Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Organocatalytic asymmetric domino Michael/O-alkylation reaction for the construction of succinimide substituted 3(2H)-furanones catalyzed by quinine†
Jing Zhou ,
Lijuan Bai,
Guojuan Liang,
Yongjie Chen
,
Lijuan Bai,
Guojuan Liang,
Yongjie Chen ,
Zongjie Gan,
Wu Wang,
Hui Zhou
,
Zongjie Gan,
Wu Wang,
Hui Zhou and
Yu Yu*
and
Yu Yu*
School of Pharmaceutical Science, Chongqing Research Center for Pharmaceutical Engineering, Chongqing Key Laboratory of Biochemistry and Molecular Pharmacology, Chongqing Medical University, Chongqing 400016, China. E-mail: zhoujing045@cqmu.edu.cn
First published on 16th August 2017
Abstract
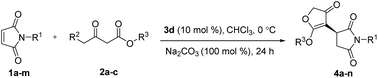
A new organocatalytic asymmetric domino Michael/O-alkylation reaction of maleimides with γ-halogenated-β-ketoesters catalyzed by simple, cheap, and commercially available quinine is described. The substrates are also commercially available. A variety of new chiral succinimide substituted 3(2H)-furanones were obtained in high yields (up to 94%) and good enantioselectivities (up to 94% ee). The absolute configuration of the new compound 4f was determined by single-crystal X-ray analysis and the proposed reaction pathway is also shown.
3(2H)-Furanones are core structural motifs that are widely present in many natural products and medicinally important agents.1 3(2H)-Furanone derivatives exhibit a wide range of biological activities such as antiulcer,2 antitumor,3 antiallergic,4 antiproliferactive,5 selective MAO-B inhibitory6 and selective COX-2 inhibitory7 activities. A variety of approaches toward the synthesis of achiral 3(2H)-furanones have been established, including metal-free processes8 as well as transition-metal-catalyzed cyclizations.9 However, protocols to construct chiral 3(2H)-furanone derivatives have been less studied. Marson and coworkers reported Sharpless's asymmetric dihydroxylation followed by a cyclization sequence of enynones to synthesize chiral 3(2H)-furanones.10 Jing and coworkers reported asymmetric Michael addition of simple 3(2H)-furanones to α,β-unsaturated ketones catalyzed by a cinchona-based tertiary–primary diamine catalyst11 or α,β-unsaturated aldehydes catalyzed by diphenylprolinol silyl ether12 to construct complex chiral 3(2H)-furanones. These reactions require harsh reaction conditions or specific substrates and complex chiral catalysts. Recently, γ-halogenated-β-ketoesters, which are commercially available, are used to construct chiral 3(2H)-furanones with imines13 and chain nitroalkenes14 via domino reactions.15 This synthetic strategy is efficient and mild. However, these reactions also need complex chiral catalysts such as tertiary amine squaramide,13a tertiary amine thiourea13b,14a and 6′-demethyl quinine catalysts.14b Moreover, the substrate scope is relatively limited. For the significance of chiral 3(2H)-furanones, it is strongly useful and desirable to discover other appropriate electrophiles such as cyclic alkenes to react with γ-halogenated-β-ketoesters catalyzed by simple, cheap, commercially available catalysts to construct diverse chiral substituted 3(2H)-furanones.
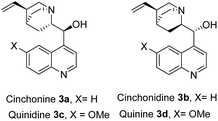
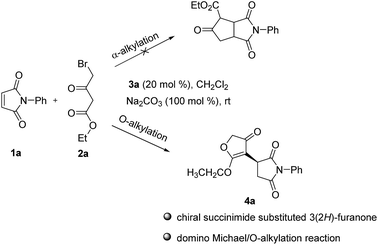
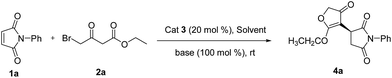
Maleimides, which are also commercially available, are an important class of cyclic alkenes. They have been extensively applied in asymmetric organocatalysis to construct chiral succinimide derivatives, which are core structural units found in natural products and clinical drug candidates.16 To date, there has been no report of asymmetric reaction of γ-halogenated-β-ketoesters with maleimides, which could afford a new class of chiral products combining biologically significant succinimides with 3(2H)-furanones. These fused chiral products might show higher or new biological activities. Recently, we reported the same transformation in racemic version catalyzed by Et3N.17 As a part of our continuing interests in the construction of more complex and novel drug candidates,18 herein, we wish to report the first asymmetric domino Michael/O-alkylation reaction of γ-halogenated-β-ketoesters with maleimides catalyzed by simple, cheap, commercially available cinchona alkaloids to access a new range of chiral succinimide substituted 3(2H)-furanones (Fig. 1). Córdova and coworkers reported asymmetric domino Michael/α-alkylation reaction of γ-halogenated-β-ketoesters with α,β-unsaturated aldehydes to afford cyclopentanone products.19 This report shows variable chemical reactivities of γ-halogenated-β-ketoesters with activated alkenes. Our preliminary studies involved maleimide 1a and ethyl 4-bromo-acetoacetate 2a as substrates, these were allowed to react in the presence of 20 mol% cinchonine with 100 mol% Na2CO3 in CH2Cl2 at room temperature. The reaction worked well and gave succinimide substituted 3(2H)-furanone 4a in 85% yield and 68% ee via domino Michael/O-alkylation process not Michael/α-alkylation process (Scheme 1).
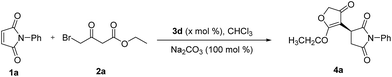
To evaluate the reactivity of this catalytic system, the reaction of N-phenyl maleimide 1a with ethyl 4-bromo-acetoacetate 2a was used as a model reaction, and four kinds of natural cinchona alkaloid catalysts were investigated in CH2Cl2 at room temperature (Table 1, entries 1–4). All catalysts gave good yields, quinine afforded best enantioselectivity than other catalysts and was chosen as the most suitable catalyst for further optimazation (Table 1, entry 4). Then an array of base additives were screened (Table 1, entries 4–6). Organic base Et3N afforded good yield but poor enantioselectivity (Table 1, entry 5). Weak inorganic base NaHCO3 decreased the reaction rate, afforded poor yield after reaction for 24 h (Table 1, entry 6). Inorganic base Na2CO3 afforded good yield and moderate enantioselectivity after reaction for 10 h. In terms of yields and enantioselectivities, Na2CO3 was chosen as the most suitable base additive for further optimization (Table 1, entry 4). Afterward, a series of solvents were evaluated (Table 1, entries 7–13). Halohydrocarbon solvents afforded good yield and moderate enantioselectivity (Table 1, entry 7). THF and toluene gave lower yields and enantioselectivities (Table 1, entries 9 and 10). Et2O gave poor yield (Table 1, entry 8) and n-hexane gave trace product (Table 1, entry 13), the possible reason might be the poor solubility of the raw materials and catalyst in these solvents. Polar solvents such as EtOAc and acetonitrile gave good yields and poor enantioselectivities (Table 1, entries 11 and 12). A survey of solvents revealed that CHCl3 was the most suitable solvent (Table 1, entry 7).
| Entry | Cat. | Base | Solvent | Time (h) | Yieldb (%) | eec (%) |
|---|---|---|---|---|---|---|
| a Unless otherwise noted, reactions were conducted with 0.2 mmol 1a, 0.2 mmol 2a, 20 mol% catalyst 3, 100 mol% base, in 1.0 mL solvent at rt.b Isolated yields.c Determined by chiral HPLC analysis.d Contrary configuration. | ||||||
| 1 | 3a | Na2CO3 | CH2Cl2 | 10 | 85 | 68d |
| 2 | 3b | Na2CO3 | CH2Cl2 | 10 | 83 | 64 |
| 3 | 3c | Na2CO3 | CH2Cl2 | 10 | 86 | 68d |
| 4 | 3d | Na2CO3 | CH2Cl2 | 10 | 88 | 71 |
| 5 | 3d | Et3N | CH2Cl2 | 2 | 92 | 49 |
| 6 | 3d | NaHCO3 | CH2Cl2 | 24 | 49 | 74 |
| 7 | 3d | Na2CO3 | CHCl3 | 10 | 90 | 75 |
| 8 | 3d | Na2CO3 | Et2O | 10 | 68 | 52 |
| 9 | 3d | Na2CO3 | THF | 10 | 82 | 47 |
| 10 | 3d | Na2CO3 | Toluene | 10 | 80 | 68 |
| 11 | 3d | Na2CO3 | EtOAc | 10 | 84 | 19 |
| 12 | 3d | Na2CO3 | CH3CN | 10 | 89 | 12 |
| 13 | 3d | Na2CO3 | n-Hexane | 10 | Trace | nd |
To further optimize the reaction conditions, reaction temperature was investigated (Table 2, entries 1–4). Decreasing reaction temperature increased enantioselectivities but slowed down the reaction rate. After reaction for 24 h, good yield could still be obtained at 0 °C (Table 2, entry 2), but further lowering the reaction temperature, the yields decreased obviously (Table 2, entries 3 and 4). 0 °C was the optimal reaction temperature. Then we examined the effect of catalyst loadings. Decreasing catalyst loadings to 10 mol% still gave good yield and enantioselectivity (Table 2, entry 5). We next examined the effect of molar ratio, increasing or lowering the molar ratio slightly influenced the yields and slightly decreased the enantioselectivities (Table 2, entries 6 and 7). The substrate concentration was also examined. It was found that increasing or lowering the substrate concentration slightly decreased the yields and enantioselectivities (Table 2, entries 8 and 9). Consequently, the following reaction conditions were recommended: 10 mol% quinine, 1 equivalent of Na2CO3 with 0.2 M of substrate concentration in CHCl3 at 0 °C.
| Entry | Temp (°C) | x | Time (h) | Yieldb (%) | eec (%) |
|---|---|---|---|---|---|
| a Unless otherwise noted, reactions were conducted with 0.2 mmol 1a, 0.2 mmol 2a, catalyst 3d, 100 mol% Na2CO3, in 1.0 mL CHCl3.b Isolated yields.c Determined by chiral HPLC analysis.d 1.5 equivs 1a was used.e 1.5 equivs 2a was used.f 0.5 mL CHCl3 was used.g 2.0 mL CHCl3 was used. | |||||
| 1 | 25 | 20 | 10 | 90 | 75 |
| 2 | 0 | 20 | 24 | 91 | 83 |
| 3 | −10 | 20 | 24 | 72 | 84 |
| 4 | −20 | 20 | 24 | 67 | 85 |
| 5 | 0 | 10 | 24 | 89 | 83 |
| 6d | 0 | 10 | 24 | 87 | 82 |
| 7e | 0 | 10 | 24 | 92 | 80 |
| 8f | 0 | 10 | 24 | 88 | 79 |
| 9g | 0 | 10 | 24 | 86 | 82 |
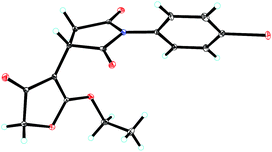
Under the optimal reaction conditions, the generality of this protocol was studied (Table 3). Firstly, a wide range of N-aromatic and aliphatic maleimides 1a–m were studied (Table 3, entries 1–13). All gave good yields and moderate to good enantioselectivities (80–94% yield, 68–94% ee). N-phenyl maleimides with strong electron-withdrawing nitro group 1g and 1i gave slightly lower yields and enantioselectivities (Table 3, entries 7 and 9). For N-aliphatic maleimides, good yields and enantioselectivities could still be obtained (Table 3, entries 11–13) and N-benzyl maleimide gave best enantioselectivity (91% yield, 94% ee, Table 3, entry 13). In addition, methyl 4-chloroacetoacetate 2b and ethyl 4-bromoacetoacetate 2c were also tested, both provided good yields and enantioselectivities (Table 3, entries 14 and 15). The absolute configuration was determined by an X-ray analysis of the single crystal of 4f, which was assigned as (S) (Fig. 2).20
| Entry | 1/R1 | 2/R2, R3 | 4 | Yieldb (%) | eec (%) |
|---|---|---|---|---|---|
| a Unless otherwise noted, reactions were conducted with 0.2 mmol 1a, 0.2 mmol 2a, 10 mol% catalyst 3d, 100 mol% Na2CO3, in 1.0 mL CHCl3 at 0 °C.b Isolated yields.c Determined by chiral HPLC analysis. | |||||
| 1 | 1a/C6H5 | 2a/Br, Et | 4a | 89 | 83 |
| 2 | 1b/4-CH3C6H4 | 2a/Br, Et | 4b | 93 | 83 |
| 3 | 1c/4-CH3OC6H4 | 2a/Br, Et | 4c | 90 | 87 |
| 4 | 1d/4-FC6H4 | 2a/Br, Et | 4d | 87 | 80 |
| 5 | 1e/4-ClC6H4 | 2a/Br, Et | 4e | 92 | 84 |
| 6 | 1f/4-BrC6H4 | 2a/Br, Et | 4f | 88 | 83 |
| 7 | 1g/4-NO2C6H4 | 2a/Br, Et | 4g | 84 | 77 |
| 8 | 1h/3-FC6H4 | 2a/Br, Et | 4h | 87 | 79 |
| 9 | 1i/3-NO2C6H4 | 2a/Br, Et | 4i | 82 | 68 |
| 10 | 1j/2-MeC6H4 | 2a/Br, Et | 4j | 80 | 83 |
| 11 | 1k/CH3 | 2a/Br, Et | 4k | 94 | 72 |
| 12 | 1l/Cyclohexyl | 2a/Br, Et | 4l | 85 | 89 |
| 13 | 1m/Bn | 2a/Br, Et | 4m | 91 | 94 |
| 14 | 1a/C6H5 | 2b/Cl, Me | 4n | 86 | 78 |
| 15 | 1a/C6H5 | 2c/Cl, Et | 4a | 83 | 80 |
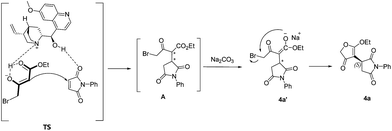
Based on the experimental results and the observed configuration of 4f, a plausible mechanism is proposed in Scheme 2.18a The tertiary amine group of quinine catalyst activates 4-bromo-acetoacetate in its enol form and the hydroxyl moiety activates maleimide through hydrogen bonding, thus the enolate would attack the maleimide from Re face via transition state TS (Scheme 2). Through the dual activation of quinine catalyst, Michael reaction is realized and generates intermediary Michael adduct A. Then in the presence of Na2CO3, intermediary Michael adducts A forms enolate ion 4a′, which undergoes an intramolecular O-alkylation process to form the product 4a with (S)-configuration.
Conclusions
In summary, we have developed a new organocatalytic asymmetric domino Michael/O-alkylation reaction of maleimides with γ-halogenated-β-ketoesters catalyzed by simple, cheap, and commercially available quinine. The substrates are also commercially available. A wide range of new chiral succinimide substituted 3(2H)-furanones were smoothly obtained in high yields (up to 94%) and good enantioselectivities (up to 94% ee). Further, expansion of these new succinimide substituted 3(2H)-furanones to access products with known biological activities or new biologically significant molecules and testing their pharmacological activities are ongoing in our laboratory.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We appreciate the financial support from National Natural Science Foundation of China (No. 21672031), Funds for Young Science and Technology Talent Cultivation Plan of Chongqing City (No. cstc2014kjrc-qnrc00004), Fundamental and Advanced Research Projects of Chongqing City (No. cstc2016jcyjA0267), Scientific and Technological Research Program of Chongqing Municipal Education Commission (No. KJ1600211 and KJ1600217) and Outstanding Young Scholars Project of Chongqing Medical University (No. 4101070059).Notes and references
- Selected examples: (a) Y. Li and K. J. Hale, Org. Lett., 2007, 9, 1267 CrossRef CAS PubMed; (b) J. M. Mitchell and N. S. Finney, Org. Biomol. Chem., 2005, 3, 4274 RSC; (c) Y. Hayashi, M. Shoji, S. Yamaguchi, T. Mukaiyama, J. Yamaguchi, H. Kakeya and H. Osada, Org. Lett., 2003, 5, 2287 CrossRef CAS PubMed; (d) K. Takao, H. Ochiai, K. Yoshida, T. Hashizuka, H. Koshimura, K. Tadano and S. Ogawa, J. Org. Chem., 1995, 60, 8179 CrossRef CAS; (e) Q. Han and D. F. Wiemer, J. Am. Chem. Soc., 1992, 114, 7692 CrossRef CAS; (f) A. C. Gyorkos, J. K. Stille and L. S. Hegedus, J. Am. Chem. Soc., 1990, 112, 8465 CrossRef CAS.
- S. W. Felman, I. Jirkovsky, K. A. Memoli, L. Borella, C. Wells, J. Russell and J. Ward, J. Med. Chem., 1992, 35, 1183 CrossRef CAS PubMed.
- P. J. Jerris and A. B. Smith III, J. Org. Chem., 1981, 46, 577 CrossRef CAS.
- R. A. Mack, W. I. Zazulak, L. A. Radov, J. E. Baer, J. D. Stewart, P. H. Elzer, C. R. Kinsolving and V. S. Georgiev, J. Med. Chem., 1988, 31, 1910 CrossRef CAS PubMed.
- S. Chimichi, M. Boccalini, B. Cosimelli, F. Dall'Acqua and G. Viola, Tetrahedron, 2003, 59, 5215 CrossRef CAS.
- A. Carotti, A. Carrieri, S. Chimichi, M. Boccalini, B. Cosimelli, C. Gnerre, A. Carotti, P. A. Carrupt and B. Testa, Bioorg. Med. Chem. Lett., 2002, 12, 3551 CrossRef CAS PubMed.
- J. L. Shamshina and T. S. Snowden, Tetrahedron Lett., 2007, 48, 3767 CrossRef CAS.
- Selected examples: (a) S. Inagaki, M. Ukaku, A. Chiba, F. Takahashi, Y. Yoshimi, T. Morita and T. Kawano, J. Org. Chem., 2016, 81, 8363 CrossRef CAS PubMed; (b) J. J. Medvedev, D. V. Semenok, X. V. Azarova, L. L. Rodina and V. A. Nikolaev, Synthesis, 2016, 48, 4525 CrossRef CAS; (c) A. G. Mal'kina, O. G. Volostnykh, K. B. Petrushenko, O. A. Shemyakina, V. V. Nosyreva, I. A. Ushakov and B. A. Trofimov, Tetrahedron, 2013, 69, 3714 CrossRef; (d) Y. Wei, S.-X. Lin, J. Zhang, Z.-H. Niu, Q. Fu and F.-S. Liang, Chem. Commun., 2011, 47, 12394 RSC; (e) Y. Wei, S.-X. Lin, H.-X. Xue, F.-S. Liang and B.-Z. Zhao, Org. Lett., 2012, 14, 712 CrossRef CAS PubMed; (f) G.-Q. Yuan, Z.-J. He, J.-H. Zheng, Z.-W. Chen, H.-W. Huang, D.-B. Shi, C.-R. Qi and H.-F. Jiang, Tetrahedron Lett., 2011, 52, 5956 CrossRef CAS.
- Selected examples: (a) H.-T. He, C.-R. Qi, X.-H. Hu, L. Ouyang, W.-F. Xiong and H.-F. Jiang, J. Org. Chem., 2015, 80, 4957 CrossRef CAS PubMed; (b) J. John, E. Târcoveanu, P. G. Jones and H. Hopf, Beilstein J. Org. Chem., 2014, 10, 1462 CrossRef PubMed; (c) J. John and H. Hopf, Eur. J. Org. Chem., 2013, 841 CrossRef CAS; (d) T. C. Kusakabe, T. Takahashi, R. Shen, A. Ikeda, Y. D. Dhage, Y. Kanno, Y. Inouye, H. Sasai, T. Mochida and K. Kato, Angew. Chem., Int. Ed., 2013, 52, 1 CrossRef PubMed; (e) F. Hu, J.-W. Yan, M. Cheng and Y.-H. Hu, Chem.–Asian J., 2013, 8, 482 CrossRef CAS PubMed; (f) C.-R. Qi, H.-F. Jiang, L.-B. Huang, G.-Q. Yuan and Y.-W. Ren, Org. Lett., 2011, 13, 5520 CrossRef CAS PubMed; (g) M. Poonoth and N. Krause, J. Org. Chem., 2011, 76, 1934 CrossRef CAS PubMed; (h) M. Egi, K. Azechi, M. Saneto, K. Shimizu and S. Akai, J. Org. Chem., 2010, 75, 2123 CrossRef CAS PubMed.
- C. M. Marson, E. Edaan, J. M. Morrell, S. J. Coles, M. B. Hursthouse and D. T. Davies, Chem. Commun., 2007, 2494 RSC.
- W.-J. He, L.-H. Jing, D.-B. Qin, R.-M. Wang, X.-H. Xie and S. Wu, Tetrahedron Lett., 2013, 54, 6363 CrossRef CAS.
- W.-J. He, L.-H. Jing, D.-B. Qin, X.-H. Xie, S. Wu and R.-M. Wang, Tetrahedron Lett., 2014, 55, 209 CrossRef CAS.
- (a) X.-B. Wang, T.-Z. Li, F. Sha and X.-Y. Wu, Eur. J. Org. Chem., 2014, 739 CrossRef; (b) N.-H. Luo, X. Sun, Y.-Y. Yan, S.-Z. Nie and M. Yan, Tetrahedron: Asymmetry, 2011, 22, 1536 CrossRef CAS.
- (a) X.-W. Dou, X.-Y. Han and Y.-X. Lu, Chem.–Eur. J., 2012, 18, 85 CrossRef CAS PubMed; (b) Y.-Y. Yan, R.-J. Lu, J.-J. Wang, Y.-N. Xuan and M. Yan, Tetrahedron, 2012, 68, 6123 CrossRef CAS.
- Selected examples: (a) L. Liu, Y. Cotelle, J. Klehr, N. Sakai, T. R. Ward and S. Matile, Chem. Sci., 2017, 8, 3770 RSC; (b) A. Bhaumik, R. S. Verma and B. Tiwari, Org. Lett., 2017, 19, 444 CrossRef CAS PubMed; (c) K. Zhao, Y. Zhi, T. Shu, A. Valkonen, K. P. Rissanen and D. Enders, Angew. Chem., Int. Ed., 2016, 55, 12104 CrossRef CAS PubMed; (d) E. S. Diez, D. L. Vesga, E. Reyes, U. Uria, L. Carrillo and J. L. Vicario, Org. Lett., 2016, 18, 1270 CrossRef PubMed.
- Selected examples: (a) R. Huang, H.-Y. Tao and C.-J. Wang, Org. Lett., 2017, 19, 1176 CrossRef CAS PubMed; (b) J. F. Ferrándiz and R. Chinchilla, Tetrahedron: Asymmetry, 2017, 28, 302 CrossRef; (c) C.-C. Zou, C.-K. Zeng, Z. Liu, M. Lu, X.-H. Sun and J.-X. Ye, Angew. Chem., Int. Ed., 2016, 55, 14257 CrossRef CAS PubMed; (d) D. Kalia, P. V. Malekar and M. Parthasarathy, Angew. Chem., Int. Ed., 2016, 55, 1432 CrossRef CAS PubMed; (e) L. Liu, B.-L. Zhao and D.-M. Du, Eur. J. Org. Chem., 2016, 2016, 4711 CrossRef CAS; (f) S. Qiu, R. Lee, B. Zhu, M. L. Coote, X.-W. Zhao and Z.-Y. Jiang, J. Org. Chem., 2016, 81, 8061 CrossRef CAS PubMed.
- W. Wang, G.-J. Liang, Y. Bai, L.-J. Bai, H. Zhou, Y. Yu and J. Zhou, ARKIVOC, 2017, 4, 236 Search PubMed.
- (a) J. Zhou, Q.-L. Wang, L. Peng, F. Tian, X.-Y. Xu and L.-X. Wang, Chem. Commun., 2014, 50, 14601 RSC; (b) J. Zhou, L.-N. Jia, Q.-L. Wang, L. Peng, F. Tian, X.-Y. Xu and L.-X. Wang, Tetrahedron, 2014, 70, 8665 CrossRef CAS; (c) J. Zhou, L.-N. Jia, L. Peng, Q.-L. Wang, F. Tian, X.-Y. Xu and L.-X. Wang, Tetrahedron, 2014, 70, 3478 CrossRef CAS; (d) Q.-L. Wang, T. Cai, J. Zhou, F. Tian, X.-Y. Xu and L.-X. Wang, Chem. Commun., 2015, 51, 10726 RSC; (e) Y.-L. Guo, L.-N. Jia, L. Peng, L.-W. Qi, J. Zhou, F. Tian, X.-Y. Xu and L.-X. Wang, RSC Adv., 2013, 3, 16973 RSC.
- R. Rios, J. Vesely, H. Sundén, I. Ibrahem, G.-L. Zhao and A. Córdova, Tetrahedron Lett., 2007, 48, 5835 CrossRef CAS.
- The CCDC number is 1524709.†.
Footnote |
| † CCDC 1524709. For crystallographic data in CIF or other electronic format see DOI: 10.1039/c7ra07317h |
| This journal is © The Royal Society of Chemistry 2017 |