein Isolates and Fractions, Cereal Chem., 1997, 74(2), 115–1118 CrossRef CAS.
- G. L. A. Makishi, R. S. Lacerda, A. M. Q. B. Bittante, H. N. M. Chambi, P. A. Costa, C. A. Gomide, R. A. Carvalho and P. J. A. Sobral, Films based on castor bean (Ricinus communis L.) proteins crosslinked with glutaraldehyde and glyoxal, Ind. Crops Prod., 2013, 50, 375–382 CrossRef CAS.
- L. Chin-Chi, A. M. Tellez-Garay and M. E. Castell-Perez, Physical and mechanical properties of peanut protein films, Lebensm.-Wiss. Technol., 2004, 37(7), 731–738 CrossRef.
- M. Anker, M. Stading and A.-M. Hermansson, Relationship between the Microstructure and the Mechanical and Barrier Properties of Whey Protein Films, J. Agric. Food Chem., 2000, 48(9), 3806–3816 CrossRef CAS PubMed.
- K. Chang, J. R. Williamson and H. S. Zarkowsky, Effect of heat on the circular dichroism of spectrin in hereditary pyropoikilocytosis, J. Clin. Invest., 1979, 64(1), 326–328 CrossRef CAS PubMed.
- F. C. D. Oliveira, J. S. D. R. Coimbra, E. B. de Oliveira, A. D. G. Zuñiga and E. E. G. Rojas, Food Protein-Polysaccharide Conjugates obtained via the Maillard Reaction: A Review, Crit. Rev. Food Sci. Nutr., 2016, 56(7), 1108–1125 CrossRef PubMed.
- D. Zhu, S. Damodaran and J. A. Lucey, Formation of whey protein isolate (WPI)-dextran conjugates in aqueous solutions, J. Agric. Food Chem., 2008, 56(16), 7113–7118 CrossRef CAS PubMed.
- Q. Wang, Peanuts: Processing Technology and Product Development, Academic Press, 1st edn, 2016 Search PubMed.
Footnote |
| † Li Liu and Wei-Jing Lin contributed equally to this article. |
| This journal is © The Royal Society of Chemistry 2017 |