Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Structural optimization of large acceptor–donor–acceptor-type molecules for improved performance of fullerene-free polymer solar cells†
Min Ju Cho‡
,
Gi Eun Park‡,
Seo Yeon Park,
Young-Un Kim and
Dong Hoon Choi *
*
Department of Chemistry, Research Institute for Natural Sciences, Korea University, 5 Anam-dong, Sungbuk-gu, Seoul 136-701, Korea. E-mail: dhchoi8803@korea.ac.kr
First published on 8th August 2017
Abstract
To control the molecular energy levels of highly π-extended n-type molecules, we synthesized two acceptor–donor–acceptor (A–D–A)-type molecules with indacenodithiophenes (IDTs) or IDT–benzodithiophene (BDT)–IDT as donating cores and 2-(2,3-dihydro-3-oxo-1H-inden-1-ylidene)propanedinitrile (IM) as terminal accepting units. These molecules showed different optical and electrochemical properties, indicating that the energy levels can be easily tuned by changing the structure of the donating core. Among two molecules, IM-BDTIDT2 showed a relatively blue shifted absorption spectrum and low-lying highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) levels. Although IM-IDT3 and IM-BDTIDT2 have a highly π-extended conjugated structure, no clear crystalline behaviour was observed in their thin films. When applied to polymer solar cells (PSCs), the device based on IM-BDTIDT2 displayed a higher PCE (5.33%) than the device bearing IM-IDT3 owing to the lower-lying energy levels of IM-BDTIDT2. Thus, the use of BDT as a donating core unit is favorable for limiting high-lying energy levels in highly π-extended A–D–A-type molecules.
Introduction
To obtain high power conversion efficiency (PCE) in non-fullerene-based bulk heterojunction (BHJ) polymer solar cells (PSCs), many kinds of n-type semiconducting organic molecules have been developed recently.1–6 Although fullerene derivatives such as [6,6]-phenyl-C61/C71-butyric acid methyl ester (PC61BM and PC71BM) have good n-type semiconducting properties and display outstanding PSC performance, they suffer from high costs and limitations in light absorption range, energy levels, and internal morphology control in blend films.7–13Recently, it has been suggested that an efficient design strategy for non-fullerene acceptors is to combine a typical conjugated donor core with acceptor units such as perylene diimide (PDI), rhodanine, and dicyanovinyl; the resultant molecules are denoted as acceptor–donor–acceptor (A–D–A)-type molecules.14–22 The PCE values of BHJ PSCs based on such n-type molecules have been rapidly improved through the development of device architectures and appropriate p-type D–A copolymers to replace polythiophene derivatives which are donor-only polymers.23–27
Among the various n-type organic semiconductors, dicyanovinyl-based electron withdrawing groups have attracted considerable attention because their combination with various building blocks can result in facile tunability of energy levels and absorption properties for specific A–D–A molecules. Additionally, it was found that the molar absorption efficiencies of such molecules, from the visible to near infrared (NIR) region, are much larger than those of fullerene derivatives.
For example, a new series of n-type semiconducting molecules consisting of indacenodithiophene (IDT) or indacenodithienothiophene derivatives as donor cores and 2-(2,3-dihydro-3-oxo-1H-inden-1-ylidene)propanedinitrile (IM) as terminal acceptor groups in A–D–A-type molecules have been demonstrated to show high PCE values in non-fullerene-based BHJ PSCs.28–36 Advantageously, the energy levels of these molecules were also easily tuned by introducing thienothiophene or thiophene bridges. On the other hand, size variation of the donating core unit in A–D–A-type molecules has been studied less frequently. Such variation is an important design strategy for governing molecular energy levels, improving film-forming properties, and controlling morphologies in donor–acceptor blend films.
In this work, we designed and synthesized highly π-extended n-type semiconducting molecules with IDT–IDT–IDT core or an IDT–benzodithiophene (BDT)–IDT core as donating units and IM as terminal accepting units, as shown in Fig. 1. IM-IDT3 was reported to show poor PCE of 1.05% in PSC device (PTB7-Th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IM-IDT3) due to similar energy level of a PTB7-Th as p-type polymer.37
IM-IDT3) due to similar energy level of a PTB7-Th as p-type polymer.37
As a new n-type molecule, IM-BDTIDT2 was prepared to modify the energy levels of IM-IDT3. These n-type semiconducting molecules showed relatively high-lying highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) energy levels compared with those of PCBM derivatives. Interestingly, IM-BDTIDT achieves favourable energy levels in PSCs which are lower-lying than those of IM-IDT3. Pure and blends films of IM-IDT3 and IM-BDTIDT2 showed lower degree of crystallinity or amorphous natures. A high PCE of 5.33% was achieved for PTB7-Th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IM-BDTIDT2-based inverted BHJ solar cells, whereas the IM-IDT3-based devices showed PCE values of 3.25%.
IM-BDTIDT2-based inverted BHJ solar cells, whereas the IM-IDT3-based devices showed PCE values of 3.25%.
Results and discussion
Synthesis and characterization
The synthetic procedure for the final n-type semiconductor IM-BDTIDT2, is shown in Scheme 1. IM-IDT3 was prepared according to literature.37 Compounds 3 bearing two aldehyde groups were prepared via Stille coupling reaction with compound 1 and compound 2. Finally, IM-BDTIDT2 was successfully synthesized quantitatively via typical Knoevenagel condensation reactions using the dialdehyde compound 3 and 2-(3-oxo-2,3-dihydro-1H-inden-1-ylidene)malononitrile. To confirm the structures of the compounds synthesized herein, NMR and MALDI-TOF analyses were performed. Two final compounds were found to have good solubility in organic solvents, such as dichloromethane, THF, chloroform, and chlorinated benzenes. | ||
| Scheme 1 Synthetic procedure for IM-BDTIDT2: (i) Pd2(dba)3, P(o-tolyl)3, toluene, 100 °C, 12 h. (ii) 2-(3-oxo-2,3-Dihydro-1H-inden-1-ylidene)malononitrile, ammonium acetate, chloroform, 70 °C, 5 h. | ||
Optical and electrochemical properties
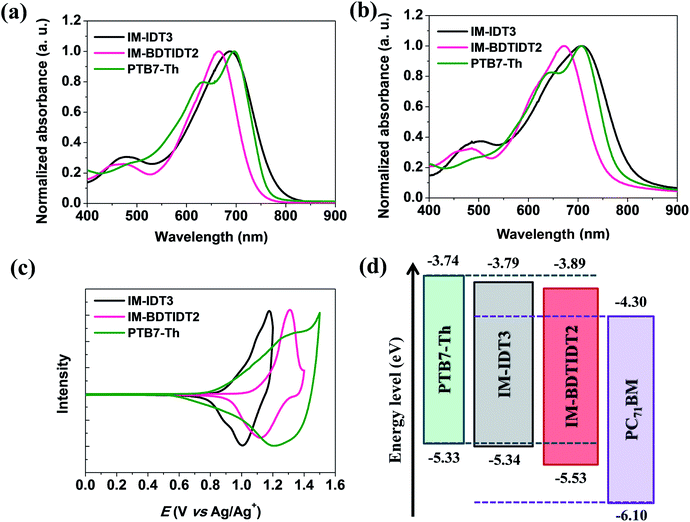
UV-vis absorption spectra of the two synthesized n-type semiconducting molecules, IM-IDT3 and IM-BDTIDT2 were obtained in both solution and thin films. The corresponding spectra and parameters are displayed in Fig. 2 and Table 1. Two synthesized compounds show a strong NIR absorption band owing to strong intramolecular charge transfer (ICT) between IDT3 or BDTIDT2 as an electron-donating group and IMs as strong electron-withdrawing groups.| n-Type molecule | Absorption (nm) | λcut-off (nm) | Eoptgb (eV) | Eoxc (V) | Energy level (eV) | ||
|---|---|---|---|---|---|---|---|
| Solutiona (ε) | Film | HOMOc | LUMOd | ||||
| a ε is a molar extinction coefficient (×10−5 M−1 cm−1).b Optical bandgap was obtained from the onset wavelength (λcut-off) of the film.c Values obtained from cyclic voltammograms.d LUMO (eV) = HOMO (eV) + Eoptg (eV). | |||||||
| IM-IDT3 | 687 (1.87) | 707 | 808 | 1.53 | 0.89 | −5.34 | −3.81 |
| IM-BDTIDT2 | 665 (2.04) | 671 | 756 | 1.64 | 1.08 | −5.53 | −3.89 |
The wavelength of maximum absorbance (λmax) of IM-IDT3 and IM-BDTIDT2 are 687 and 665 nm, respectively, in chloroform solution and 707 and 671 nm, respectively, in thin films. Although IM-BDTIDT2 has a highly π-extended backbone, similar to IM-IDT3, the absorption spectrum of IM-BDTIDT2 in solution shows a blue shift relative to that of IM-IDT3. Thus, it seems that the electron-donating ability of the BDT unit might be weaker than that of IDT.
Compared with those of IM-IDT3, the absorption spectrum of the IM-BDTIDT2 film shows relatively smaller bathochromic shift. This observation indicates that the IDT–BDT–IDT core in IM-BDTIDT2 might suppress intermolecular interactions (Fig. S3†). Additionally, molar extinction coefficient of IM-BDTIDT2 is 2.04 × 105 M−1 cm−1, which is relatively higher than that of IM-IDT3 as shown in Fig. S4a.† The optical bandgaps of IM-IDT3 and IM-BDTIDT2 calculated from the absorption edges in the absorption spectra of the thin films were 1.53 and 1.64 eV, respectively.
The redox potentials of synthesized IM-IDT3 and IM-BDTIDT2 were examined using CV measurements, as shown in Fig. 2c. The oxidation potentials (Eox) of IM-IDT3 and IM-BDTIDT2 were 0.89 and 1.08 V, respectively, and the corresponding HOMO levels were located at −5.34 and −5.53 eV, respectively. The LUMO levels, as calculated from the HOMO levels and the optical bandgaps, were located at −3.81 and −3.89 eV for IM-IDT3 and IM-BDTIDT2, respectively. Compared with PC71BM derivatives, the LUMO levels of the synthesized molecules as acceptors are suitable for fabricating BHJ PSCs, as shown in Fig. 2d. It could lead us to expect a higher open circuit voltage (Voc) value in a PSC with the blend film containing PTB7-Th as p-type polymer.
However, as the energy levels of IM-IDT3 are very similar to those of PTB7-Th, (Fig. 2d), efficient charge separation and transport might not be achieved in an IM-IDT3 and PTB7-Th interface. Interestingly, the energy levels of IM-BDTIDT2 are slightly lower lying than those of IM-IDT3 owing to the effect of the BDT core.
Performance of bulk heterojunction polymer solar cells
BHJ PSCs were fabricated using the synthesized n-type molecules and PTB7-Th as a well-known p-type semiconducting polymer. We chose an inverted solar cell structure with the configuration of ITO/ZnO/PTB7-Th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n-type molecule/MoO3/Ag. To obtain the best device performance, the donor and acceptor blend ratio and the amount of DPE as a solvent additive were optimized, as shown in Table S1.†
n-type molecule/MoO3/Ag. To obtain the best device performance, the donor and acceptor blend ratio and the amount of DPE as a solvent additive were optimized, as shown in Table S1.†
Fig. 3a and b show the current density–voltage (J–V) and external quantum efficiency (EQE) characteristics of PTB7-Th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n-type-molecule-based devices obtained under simulated AM 1.5 G solar illumination at an intensity of 100 mW cm−2, and the resultant device performance parameters are summarized in Tables 2 and S1.† The best PCE value for PTB7-Th
n-type-molecule-based devices obtained under simulated AM 1.5 G solar illumination at an intensity of 100 mW cm−2, and the resultant device performance parameters are summarized in Tables 2 and S1.† The best PCE value for PTB7-Th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IM-IDT3 with a 1
IM-IDT3 with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w) blend ratio and 4 vol% DPE additive was 3.25%, which is three times higher than that of published PSCs made of an o-DCB solution of PTB7-Th
1 (w/w) blend ratio and 4 vol% DPE additive was 3.25%, which is three times higher than that of published PSCs made of an o-DCB solution of PTB7-Th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IM-IDT3 (1
IM-IDT3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5) blend without adding any additives.37 Interestingly, the PTB7-Th
1.5) blend without adding any additives.37 Interestingly, the PTB7-Th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IM-BDTIDT2 – based device (1
IM-BDTIDT2 – based device (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2 vol% DPE additive) exhibited the highest PCE of 5.3%. Interestingly, two devices showed the high Voc value from 0.96 to 0.97 V, which is consistent with the difference between the LUMO level of the acceptor and the HOMO level of PTB7-Th obtained from the CV results. Compared to IM-IDT3, IM-BDTIDT2-based device with 2% DPE exhibited high Jsc value of 11.28 mA cm−2.
1, 2 vol% DPE additive) exhibited the highest PCE of 5.3%. Interestingly, two devices showed the high Voc value from 0.96 to 0.97 V, which is consistent with the difference between the LUMO level of the acceptor and the HOMO level of PTB7-Th obtained from the CV results. Compared to IM-IDT3, IM-BDTIDT2-based device with 2% DPE exhibited high Jsc value of 11.28 mA cm−2.
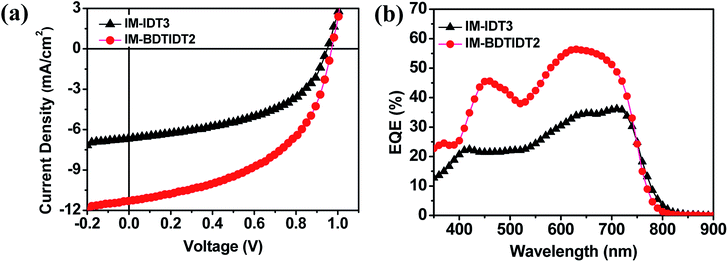 | ||
Fig. 3 J–V curves (a) and EQE spectra (b) of PTB7-Th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IM-IDT3 (1 IM-IDT3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) with 4 vol% DPE, and PTB7-Th 1) with 4 vol% DPE, and PTB7-Th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IM-BDTIDT2 (1 IM-BDTIDT2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) with 2 vol% DPE. 1) with 2 vol% DPE. | ||
| n-Type molecule | D/A ratio (wt%) | Solvent/additive | Voc (V) | Jsc (mA cm−2) | FF (%) | PCE a (%) |
|---|---|---|---|---|---|---|
| a Average PCE values, which were obtained from more than 10 devices, are shown in parentheses. | ||||||
| IM-IDT3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
CB | 0.95 (0.94 ± 0.01) | 5.72 (5.68 ± 0.04) | 49.87 (49.63 ± 0.24) | 2.70 (2.65 ± 0.05) |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
CB/DPE 4% | 0.95 (0.94 ± 0.01) | 7.15 (7.06 ± 0.09) | 47.82 (47.71 ± 0.11) | 3.25 (3.17 ± 0.08) | |
| IM-BDTIDT2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
CB | 0.96 (0.95 ± 0.01) | 9.42 (8.97 ± 0.45) | 50.79 (50.67 ± 0.12) | 4.57 (4.32 ± 0.25) |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
CB/DPE 2% | 0.97 (0.96 ± 0.01) | 11.28 (10.89 ± 0.39) | 48.72 (48.58 ± 0.14) | 5.33 (5.08 ± 0.25) | |
The EQE spectra of the corresponding devices are shown in Fig. 3b. EQE spectrum shapes of them correspond to the absorption band of their blend films, as shown in Fig. S5.† The IM-BDTIDT2-based devices with higher PCE values show high photon-to-current responses from 500 to 800 nm, whereas the IM-IDT3-based device exhibit relatively low EQE values. This observation indicates that the PTB7-Th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IM-BDTIDT2 blend achieves more efficient photoelectron conversion.
IM-BDTIDT2 blend achieves more efficient photoelectron conversion.
Moreover as shown in Fig. S6 and Table S2,† the device fabricated with the PTB7-Th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IM-BDTIDT2 blend had a relatively low ratio of hole and electron mobility (μh/μe = 6.32) compared to that of PTB7-Th
IM-BDTIDT2 blend had a relatively low ratio of hole and electron mobility (μh/μe = 6.32) compared to that of PTB7-Th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IM-IDT3-based device (μh/μe = 10.65). This result may explain the relatively high Jsc of the PTB7-Th
IM-IDT3-based device (μh/μe = 10.65). This result may explain the relatively high Jsc of the PTB7-Th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IM-BDTIDT2 BHJ device.
IM-BDTIDT2 BHJ device.
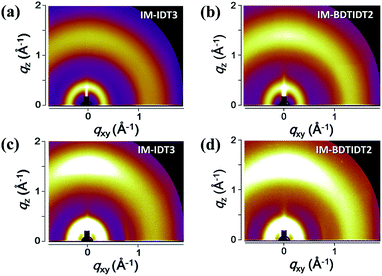
The microstructural features of the pure molecule films and their optimized blend films were investigated by two-dimensional GIWAXS, as shown in Fig. 4, S7 and S8.† IM-IDT3 and IM-BDTIDT2 showed poor crystallinity, both in pristine and PTB7-Th blend films. This behavior might be attributable to the longer length of these molecules and high rotational freedom in the structure of IDTs or IDT–BDT–IDT. From this result, we could not find significantly different crystalline behavior in pure and blend films.
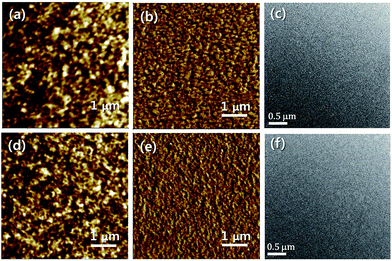
To find other evidences of the PSC performance, the surface and internal morphologies of the optimized blend films were investigated by AFM and TEM, respectively. The IM-IDT3 and IM-BDTIDT2 films display very similar surface morphologies as shown in Fig. 5. Additionally, no clear phase segregation behaviors are observed in the blend films of PTB7-Th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IM-IDT3 and PTB7-Th
IM-IDT3 and PTB7-Th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IM-BDTIDT2 as shown in TEM images (Fig. 5c and f). The formation of fine domains and the occurrence of nanophase separation are crucial factors for improving Jsc values by achieving efficient exciton diffusion and increasing the interfacial area for efficient exciton dissociation between the p- and n-type molecules. However, although PTB7-Th
IM-BDTIDT2 as shown in TEM images (Fig. 5c and f). The formation of fine domains and the occurrence of nanophase separation are crucial factors for improving Jsc values by achieving efficient exciton diffusion and increasing the interfacial area for efficient exciton dissociation between the p- and n-type molecules. However, although PTB7-Th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IM-IDT3 showed favorable morphology with a fine structure, the corresponding device exhibited relatively poor photovoltaic performance. It is probably that the different PSC performance observed for IM-IDT3- and IM-BDTIDT2-based devices could be governed by their molecular energy levels.38
IM-IDT3 showed favorable morphology with a fine structure, the corresponding device exhibited relatively poor photovoltaic performance. It is probably that the different PSC performance observed for IM-IDT3- and IM-BDTIDT2-based devices could be governed by their molecular energy levels.38
Conclusions
To investigate the correlation between HOMO/LUMO energy levels and donating cores, we synthesized two different A–D–A-type large molecules containing the same or different donating cores. IM-IDT3 and IM-BDTIDT2 showed NIR absorption spectra and higher-lying HOMO and LUMO levels compared to those of PCBM. However, the energy levels of IM-IDT3 were very close to those of the p-type polymer PTB7-Th. In contrast, IM-BDTIDT2 with an IDT–BDT–IDT core exhibited a significantly blue-shifted absorption spectrum and lower-lying energy levels, which overcomes the disadvantages of IM-IDT3 bearing an IDT–IDT–IDT core. PTB7-Th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IM-BDTIDT2 exhibited significantly improved device performance compared with the IM-IDT3-based device owing to the fine surface and internal morphology of blend films and favorable energy levels.
IM-BDTIDT2 exhibited significantly improved device performance compared with the IM-IDT3-based device owing to the fine surface and internal morphology of blend films and favorable energy levels.
Experimental
Materials
All starting reagents were purchased from Tokyo Chemical Industry, Sigma-Aldrich, and Acros Organics. The reagent-grade solvents used in this study were freshly dried using standard methods. Compounds 1, 2, and IM-IDT3 were synthesized according to literature methods.37,39Synthesis of compound 3
Pd2(dba)3 (11.4 mg, 10.0 μmol) and P(o-tolyl)3 (11.4 mg, 10.0 μmol) were added to a solution of compound 1 (0.09 g, 0.1 mmol) and compound 2 (0.203 g, 0.2 mmol) in toluene (20 mL) at room temperature under an argon atmosphere. The mixture was heated to 100 °C and stirred at this temperature for 12 h. After cooling the mixture to room temperature, the mixture was poured into water (50 mL) and then extracted with dichloromethane. The organic layer was dried over MgSO4 and the solvent was evaporated under reduced pressure. The resulting product was purified by silica gel column chromatography (methylene chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane = 1
hexane = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) to yield 0.212 g (87%) of compound 3. 1H NMR (500 MHz, CDCl3): δ (ppm) 9.81 (s, 2H), 7.64 (s, 2H), 7.62 (s, 2H), 7.54 (s, 2H), 7.41 (s, 2H), 7.27 (d, J = 4.5 Hz, 2H), 7.07–7.18 (m, 34H), 6.91 (d, J = 4.5 Hz, 2H), 2.88 (d, 4H), 2.57 (t, 16H), 1.71 (m, 2H), 1.59 (m, 16H), 1.29–1.36 (m, 64H), 0.86–0.92 (t, 36H). 13C NMR (125 MHz, CDCl3): δ (ppm) 182.76, 157.74, 156.11, 154.95, 153.85, 151.35, 146.07, 145.45, 141.96, 141.83, 141.06, 140.91, 140.86, 138.61, 138.36, 137.32, 137.27, 136.67, 133.73, 132.07, 128.53, 128.48, 127.81, 127.65, 125.47, 123.26, 121.19, 118.92, 117.49, 63.11, 62.84, 41.40, 35.55, 35.53, 34.30, 32.49, 31.69, 31.30, 29.11, 29.08, 28.93, 25.77, 23.04, 22.57, 14.21, 14.07, 10.91. MALDI-TOF: m/z 2442.49 [M+]. Anal. calcd for C164H186N4O2S8: C, 80.54; H, 7.67; S, 10.49. Found: C, 80.37; H, 7.61; S, 10.45%.
4) to yield 0.212 g (87%) of compound 3. 1H NMR (500 MHz, CDCl3): δ (ppm) 9.81 (s, 2H), 7.64 (s, 2H), 7.62 (s, 2H), 7.54 (s, 2H), 7.41 (s, 2H), 7.27 (d, J = 4.5 Hz, 2H), 7.07–7.18 (m, 34H), 6.91 (d, J = 4.5 Hz, 2H), 2.88 (d, 4H), 2.57 (t, 16H), 1.71 (m, 2H), 1.59 (m, 16H), 1.29–1.36 (m, 64H), 0.86–0.92 (t, 36H). 13C NMR (125 MHz, CDCl3): δ (ppm) 182.76, 157.74, 156.11, 154.95, 153.85, 151.35, 146.07, 145.45, 141.96, 141.83, 141.06, 140.91, 140.86, 138.61, 138.36, 137.32, 137.27, 136.67, 133.73, 132.07, 128.53, 128.48, 127.81, 127.65, 125.47, 123.26, 121.19, 118.92, 117.49, 63.11, 62.84, 41.40, 35.55, 35.53, 34.30, 32.49, 31.69, 31.30, 29.11, 29.08, 28.93, 25.77, 23.04, 22.57, 14.21, 14.07, 10.91. MALDI-TOF: m/z 2442.49 [M+]. Anal. calcd for C164H186N4O2S8: C, 80.54; H, 7.67; S, 10.49. Found: C, 80.37; H, 7.61; S, 10.45%.
Synthesis of compound 4 (IM-BDTIDT2)
IM-BDTIDT2 was synthesized via Knoevenagel condensation of compound 3 (0.20 g, 0.08 mmol) and 2-(3-oxo-2,3-dihydro-1H-inden-1-ylidene)malononitrile (0.06 g, 0.32 mmol) with ammonium acetate as a catalyst. The mixture was heated at 70 °C for 5 h. The resulting product was purified by silica gel column chromatography (methylene chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane = 1
hexane = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) to yield 0.181 g (81%) of IM-BDTIDT2 as a dark green solid. 1H NMR (500 MHz, CDCl3): δ (ppm) 8.88 (s, 2H), 8.68 (d, J = 7.5 Hz, 2H), 7.88 (d, J = 7.5 Hz, 2H), 7.72 (m, 4H), 7.69 (s, 2H), 7.67 (s, 2H), 7.65 (s, 2H), 7.42 (s, 2H), 7.29 (d, J = 4.5 Hz, 2H), 7.23 (s, 2H), 7.09–7.17 (m, 32H), 6.92 (d, J = 4.5 Hz, 2H), 2.88 (d, 4H), 2.58 (t, 16H), 1.71 (m, 2H), 1.60 (m, 16H), 1.29–1.36 (m, 64H), 0.87–0.92 (t, 36H). 13C NMR (125 MHz, CDCl3): δ (ppm) 188.39, 160.79, 160.48, 158.45, 157.09, 156.27, 154.22, 146.14, 142.10, 141.95, 141.90, 140.92, 140.63, 140.40, 139.91, 139.60, 138.89, 138.70, 138.52, 138.32, 137.30, 136.84, 136.58, 134.90, 134.24, 133.73, 128.62, 128.56, 127.81, 127.74, 127.70, 125.49, 125.18, 124.60, 123.57, 123.35, 121.57, 119.77, 119.10, 117.53, 114.77, 68.41, 63.08, 62.84, 41.40, 35.55, 35.53, 34.30, 32.50, 31.68, 31.28, 29.07, 28.93, 25.77, 23.04, 22.55, 14.20, 14.07, 10.91. MALDI-TOF: m/z 2794.09 [M+]. Anal. calcd for C188H194N4O2S8: C, 80.70; H, 6.99; N, 2.00; S, 9.17. Found: C, 80.68; H, 6.92; N, 2.04; S, 9.13.
4) to yield 0.181 g (81%) of IM-BDTIDT2 as a dark green solid. 1H NMR (500 MHz, CDCl3): δ (ppm) 8.88 (s, 2H), 8.68 (d, J = 7.5 Hz, 2H), 7.88 (d, J = 7.5 Hz, 2H), 7.72 (m, 4H), 7.69 (s, 2H), 7.67 (s, 2H), 7.65 (s, 2H), 7.42 (s, 2H), 7.29 (d, J = 4.5 Hz, 2H), 7.23 (s, 2H), 7.09–7.17 (m, 32H), 6.92 (d, J = 4.5 Hz, 2H), 2.88 (d, 4H), 2.58 (t, 16H), 1.71 (m, 2H), 1.60 (m, 16H), 1.29–1.36 (m, 64H), 0.87–0.92 (t, 36H). 13C NMR (125 MHz, CDCl3): δ (ppm) 188.39, 160.79, 160.48, 158.45, 157.09, 156.27, 154.22, 146.14, 142.10, 141.95, 141.90, 140.92, 140.63, 140.40, 139.91, 139.60, 138.89, 138.70, 138.52, 138.32, 137.30, 136.84, 136.58, 134.90, 134.24, 133.73, 128.62, 128.56, 127.81, 127.74, 127.70, 125.49, 125.18, 124.60, 123.57, 123.35, 121.57, 119.77, 119.10, 117.53, 114.77, 68.41, 63.08, 62.84, 41.40, 35.55, 35.53, 34.30, 32.50, 31.68, 31.28, 29.07, 28.93, 25.77, 23.04, 22.55, 14.20, 14.07, 10.91. MALDI-TOF: m/z 2794.09 [M+]. Anal. calcd for C188H194N4O2S8: C, 80.70; H, 6.99; N, 2.00; S, 9.17. Found: C, 80.68; H, 6.92; N, 2.04; S, 9.13.
Instrumentation
1H NMR spectra were acquired using a Varian Mercury a Bruker 500 MHz spectrometer. Elemental analyses were performed using an EA1112 elemental analyzer to establish C, H, N, and S contents. The masses of the synthesized compounds were determined by matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrometry (LRF20, Bruker Daltonics). Absorption spectra were recorded on a UV-vis absorption spectrophotometer (HP 8453, photodiode array). Cyclic voltammetry (CV) experiments were performed using an eDAQ EA161 potentiostat. The reference and counter electrodes were Ag/AgCl and Pt wire (0.5 mm in diameter), respectively. The working electrodes were obtained by coating the prepared polymer films onto Pt plates. The electrolyte solution consisted of tetrabutylammonium hexafluorophosphate (Bu4NPF6) (0.1 M) in acetonitrile. The morphology of each polymer film surface was analyzed by atomic force microscopy (AFM; Advanced Scanning Probe Microscope, XE-100, PSIA). The internal morphologies of each polymer film and the film blends were investigated using transmission electron microscopy (TEM; Tecnai G2 F30, FEI Inc.) at an accelerating voltage of 300 kV. GIWAXS measurements were carried out at the PLS-II 9A ultra-small-angle X-ray scattering beamline at the Pohang Accelerator Laboratory (energy = 11.04 keV, wavelength (λ) = 1.126 Å, 2θ = 0–20°). The parameters qxy and qz represent the components of the scattering vectors parallel and perpendicular to the film surface, respectively.Fabrication of polymer solar cells
BHJ PSCs were fabricated with an inverted device configuration (indium tin oxide (ITO)/ZnO/PTB7-Th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A–D–A/MoO3/Ag). A thin layer of ZnO was fabricated on the surface of ITO-patterned glass, which was treated with UV-ozone for 20 min. After annealing the ZnO layer thermally at 200 °C for 1 h, the active layer was prepared on top of the ZnO layer by spin-coating the blended solution of PTB7-Th
A–D–A/MoO3/Ag). A thin layer of ZnO was fabricated on the surface of ITO-patterned glass, which was treated with UV-ozone for 20 min. After annealing the ZnO layer thermally at 200 °C for 1 h, the active layer was prepared on top of the ZnO layer by spin-coating the blended solution of PTB7-Th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A–D–A dissolved in chlorobenzene with or without diphenyl ether (DPE) as a solvent additive. Subsequently, the electrodes were subjected to thermal evaporation to form a 10 nm MoO3 layer and a 100 nm Ag layer (0.04 cm2 photoactive area). A Keithley 2400 source meter was used to investigate the current density–voltage (J–V) characteristics in the dark and under AM 1.5 G illumination at 100 mW cm−2, as supplied by a solar simulator (Oriel, 1000 W). An AM 1.5 filter (Oriel) and a neutral density filter were used to adjust the light intensity. The incident light intensity was measured with a calibrated broadband optical power meter (Spectra Physics, Model 404).
A–D–A dissolved in chlorobenzene with or without diphenyl ether (DPE) as a solvent additive. Subsequently, the electrodes were subjected to thermal evaporation to form a 10 nm MoO3 layer and a 100 nm Ag layer (0.04 cm2 photoactive area). A Keithley 2400 source meter was used to investigate the current density–voltage (J–V) characteristics in the dark and under AM 1.5 G illumination at 100 mW cm−2, as supplied by a solar simulator (Oriel, 1000 W). An AM 1.5 filter (Oriel) and a neutral density filter were used to adjust the light intensity. The incident light intensity was measured with a calibrated broadband optical power meter (Spectra Physics, Model 404).
Acknowledgements
The authors acknowledge the financial support from the National Research Foundation of Korea (NRF2012R1A2A1A01008797) and from the Key Research Institute Program (NRF20100020209). We are grateful to Pohang Accelerator Laboratory (Pohang, Korea) for allowing us to conduct the grazing incidence X-ray diffraction (GI-XRD) measurements.Notes and references
- W. Chen and Q. Zhang, J. Mater. Chem. C, 2017, 5, 1275 RSC.
- S. M. McAfee, J. M. Topple, I. G. Hill and G. C. Welch, J. Mater. Chem. A, 2015, 3, 16393 CAS.
- H. Li, T. Earmme, G. Ren, A. Saeki, S. Yoshikawa, N. M. Murari, S. Subramaniyan, M. J. Crane, S. Seki and S. A. Jenekhe, J. Am. Chem. Soc., 2014, 136, 14589 CrossRef CAS PubMed.
- O. K. Kwon, M. A. Uddin, J. H. Park, S. K. Park, T. L. Nguyen, H. Y. Woo and S. Y. Park, Adv. Mater., 2016, 28, 910 CrossRef CAS PubMed.
- K. Wang, Y. Firdaus, M. Babics, F. Cruciani, Q. Saleem, A. E. Labban, M. A. Alamoudi, T. Marszalek, W. Pisula, F. Laquai and P. M. Beaujuge, Chem. Mater., 2016, 28, 2200 CrossRef CAS.
- G. E. Park, S. Choi, D. H. Lee, M. Godumala, M. A. Uddin, H. Y. Woo, M. J. Cho and D. H. Choi, J. Mater. Chem. A, 2017, 5, 663 CAS.
- J.-D. Chen, C. Cui, Y.-Q. Li, L. Zhou, Q.-D. Ou, C. Li, Y. Li and J.-X. Tang, Adv. Mater., 2015, 27, 1035 CrossRef CAS PubMed.
- B. Kan, M. Li, Q. Zhang, F. Liu, X. Wan, Y. Wang, W. Ni, G. Long, X. Yang, H. Feng, Y. Zuo, M. Zhang, F. Huang, Y. Cao, T. P. Russell and Y. Chen, J. Am. Chem. Soc., 2015, 137, 3886 CrossRef CAS PubMed.
- W. Zhao, D. Qian, S. Zhang, S. Li, O. Inganäs, F. Gao and J. Hou, Adv. Mater., 2016, 28, 4734 CrossRef CAS PubMed.
- Y.-J. Hwang, H. Li, B. A. E. Courtright, S. Subramaniyan and S. A. Jenekhe, Adv. Mater., 2016, 28, 124 CrossRef CAS PubMed.
- G. E. Park, S. Choi, J. Shin, M. J. Cho and D. H. Choi, Org. Electron., 2016, 34, 157 CrossRef CAS.
- D. Meng, D. Sun, C. Zhong, T. Liu, B. Fan, L. Huo, Y. Li, W. Jiang, H. Choi, T. Kim, J. Y. Kim, Y. Sun, Z. Wang and A. J. Heeger, J. Am. Chem. Soc., 2016, 138, 375 CrossRef CAS PubMed.
- Y. J. He and Y. F. Li, Phys. Chem. Chem. Phys., 2011, 13, 1970 RSC.
- X. Zhang, C. L. Zhan and J. N. Yao, Chem. Mater., 2015, 27, 166 CrossRef CAS.
- S. Holliday, R. S. Ashraf, A. Wadsworth, D. Baran, S. A. Yousaf, C. B. Nielsen, C. H. Tan, S. D. Dimitrov, Z. R. Shang, N. Gasparini, M. Alamoudi, F. Laquai, C. J. Brabec, A. Salleo, J. R. Durrant and I. McCulloch, Nat. Commun., 2016, 7, 11585 CrossRef CAS PubMed.
- H. T. Bai, Y. Wu, Y. F. Wang, Y. Wu, R. Li, P. Y. Cheng, M. Y. Zhang, J. Y. Wang, W. Ma and X. W. Zhan, J. Mater. Chem. A, 2015, 3, 20758 CAS.
- M. M. Li, Y. T. Liu, W. Ni, F. Liu, H. R. Feng, Y. M. Zhang, T. T. Liu, H. T. Zhang, X. J. Wan, B. Kan, Q. Zhang, T. P. Russell and Y. S. Chen, J. Mater. Chem. A, 2016, 4, 10409 CAS.
- K. E. Yi, G. E. Park, J. H. Lee, H. J. Kim, D. H. Lee, H. Ahn, M. A. Uddin, H. Y. Woo, M. J. Cho and D. H. Choi, ACS Appl. Mater. Interfaces, 2017, 9, 8838 Search PubMed.
- Y. Lin and X. Zhan, Mater. Horiz., 2014, 1, 470 RSC.
- Y. Lin and X. Zhan, Acc. Chem. Res., 2016, 49, 175 CrossRef CAS PubMed.
- P. Cheng, M. Zhang, T.-K. Lau, Y. Wu, B. Jia, J. Wang, C. Yan, M. Qin, X. Lu and X. Zhan, Adv. Mater., 2017, 29, 1605216 CrossRef PubMed.
- W. Wang, C. Yan, T.-K. Lau, J. Wang, K. Liu, Y. Fan, X. Lu and X. Zhan, Adv. Mater., 2017 DOI:10.1002/adma.201701308.
- Y. Qin, M. A. Uddin, Y. Chen, B. Jang, K. Zhao, Z. Zheng, R. Yu, T. J. Shin, H. Y. Woo and J. Hou, Adv. Mater., 2016, 28, 9416 CrossRef CAS PubMed.
- H. Bin, L. Gao, Y. Yang, Y. Zhang, C. Zhang, S. Chen, L. Xue, C. Yang, M. Xiao and Y. Li, Nat. Commun., 2016, 7, 13651 CrossRef CAS PubMed.
- G. E. Park, H. J. Kim, S. Choi, D. H. Lee, M. A. Uddin, H. Y. Woo, M. J. Cho and D. H. Choi, Chem. Commun., 2016, 52, 8873 RSC.
- J. Yuan, L. Qiu, Z.-G. Zhang, Y. Li, Y. Chen and Y. Zou, Nano Energy, 2016, 30, 312 CrossRef CAS.
- D. Liu, B. Yang, B. Jang, B. Xu, S. Zhang, C. He, H. Y. Woo and J. Hou, Energy Environ. Sci., 2017, 10, 546 CAS.
- Y. Z. Lin, Z. G. Zhang, H. Bai, J. Wang, Y. Yao, Y. Li, D. Zhu and X. Zhan, Energy Environ. Sci., 2015, 8, 610 CAS.
- Y. Lin, J. Wang, Z. Zhang, H. Bai, Y. Li, D. Zhu and X. Zhan, Adv. Mater., 2015, 27, 1170 CrossRef CAS PubMed.
- L. Gao, Z. G. Zhang, H. Bin, L. Xue, Y. Yang, C. Wang, F. Liu, T. P. Russell and Y. Li, Adv. Mater., 2016, 28, 8288 CrossRef CAS PubMed.
- H. Bin, Z.-G. Zhang, L. Gao, S. Chen, L. Zhong, L. Xue, C. Yang and Y. Li, J. Am. Chem. Soc., 2016, 138, 4657 CrossRef CAS PubMed.
- Y. Lin, F. Zhao, Q. He, L. Huo, Y. Wu, T. C. Parker, W. Ma, Y. Sun, C. Wang, D. Zhu, A. J. Heeger, S. R. Marder and X. Zhan, J. Am. Chem. Soc., 2016, 138, 4955 CrossRef CAS PubMed.
- Y. Lin, Q. He, F. Zhao, L. Huo, J. Mai, X. Lu, C. J. Su, T. Li, J. Wang, J. Zhu, Y. Sun, C. Wang and X. Zhan, J. Am. Chem. Soc., 2016, 138, 2973 CrossRef CAS PubMed.
- S. Dai, F. Zhao, Q. Zhang, T. Lau, T. Li, K. Liu, Q. Ling, C. Wang, X. Lu, W. You and X. Zhan, J. Am. Chem. Soc., 2017, 139, 1336 CrossRef CAS PubMed.
- F. Zhao, S. Dai, Y. Wu, Q. Zhang, J. Wang, L. Jiang, Q. Ling, Z. Wei, W. Ma, W. You, C. Wang and X. Zhan, Adv. Mater., 2017, 29, 1700144 CrossRef PubMed.
- Y. Lin, F. Zhao, Y. Wu, K. Chen, Y. Xia, G. Li, S. K. K. Prasad, J. Zhu, L. Huo, H. Bin, Z.-G. Zhang, X. Guo, M. Zhang, Y. Sun, F. Gao, Z. Wei, W. Ma, C. Wang, J. Hodgkiss, Z. Bo, O. Inganas, Y. Li and X. Zhan, Adv. Mater., 2017, 29, 1604155 CrossRef PubMed.
- Y. Lin, T. Li, F. Zhao, L. Han, Z. Wang, Y. Wu, Q. He, J. Wang, L. Huo, Y. Sun, C. Wang, W. Ma and X. Zhan, Adv. Energy Mater., 2016, 6, 1600854 CrossRef.
- D. Veldman, S. C. J. Meskers and R. A. J. Janssen, Adv. Funct. Mater., 2009, 19, 1939 CrossRef CAS.
- J. Y. Kim, Y. U. Kim, H. J. Kim, H. A. Um, J. Shin, M. J. Cho and D. H. Choi, Macromol. Res., 2016, 24, 980 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: MALDI-TOF, device performance, GI-XRD. See DOI: 10.1039/c7ra06879d |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2017 |