Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Alq3 (tris(8-hydroxyquinolinato)aluminium) as a selective n-type contact for FAMAPIBr perovskite solar cells with efficient energy transfer to increase the solar cell photocurrent†
Maria Méndez a and
Emilio Palomares
a and
Emilio Palomares *ab
*ab
aThe Institute of Chemical Research of Catalonia (ICIQ), The Barcelona Institute of Science and Technology (BIST), Avda. Països Catalans, 16, Tarragona, E-43007, Spain. E-mail: epalomares@iciq.es
bICREA, Passeig Lluís Companys, 23, Barcelona, E-08010, Spain
First published on 17th July 2017
Abstract
We investigated the use of tris(8-hydroxyquinolinato)aluminium, known as Alq3, as a selective contact for electrons in functional FAMAPIBr (formamidinium/methylammonium lead iodide bromide) solar cells. The solar cells with Alq3 interfacial layers show excellent photocurrent upon standard 1 sun illumination (100 mW cm−2@1.5 AM G sun simulated light) due to efficient energy transfer from the selective contact layer to the perovskite.
Solar cells based on solution processed perovskite materials are currently receiving much attention due to their rapid preparation, and light-to-energy conversion efficiencies as high as 22.1%
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,2 under standard measurement conditions have been reported.
1,2 under standard measurement conditions have been reported.
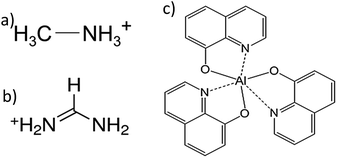
Much of the progress has been possible through composition tuning of the perovskite.3,4 One of the highest and most stable perovskite solar cells uses a combination of formamidinium and methylammonium cations (Fig. 1). Yet, the exploration of suitable selective contacts, either n-type or p-type, is less investigated and often, despite changing the perovskite, the contacts remain the same.5
In this communication, we have prepared formamidinium/methylammonium lead iodide bromide (FAMAPIBr)3,4,6 as photoactive semiconductor. Moreover, we have used a well-known n-type material as interfacial layer and electron selective contact, the tris(8-hydroxyquinolinato)aluminium7,8 (Alq3 in Fig. 1). The deposition of Alq3, using thermal evaporation under high vacuum, allowed us to careful control the interfacial layer thickness between the compact TiO2 layer (c-TiO2) and the FAMAPIBr perovskite. The solar cells structure used was FTO/c-TiO2/Alq3/FAMAPIBr/spiro-OMeTAD/Ag, where FTO is fluorine doped tin oxide, the spiro-OMeTAD is the hole transport material (HTM) and Ag is silver metal.
In Table 1 we have listed the parameters for the different solar cells having different Alq3 thickness (see S1† for statistical data).
| Device – ETL | Scan | Jsc (mA cm−2) | Voc (V) | FF | PCE (%) |
|---|---|---|---|---|---|
| TiO2-c/Alq3-10 nm | Fw | 23.0 | 0.775 | 0.43 | 7.71 |
| Rev | 22.85 | 0.909 | 0.53 | 11.02 | |
| TiO2-c/Alq3-25 nm | Fw | 22.45 | 0.853 | 0.44 | 8.51 |
| Rev | 22.36 | 0.950 | 0.53 | 11.45 | |
| TiO2-c/Alq3-50 nm | Fw | 24.93 | 0.793 | 0.41 | 8.13 |
| Rev | 24.24 | 0.954 | 0.53 | 12.48 | |
| Ref cell | Fw | 24.87 | 1.020 | 0.42 | 10.76 |
| Rev | 24.30 | 1.065 | 0.53 | 13.83 |
As can be seen, the best efficiencies were achieved with Alq3 thicknesses of 50 nm. Interestingly, increasing the Alq3 thickness leads to better device photocurrents. Yet, the overall efficiency is similar to the standard cell due to the lower fill factor. The hysteresis effect on the measured JV curves (forward/reverse sweeps) is also comparable. Fig. 2 illustrates the best JV (current density vs. voltage) curves for the devices containing the Alq3 interfacial layer and the reference solar cell (forward/reverse sweeps).
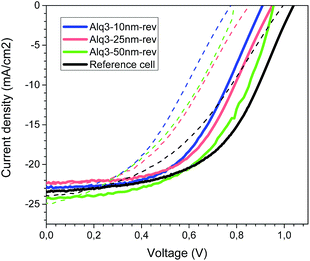 | ||
| Fig. 2 The JV curves measured at 1 sun (1.5 AM G sun simulated light) under forward (dashed line) and reverse (solid line) bias. | ||
We would like to point out that in both type of solar cells we observed the hysteresis phenomena, which has been recently associated to the accumulation of ionic species and the cell voltage.9 We carried out PIT-PV (Photo Induced Transient Photo-Voltage) measurements that consist on measuring photo-voltage transient upon a short perturbation of the equilibrated Voc.10 As can be seen (Fig. 3), the PIT-PV decay results are almost identical for the different solar cells, which implies that, in principle, there are not differences in the accumulation of charges at the interfaces and, moreover, in the carrier recombination kinetics.
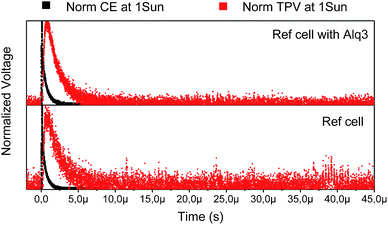 | ||
| Fig. 3 Comparison between PICE (black square) and PIT-PV (red square) decays at 1 sun for perovskite solar cells with c-TiO2 (bottom) and c-TiO2/Alq3 (top) as selective contacts. | ||
To understand if the presence of the interfacial Alq3 layer effects a change over the accumulation of ionic charges at the interface between the perovskite and the Alq3 surface we measured PICE (Photo-Induced Charge Extraction) and PIDC (Photo-Induced Differential Charging). Although both experiments allow the estimation of the accumulated charge at the device under illumination,11,12 the main difference is that PIDC allows measuring the accumulated charge when the PICE decay is much slower than the carrier recombination lifetime and, thus, carriers can recombine before extracted. We have previously shown that, for example, in MAPI (methylammonium lead iodide) perovskite solar cells both measurements lead to considerable differences for charges measured.13
As shown in Fig. 4 the measured charge is alike on both types of devices.
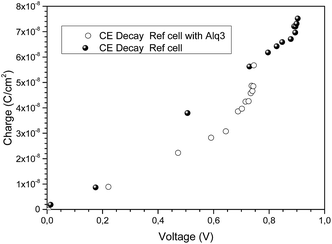 | ||
| Fig. 4 Measured charge accumulated at the c-TiO2 and c-TiO2/Alq3/FAMAPIBr perovskite solar cells under different light bias. | ||
Indeed, it seems evident that the presence of the Alq3 layer does not affects the extraction of charges and, in fact, in all cases the charge extraction was much faster than the PIT-PV (Fig. 3).
Once we have evaluated the transient photo-voltage and the charge extraction processes in the solar cells containing the interfacial layer of Alq3 and the reference cell we turned onto the differences observed in photocurrent. As listed in Table 1, the increase in Alq3 thickness leads to an increase in photocurrent.
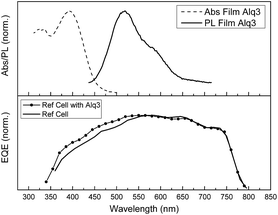
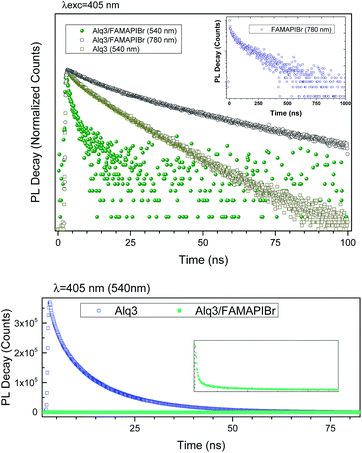
We measured the absorption and fluorescence emission of an Alq3 film (Fig. 5). As shown in Fig. 5 (top), the emission of Alq3 overlaps with the FAMAPIBr absorption (Fig. S2†), which in principle will favour efficient energy transfer from the Alq3 to the perovskite. The normalized EQE measurements showed an increase in the incident photon-to-electron conversion efficiency in the region between 400 to 600 nm corresponding to the absorption and emission regions of Alq3 (Fig. 5 bottom). Hence, we postulate that the increase in device photocurrent is due to efficient energy transfer between the Alq3 thin film and the FAMAPIBr perovskite. To further ascertain the energy transfer process we carried out photoluminescence emission lifetime measurements. As can be seen, the emission lifetime and the signal amplitude changes for the Alq3 film when used as selective contact (Fig. 6).
The Alq3 thin film has an emission maximum at 540 nm when excited at 405 nm. The measured Alq3 thin film fluorescence lifetime is of τ = 10.7 ns. In contrast, the emission of the perovskite at the same excitation wavelength (405 nm) but monitoring at 780 nm is of τ = 0.1 μs (S3† shows the perovskite PL emission). When both layers are in contact, the emission lifetime for the former is reduced to τ = 1.7 ns and also the emission of the later is reduced to τ = 19.9 ns. In the case of the Alq3 contact is due to the efficient energy transfer to the perovskite while in the case of the perovskite is due to the efficient charge injection into the Alq3. Furthermore, measuring the fluorescence lifetime at the same acquisition time for the Alq3/perovskite thin film and comparing to a standard Alq3 thin film it is clear that the emission is quenched almost at 100% due to the energy transfer to the perovskite (Fig. 6 bottom).
In conclusion, we have studied Alq3 as selective contact for electrons in FAMAPIBr perovskite solar cells. The decrease in photovoltage for the FTO/c-TiO2/Alq3/FAMAPIBr/spiro OMeTAD/Ag is not significant. Studies in depth of the factors that affect the Voc namely, charge accumulation and carrier recombination lifetime also show that there are not remarkable differences. The measurement of the luminescence emission in Alq3 films and the EQE spectra of the different solar cells lead us to conclude that the increase in photocurrent is due to an efficient energy transfer process between the Alq3 interfacial layer and the perovskite film. Further efforts to increase the fill factor in these solar cells having the Alq3 interfacial layer are being carried out to achieve higher efficiencies through efficient energy transfer from the selective contact.
Acknowledgements
The authors would like to acknowledge ICIQ-BIST (Severo Ochoa Excellence Accreditation 2014-2018 SEV-2013-0319) and ICREA for economical support. E. P. is also grateful to Spanish MINECO for the CTQ2016-80042-R project, Generalitat de Catalunya for the AGAUR funding and finally to the COFUND European Commission for the COFUND programme (ICIQ-IPMP).References
- M. A. Green, K. Emery, Y. Hishikawa, W. Warta, E. D. Dunlop, D. H. Levi and A. W. Y. Ho-Baillie, Progress in Photovoltaics: Research and Applications, 2017, 25, 3–13 CrossRef.
- https://www.nrel.gov.
- N. J. Jeon, J. H. Noh, W. S. Yang, Y. C. Kim, S. Ryu, J. Seo and S. I. Seok, Nature, 2015, 517, 476–480 CrossRef CAS PubMed.
- D. Bi, W. Tress, M. I. Dar, P. Gao, J. Luo, C. Renevier, K. Schenk, A. Abate, F. Giordano, J.-P. Correa Baena, J.-D. Decoppet, S. M. Zakeeruddin, M. K. Nazeeruddin, M. Grätzel and A. Hagfeldt, Sci. Adv., 2016, 2, 1–7 Search PubMed.
- S. Ameen, M. A. Rub, S. A. Kosa, K. A. Alamry, M. S. Akhtar, H.-S. Shin, H.-K. Seo, A. M. Asiri and M. K. Nazeeruddin, ChemSusChem, 2016, 9, 10–27 CrossRef CAS PubMed.
- N. Pellet, P. Gao, G. Gregori, T.-Y. Yang, M. K. Nazeeruddin, J. Maier and M. Grätzel, Angew. Chem., Int. Ed., 2014, 53, 3151–3157 CrossRef CAS PubMed.
- X. Ma, G. K. Lim, K. D. M. Harris, D. C. Apperley, P. N. Horton, M. B. Hursthouse and S. L. James, Cryst. Growth Des., 2012, 12, 5869–5872 CAS.
- L. Yao, L. Li, L. Qin, Y. Ma, W. Wang, H. Meng, W. Jin, Y. Wang, W. Xu, G. Ran, L. You and G. Qin, Nanotechnology, 2017, 28, 105201–105211 CrossRef PubMed.
- S. Ravishankar, O. Almora, C. Echeverría-Arrondo, E. Ghahremanirad, C. Aranda, A. Guerrero, F. Fabregat-Santiago, A. Zaban, G. Garcia-Belmonte and J. Bisquert, J. Phys. Chem. Lett., 2017, 8, 915–921 CrossRef CAS PubMed.
- J. M. Marin-Beloqui, L. Lanzetta and E. Palomares, Chem. Mater., 2016, 28, 207–213 CrossRef CAS.
- B. C. O'Regan, P. R. F. Burnes, X. Li, C. Law, E. Palomares and J. M. Marin-Beloqui, J. Am. Chem. Soc., 2015, 137, 5087–5099 CrossRef PubMed.
- L. Cabau, I. Garcia-Benito, A. Molina-Ontoria, N. F. Montcada, N. Martin, A. Vidal-Ferran and E. Palomares, Chem. Commun., 2015, 51, 13980–13982 RSC.
- N. F. Montcada, J. M. Marín-Beloqui, W. Cambarau, J. Jiménez-López, L. Cabau, K. T. Cho, M. K. Nazeeruddin and E. Palomares, ACS Energy Lett., 2017, 2, 182–187 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section and perovskite UV-Visible and TCSPC. See DOI: 10.1039/c7ra06645g |
| This journal is © The Royal Society of Chemistry 2017 |