Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The in vivo anti-tumor effect of curcumin derivative (2E,6E)-2,6-bis(4-hydroxy-3-methoxybenzylidene)cyclohexanone (BHMC) on 4T1 breast cancer cells
Nursyamirah Abd Razaka,
M. Nadeem Akhtarb,
Nadiah Abuc,
Wan Yong Hod,
Sheau Wei Tana,
Seema Zareenb,
Saiful Nizam bin Taj-ud-dinb,
Kamariah Long e,
Noorjahan Banu Alitheen*f and
Swee Keong Yeap
e,
Noorjahan Banu Alitheen*f and
Swee Keong Yeap *ag
*ag
aInstitute of Bioscience, Universiti Putra Malaysia, Serdang, Selangor, Malaysia. E-mail: skyeap2005@gmail.com; Tel: +60-3-88006901
bBio-aromatic Research Center of Excellence, Faculty of Industrial Sciences & Technology, Universiti Malaysia Pahang, Lebuhraya Tun Razak, Kuantan 26300, Pahang, Malaysia
cUKM Molecular Biology Institute (UMBI), UKM Medical Centre, Jalan Yaa'cob Latiff, Bandar Tun Razak, Cheras 56000, Kuala Lumpur, Malaysia
dSchool of Biomedical Sciences, The University of Nottingham Malaysia Campus, Jalan Broga, Semenyih 43500, Selangor, Malaysia
eMalaysian Agricultural Research and Development Institute (MARDI), Serdang, Selangor 43400, Malaysia
fDepartment of Cell and Molecular Biology, Faculty of Biotechnology and Biomolecular Sciences, Universiti Putra Malaysia, Serdang, Selangor 43400, Malaysia. E-mail: noorjahan@upm.edu.my
gChina-ASEAN College of Marine Sciences, Xiamen University Malaysia, Jalan Sunsuria, Bandar Sunsuria, Sepang 43900, Selangor, Malaysia
First published on 20th July 2017
Abstract
Curcumin is one of the promising natural products extracted from the rhizomes of curcuma longa and has been extensively investigated by researchers to explore its potential as a chemopreventive and therapeutic agent against several chronic diseases. To further enhance the cytotoxic potential of curcumin, its derivative (2E,6E)-2,6-bis(4-hydroxy-3-methoxybenzylidene)cyclohexanone (BHMC) has been synthesized and investigated, and its antitumor effect on tested on 4T1 challenged mice. BHMC was recorded with in vitro cytotoxicity on murine 4T1 breast cancer cells with IC50 value 13.66 μM, which was 2 times lower than curcumin after 72 hours of treatment. An in vivo study indicated that BHMC possessed antitumor effect on the 4T1 cells of the challenged mice by induction of apoptosis, antiproliferation, anti-inflammation and antimetastasis. This effect is better compared to curcumin treatment at the same evaluated concentration. Thus, BHMC is a potential antitumor agent against breast cancer.
1. Introduction
Cancer is still among the leading causes of death worldwide with breast cancer as the primary cancer incidence for women.1 Triple negative breast cancer (TNBC) is the subtype of breast cancer with absence of estrogen receptor, progesterone receptor and human epidermal growth factor receptor 2 (HER2). TNBC is highly aggressive associated with high event of relapse, metastasis and poor survival. Chemotherapy is the common strategy among the limited treatment options for TNBC. However, TNBC may possess resistance against standard chemotherapeutic drugs such as anthracycline and taxane if they have been previously used as adjuvant or neoadjuvant. Thus, there is a need to discover potential treatment for TNBC.2 Natural compound has been proposed as safe and potential agent against TNBC.3 Among the natural compounds, curcumin that present in the spice turmeric has been reported with antitumor effect on TNBC.4,5 Resolving cancer challenged by natural product hold better potential with the introduction of chemical synthesis, which resolve the availability of the compounds of interest.6 On the other hand, synthetic compounds also help to improve the drug sensitivity and specificity while reduce the toxicity via chemical modification.7Curcumin (Fig. 1A) is one of the classical natural product extracted from the rhizomes of Curcuma longa (turmeric) and named it curcumin.8 Curcumin mainly identified as antioxidant, anti-inflammatory,9–12 anti-cancer9,13–17 and anti-acetylcholinesterase18 activities. Several biological activities including the hepato- and nephro-protective,19–22 thrombosis suppressing,23 myocardial infarction protective,24,25 curcumin has proved to be potential candidate for future drug discovery. In spite of several pharmacological properties, curcumin that was classified under “pans-assay interference compounds” and “invalid metabolic panaceas” still facing several liabilities including instability, poor solubility, poor selectivity and multiple modes of assay interference particularly to the in vitro based assays.26,27 Thus, more efforts to improve efficacy, selectivity of curcumin are needed. In recent years, chemical modification of curcuminoids has been increasingly investigated. For example, various curcumin analogues have been reported with enhance antitumor effect than curcumin.28 A curcumin derivative, 2,6-bis(4-hydroxy-3-methoxybenzylidene)cyclohexanone (BHMC) (Fig. 1B) has been synthesised with diketone moiety of curcumin has been replaced with mono cyclic ketone. To avoid false positive results contributed by the assay interference character of curcumin, in vivo validation are especially on those potential curcumin analog.27 This synthetic curcuminoid analogue has been reported with antinociceptive effect in mice through inhibition of various inflammatory mediators.29–31 Inflammation has been correlated with progression of triple negative breast cancer including promotion of cancer cells invasion and migration.32 The anti-inflammatory effect of BHMC has been evaluated in vitro29 and in vivo.30 BHMC has been reported with cytotoxic effect on estrogen dependent MCF-7 cells.33 In addition, BHMC was reported with greater in vitro cytotoxic effect than curcumin to TNBC MDA-MB-231 cells.34 However, the in vivo antitumor effect of BHMC particularly on TNBC was not evaluated. 4T1 has been reported as excellent model system for TNBC study as its physical location, proliferative, metastatic and inflammatory characteristics mimic human TNBC.32,35 In this study, 2,6-bis(4-hydroxy-3-methoxybenzylidene)cyclohexanone (BHMC) was synthesized and its antitumor effect was compared with curcumin using in vivo 4T1 mouse model.
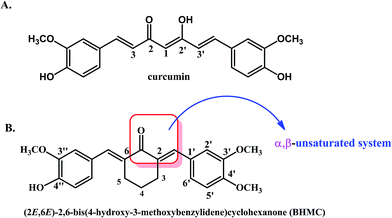 | ||
| Fig. 1 The chemical structures of (A) curcumin and (B) 2,6-bis-(4-hydroxy-3-methoxybenzylidene)cyclohexanone (BHMC). | ||
2. Materials and methods
2.1. Synthesis of BHMC
Curcumin (C7727) was purchased from Sigma-Aldrich (USA). BHMC was chemically synthesized from 4-hydroxy-3-methoxybenzaldehyde (cyclohexanone). Briefly, a mixture of 3-methoxy-4-hydroxy (vanillin) (40 mmol, 2 equiv.) and cyclohexanone (20 mmol, 1 equiv.) was dissolved in 25 mL of absolute ethanol. The mixture was heated at 50 °C for half hour and conc. HCl (2.0 mL) was added drop wise over 5 min to the stirred mixture. The mixture was further stirred for one hours and left over night in refrigerator. The mixture was dissolved in ice water (500 mL) and transferred into separating funnel. A yellow viscous product was floating on the surface of water, which was extracted with ethyl acetate (250 mL × 3 times). The ethyl acetate layer was collected, dried over rotary evaporator and then pass to sodium sulphate anhydrous. The crude product was subjected to further purification by silica gel column chromatography. The column was eluted with ethyl acetate and hexane (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60%). Yield 76%; yellow powder, UV (CHCI3): λmax 302, 339 nm; IR (CHCI3 cm−1): νmax 3393 (OH), 2928 (Ar-C–H) 1618 (C
60%). Yield 76%; yellow powder, UV (CHCI3): λmax 302, 339 nm; IR (CHCI3 cm−1): νmax 3393 (OH), 2928 (Ar-C–H) 1618 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1527 (Ar C
O), 1527 (Ar C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C str.); 1H-NMR (600 MHz, CDCI3): δ in ppm: 7.78 (bs, 2H, –C
C str.); 1H-NMR (600 MHz, CDCI3): δ in ppm: 7.78 (bs, 2H, –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H), 7.12 (dd, 2H, J = 8.34, 1.92 Hz, H-6′, H-6′′), 7.02 (d, 2H, J = 1.92 Hz, H-2′, H-2′′), 6.90 (d, 2H, J = 7.90 Hz, J = 8.34, H-5′, H-5′′), 3.91 (s, 6H, 2 × OCH3, C-3′, C-3′′), 2.90 (m, 4H, H-3, H-5), 1.82 (m, 2H, H-4). 13C-NMR (150 MHz, CDCI3) δ in ppm: 190.41, (C
C–H), 7.12 (dd, 2H, J = 8.34, 1.92 Hz, H-6′, H-6′′), 7.02 (d, 2H, J = 1.92 Hz, H-2′, H-2′′), 6.90 (d, 2H, J = 7.90 Hz, J = 8.34, H-5′, H-5′′), 3.91 (s, 6H, 2 × OCH3, C-3′, C-3′′), 2.90 (m, 4H, H-3, H-5), 1.82 (m, 2H, H-4). 13C-NMR (150 MHz, CDCI3) δ in ppm: 190.41, (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 151.89 (C-4′, C-4′′), 149.71 (C-3′, 3′′), 136.83, (–C
O), 151.89 (C-4′, C-4′′), 149.71 (C-3′, 3′′), 136.83, (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H), 134.80 (C-2, 6), 132.23 (C-1′, 1′′) 128.36, (C-6′, 6′′, 2′, 2′′), 115.34, (C-5′, 5′′), (56.07, OCH3), 28.74, (C-3, C-5), (23.42, C-4). MS: m/z = 366 (100%) [M]+, EI-MS m/z (rel. int.) 351 (56), 335 (68). 321 (17), 309 (12), 161 (24), 131 (15). HR-EIMS C22H22O5. 366.231 calc. for 366.229.
C–H), 134.80 (C-2, 6), 132.23 (C-1′, 1′′) 128.36, (C-6′, 6′′, 2′, 2′′), 115.34, (C-5′, 5′′), (56.07, OCH3), 28.74, (C-3, C-5), (23.42, C-4). MS: m/z = 366 (100%) [M]+, EI-MS m/z (rel. int.) 351 (56), 335 (68). 321 (17), 309 (12), 161 (24), 131 (15). HR-EIMS C22H22O5. 366.231 calc. for 366.229.
The purity of compound was determined by using JASCO-HPLC attached with ChromNAV-software (JASCO Corporation, Tokyo, Japan). The column used XBridge RP-18 (5 μm particle size, 4.6 × 150 mm i.d.; Waters Corporation, Wexford, Ireland) kept at ambient temperature. Injection volume of 20 μL, flow rate at 1 mL min−1. and detector wavelength was adjusted at 250 and 366 nm, mobile phase H2O and MeOH (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60), retention time (tR) 11.66 min. The percentage of purity was determined by calculating the peak purity method automatically.36
60), retention time (tR) 11.66 min. The percentage of purity was determined by calculating the peak purity method automatically.36
2.2. In vitro cell viability assay
Murine 4T1 TNBC cells were purchased from American Type Culture Collection and cultured in RPMI-1640 medium (Sigma-Aldrich, USA) supplemented with 10% Fetal Bovine Serum (FBS) (Gibco, Thermo Fisher Scientific, USA) at 37 °C, 5% CO2. Cell viability was determined by MTT assay where 4T1 cells were seeded (0.8 × 105 cells per mL) in 96 well plate overnight. Then, BHMC and curcumin (Sigma-Aldrich, USA) were added to the cells at concentration ranging within 30–0.47 μg mL−1 by two fold serial dilution and further incubated at 37 °C, 5% CO2 for 24, 48 or 72 hours. After the incubation time, MTT solution (5 mg mL−1) was added into all wells and further incubated for 4 hours. The purple formazon formed was then dissolved by 100 μL of DMSO (Sigma-Aldrich, USA) and the absorbance was measured at 570 nm using μQuant plate reader (Bio-Tek Instrument, USA). Cell viability was calculated as below:| Cell viability (%) = (absorbance of treated well)/(absorbance of control well) × 100% |
IC50 (concentration that reduce 50% of cell viability comparing to untreated cells) was obtained from the graph of cell viability vs. concentration.
2.3. In vivo antitumor effect of BHMC
Female mice (n = 18, 6 weeks old) were purchased from Comparative Medicine and Technology Unit (COMeT), Institute of Bioscience, Universiti Putra Malaysia. The mice were fed with distilled water and standard pellets ad libitum at room temperature and 12 hours of day/dark light cycles. This study was conducted according to the guidelines and was approved by Institution of Animal Care and Use Committee (IACUC), Universiti Putra Malaysia (UPM/IACUC/AUP-R009/2015). All animal experiment was carried out in accordance with the Malaysia Animals Act 1953 (Revised-2006) under Malaysian Veterinary Council (MVC) for animal experiments.After 2 weeks of acclimatization of mice (body weight ∼22 g), mice were separated into 3 groups and challenged with 1 × 105 4T1 cells per mice subcutaneously. BHMC and curcumin were dissolved in olive oil. Untreated mice (n = 6) were fed with olive oil, curcumin 50 mice were orally fed with 50 mg kg−1 body weight (BW) of curcumin (Sigma-Aldrich, USA) and BHMC 50 mice were orally fed with 50 mg kg−1 BW of BHMC for 28 days. Throughout the experiment period, tumors were measured using a caliper and the tumor volumes were calculated by the following formula:
| Tumor volume = (d2 × a)/2 |
After the 28 days of treatment, mice were amnestied with isoflurane, and euthanised by cervical dislocation. Serum, lung and tumor were collected and subjected to the following assays. Tumor per body weight ratio was calculated.
2.4. Hematoxylin and eosin (H&E) histology analysis of tumor
Harvested tumor was fixed in 10% formalin, embedded in paraffin, section and stained with H&E. Histology of the tumor was viewed under bright-field microscope (Nikon, Japan). Even of mitotic cells were counted from five random fields of the slides.2.5. Lung clonogenic assay
Lung was harvested, washed with ice-cold PBS, minced, treated with 2 mg mL−1 collagenase type IV for 1 hour at 37 °C and filtered through 70 μm strainer. Then, the filtrate was pelleted at 2000 rpm for 5 minutes. The lung pellet was cultured in 10 mL of RPMI-1640 supplemented with 10% Fetal Bovine Serum (FBS) (Gibco, Thermo Fisher Scientific, USA) at 37 °C, 5% CO2 for 10 days. After the incubation period, all wells were fixed with methanol for 1 hour and stained with 0.5% crystal violet for 2 hours. Number of colonies formed per organ was counted for untreated, curcumin treated and BHMC treated groups.2.6. Expression of MMP9, TNF-α, IL-1β, IL-4, G-CSF and NF-kB in tumor by quantitative reverse transcription PCR (RT-qPCR)
Total RNA from the tumor was extracted using RNeasy mini kit (Qiagen, USA) according to the manufacturer's protocol. cDNA was synthesized from 1 μg of total RNA using NEXscript cDNA synthesis kit (NEX Diagnostics, Korea) according to the manufacturer's protocols. Primers for target genes MMP9, TNF-α, IL-1β, IL-4, G-CSF, NF-kB and house-keeping gene β-actin were listed in Table 2. Evaluation of primer efficiency and expression of the targeted genes were performed using NEXpro qPCR Evagreen Master Mix (NEX Diagnostics, Korea) using Eco Real Time PCR system (Illumina, USA) by the following steps: 95 °C for 2 min, 40 cycles of 95 °C for 10 s, 60 °C for 45 s and acquisition of fluorescent signal. Expression of the targeted genes in the samples were normalized by β-actin and the fold change in the expression of each target gene was calculated by the Eco 48 Software (Illumina, USA) using the efficiency-corrected method.2.7. Serum IL-1β and TNF-α levels
IL-1β and TNF-α cytokines level in the serum were measured by ELISA kits (R&D system, USA) according to the manufacturer's protocol.2.8. Secondary tumor regeneration
Tumor was harvested and dissociated using accutase (Innovative Cell Tech, USA). Mice (n = 10, 8 weeks old) were separated into two groups. All mice were injected subcutaneously with 1 × 106 cells harvested from untreated tumor in the lower left abdomen. On the other hand, mice in group 1 and 2 were injected subcutaneously with 1 × 106 cells harvested from the tumor of curcumin 50 mg kg−1 BW mice and BHMC 50 mg kg−1 BW tumor in the lower right abdomen, respectively. Tumor progression was then observed for 21 days.3. Results
3.1. In vitro cytotoxicity of BHMC on 4T1 cells
BHMC was prepared as previously described.31 The in vitro cytotoxicity of BHMC was compared with curcumin on 4T1 cells by MTT assay. As shown in Table 1, both BHMC and curcumin were cytotoxic to 4T1 cells in time dependent manner where the IC50 value of both treatment reduced from 24 to 72 hours. More interestingly, IC50 value of BHMC at 48 and 72 hours were approximately 2 folds lower than curcumin on 4T1 cells.| 24 h | 48 h | 72 h | |
|---|---|---|---|
| BHMC (μM) | 54.64 ± 2.33 | 21.66 ± 2.66 | 13.66 ± 3.24 |
| Curcumin (μM) | 81.44 ± 2.44 | 48.86 ± 3.46 | 27.15 ± 2.36 |
| Gene | Primer sequence (5′–3′) | |
|---|---|---|
| Forward | Reverse | |
| MMP9 | GTCTTCCTGGGCAAGCAGTA | CTGGACAGAAACCCCACTTC |
| TNF-α | CATCTTCTCAAAATTCGAGTGACAA | TGGGAGTAGACAAGGTACAACCC |
| IL-1β | CGCCAATGACTCAGAGGAAGA | AGGGCGTCATTCAGGATGAA |
| IL-4 | AGATGGATGTGCCAAACGTCCTCA | AATATGCGAAGCACCTTGGAAGCC |
| G-CSF | CTCAGAAATGTTTGACCTCCAG | TGACAAGCAGAAAGTCCTTCAG |
| NF-kB | GTG CGTCTGGCCTGGTAAG | CCCAGGATGTG TACTCAGAGC |
| β-Actin | TCCTTCCTGGGCATGGAG | AGGAGGAGCAATGATCTTGATCTT |
3.2. In vivo antitumor effect of BHMC on 4T1 challenged mice
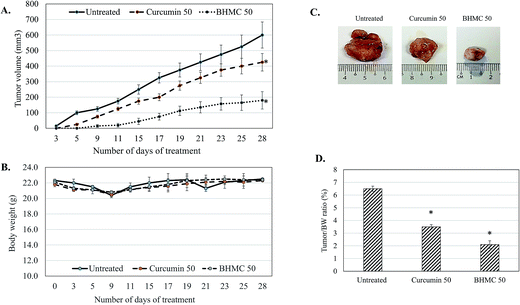
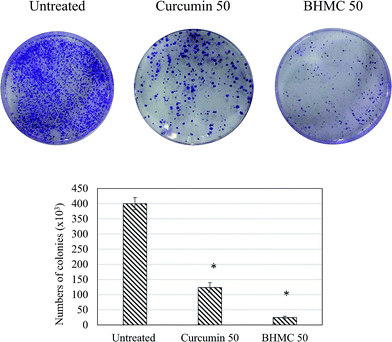
To evaluate the antitumor effect of BHMC on breast cancer, mice challenged with 4T1 breast cancer cells were subjected to 50 mg kg−1 BW of curcumin or BHMC treatment. Tumor start to observe at day 5 in untreated mice post-inoculation of 4T1 cells. Both curcumin and BHMC treated mice were observed with 5 and 9 days delay of tumor formation comparing to the untreated mice (Fig. 2A). After 28 days of treatment, without changes of the body weight were observed in all the mice (Fig. 2B), tumor burden of curcumin and BHMC treated mice were 1.86 and 3.10 folds smaller than the untreated mice (Fig. 2C and D). Based on the histology analysis, tumor harvested from untreated mice was observed with highest number mitotic cells. On the other hand, curcumin and BHMC treated mice were recorded with 1.67 and 3.75 folds less number of mitotic cells in the tumor (Fig. 3), which indicated that lower tumor burden in the curcumin and BHMC treated mice maybe contributed by anti-proliferation effect. In addition, clonogenic assay has shown that high number of 4T1 breast cancer cells have metastasized to lung in the untreated mice. On the other hand, mice treated with curcumin and BHMC were observed with lower event of 4T1 metastasis into lung indicating that both treatment possess anti-metastasis effect (Fig. 4). Overall, antitumor effect of BHMC was better comparing to curcumin as the BHMC treated mice was recorded with lower tumor burden (Fig. 2), mitotic cells in the tumor (Fig. 3) and lung metastasis (Fig. 4) comparing to the curcumin treated mice.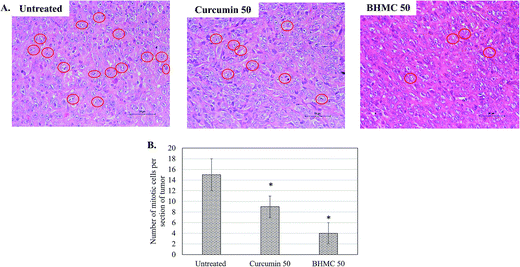 | ||
| Fig. 3 (A) Histological staining of the tumor. Red circles indicate mitotic cells. (B) Numbers of mitotic cells per groups. Significant values were calculated against untreated group (*P < 0.05). | ||
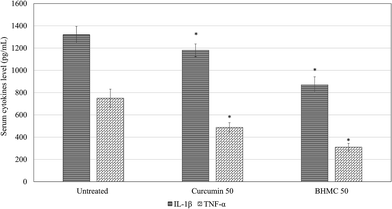
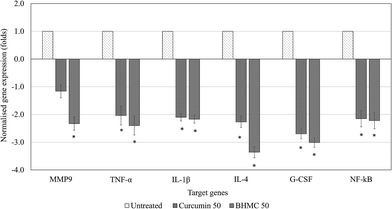
To understand the regulation of inflammation and metastasis related genes by both curcumin and BHMC treatment, which contribute to the delay of tumor progression, expression of MMP9, TNF-α, IL-1β, IL-4, G-CSF and NF-kB genes in the tumor of untreated, curcumin 50 and BHMC 50 treated mice were evaluated by RT-qPCR (Fig. 5). BHMC treatment was able to suppress expression of MMP9, TNF-α, IL-1β, IL-4, G-CSF and NF-kB genes comparing to the untreated mice. On the other hand, curcumin treated was able to suppress expression of TNF-α, IL-1β, IL-4, G-CSF and NF-kB genes comparing to the untreated mice. However, expression of MMP9 was not significantly regulated by curcumin treatment (Fig. 5). Level of IL-1β and TNF-α were further validated by checking serum IL-1β and TNF-α by ELISA. Both curcumin and BHMC treatment were able to reduce the serum level of IL-1β and TNF-α comparing to the untreated mice (Fig. 6).
 | ||
| Fig. 5 Serum IL-1β and TNF-α level of untreated, curcumin 50 mg kg−1 BW mice and BHMC 50 mg kg−1 BW 4T1 challenged mice. Significant values were calculated against untreated group (*P < 0.05). | ||
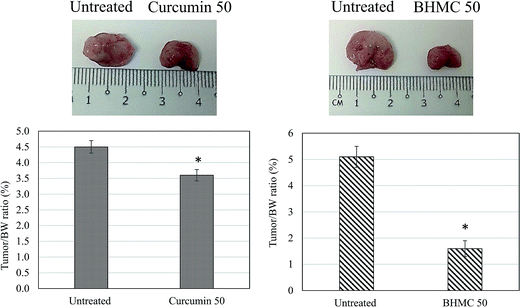
Furthermore, regeneration capacity of the tumor harvested from untreated and curcumin or BHMC treated mice were evaluated by transplanting 1 million cells to healthy mice. When injected with tumor cells harvested from untreated and BHMC treated 4T1 challenged mice, tumor/BW ratio generated from the untreated tumor cells was approximate 2.8 fold higher than the tumor generated from BHMC group. On the other hand, tumor generated from curcumin treated mice was only 1.25 fold lower than tumor generated from the untreated mice (Fig. 7).
4. Discussion
Prognosis of TNBC remains poor in spite of advance in understanding of breast cancer pathologic features and molecular characteristics.37 TNBC are more difficult to treat as they carry fewer targets and generally develop resistant to conventional chemotherapeutic agents.38 Thus, there is a need to continue the discovery of novel effective cytotoxic agents against TNBC with minimum or no toxic side effect. Curcumin is a polyphenol presents in the turmeric Curcumina longa. It belongs to the 1,3-dicarbonyl or β-diketone class of compounds, which possess several biological activities including important building blocks for the synthesis of core heterocycles such as pyrazole, isoxazole, and triazole in medicinal chemistry.39 Curcumin has been identified as strong anti-inflammatory agents that has broad spectrum of antitumor activity.35 Structure activity relationship of curcumin and its analogous is well established in the literatures.40,41 Curcumin was reported as potential agents targeting TNBC as recent study has reported that it was more sensitive to human TNBC MDA-MB-231 cells than human estrogen dependent MCF-7 breast cancer cells.42 A part of interesting biological activities, still curcumin not recommended for therapeutic potential because of low bioavailability.26,27 Therefore, there is need to modify its structure to improve the bioavailability, stability and selectivity. Recent study further support the idea that synthetic chemistry help to enhance the efficacy and safety of known natural metabolites.43 For examples, curcumin analogues were reported with enhance cytotoxicity on various types of cancer cells.28,44 In this study, BHMC that is the analog of curcumin was synthesized. Previously, the antinociceptive activity31 and anti-inflammatory effect29,30 of BHMC were evaluated. Unlike curcumin that contain enol moiety, BHMC possessing α,β-unsaturated bis-enone system which is an essential for the several biological activities including anticancer effect as in chalcones and bis-chalcones.41 Such compounds with conjugated enones or enone-like compounds exhibited potential interaction with Michael acceptor can react selectively with target nucleophiles.41 This structure may contributed to the greater cytotoxic effect of BHMC on 4T1 cells in vitro, which was similar to the previous in vitro studies on human TBNC MDA-MB-231 cells.34In addition, in vivo antitumor effect of BHMC was compared with curcumin using mouse 4T1 model. Mice challenged with 4T1 has been used as in vivo model to evaluate antitumor and antimetastasis effect of chemicals on TNBC.45,46 In this study, untreated mice was recorded with tumor development on day 3 post challenged. In addition, high tumor mitotic event and lung metastasis were observed too. On the other hand, both curcumin and BHMC treatment were found with delayed tumor formation. Curcumin was reported to suppress cell proliferation of TNBC in vitro.47 Lower number of mitotic cells observed in the tumor of curcumin and BHMC treated mice supported the report on the in vitro antiproliferation effect and given the idea that lower tumor burden in the treated mice maybe contributed by the inhibition of 4T1 cells proliferation. The antiproliferative effect of BHMC has been correlated to its interactions with nuclear type II sites.33
TNBC is a type of highly metastatic cancer.48 Inflammation was found to promote tumor cell proliferation and expression of MMP9 that support the cancer metastasis.49 Curcumin has been reported with in vitro antimetastatic effect on breast cancer via suppression of MMP-9 (ref. 50) and suppression of inflammation.51 In this study, both curcumin and BHMC were found with lower lung metastasis comparing to untreated mice. This antimetastatic effect maybe contributed by the suppression of inflammatory and MMP9 as observed in the gene expression study. Although both curcumin and BHMC show similar inhibition on inflammatory related genes, significant suppression of MMP9 expression by BHMC may contribute to its better antimetastatic effect than curcumin. Previous study has shown that curcumin possessed substantial antitumor and antimetastasis when treated at 800 mg kg−1 BW.35 Encapsulation of curcumin with dendrosome help to reduce the effective dosage to 40 mg kg−1 BW.52 In addition, TNBC was also known with high rate of recurrence.48 In this study, recurrence of untreated tumor with tumor harvested from curcumin or BHMC treatment were compared by injected them in the secondary healthy mice. After 21 days of secondary challenge, tumor burden originated from BHMC treated mice were remain lowest comparing those from untreated or curcumin treated mice. This result indicate that BHMC may even help to delay recurrence of TNBC even better than curcumin.
5. Conclusions
This study reported that the synthesis BHMC, which is an analogue of curcumin showed better in vitro cytotoxicity and in vivo antitumor effect on 4T1 TNBC model than curcumin via suppression of inflammation, cancer cells proliferation and metastasis.Conflict of interest
All authors in this article declare no conflict of interest.Acknowledgements
This study was supported by Universiti Malaysia Pahang (internal grant no. RDU 150109 and 150349), MARDI internal grant (vote no. 6300300) and MAKNA cancer research award 2013 (vote no. 6300123).References
- C. Fitzmaurice, C. Allen, R. M. Barber, L. Barregard, Z. A. Bhutta and H. Brenner, et al., JAMA Oncol., 2015, 1, 505–527 CrossRef PubMed.
- F. Andre and C. C. Zielinski, Ann. Oncol., 2012, 23, vi46–vi51 Search PubMed.
- M. T. Cook, Y. Liang, C. Besch-Williford and S. M. Hyder, Breast Cancer, 2016, 9, 9–19 Search PubMed.
- P. Thulasiraman, D. J. McAndrews and I. Q. Mohiudddin, BMC Cancer, 2014, 14, 724 CrossRef PubMed.
- X. D. Sun, X. E. Liu and D. S. Huang, Mol. Med. Rep., 2012, 6, 1267–1270 CAS.
- S. Neidle and D. E. Thurston, Nat. Rev. Cancer, 2005, 5, 285–296 CrossRef CAS PubMed.
- A. L. Demain and P. Vaishnav, Microb. Biotechnol., 2011, 4, 687–699 CrossRef PubMed.
- S. C. Gupta, S. Patchva, W. Koh and B. B. Aggarwal, Clin. Exp. Pharmacol. Physiol., 2012, 39, 283–299 CrossRef CAS PubMed.
- B. B. Aggarwal, A. Kumar and A. C. Bharti, Anticancer Res., 2003, 23, 363–398 CAS.
- O. P. Sharma, Biochem. Pharmacol., 1976, 25(15), 1811–1812 CrossRef CAS PubMed.
- A. J. Ruby, G. Kuttan, K. D. Babu, K. N. Rajasekharan and R. Kuttan, Cancer Lett., 1995, 94, 79–83 CrossRef CAS PubMed.
- Y. Sugiyama, S. Kawakishi and T. Osawa, Biochem. Pharmacol., 1996, 52, 519–525 CrossRef CAS PubMed.
- R. C. Srimal and B. N. Dhawan, J. Pharm. Pharmacol., 1973, 25, 447–452 CrossRef CAS PubMed.
- W. C. Jordan and C. R. Drew, J. Natl. Med. Assoc., 1996, 88, 333 CAS.
- M. K. Kim, G. J. Choi and H. S. Lee, J. Agric. Food Chem., 2003, 51, 1578–1581 CrossRef CAS PubMed.
- R. C. Reddy, P. G. Vatsala, V. G. Keshamouni, G. Padmanaban and P. N. Rangarajan, Biochem. Biophys. Res. Commun., 2005, 326(2), 472–474 CrossRef CAS PubMed.
- R. Kuttan, P. Bhanumathy, K. Nirmala and M. C. George, Cancer Lett., 1985, 29(2), 197–202 CrossRef CAS PubMed.
- X. Zhang, Y. Tian, Z. Li, X. Tian, H. Sun, H. Liu, A. Moore and C. Ran, J. Am. Chem. Soc., 2013, 135, 16397–16409 CrossRef CAS PubMed.
- Y. Kiso, Y. Suzuki, N. Watanabe, Y. Oshima and H. Hikino, Planta Med., 1983, 49, 185–187 CrossRef CAS PubMed.
- N. Venkatesan, Br. J. Pharmacol., 1998, 124, 425–427 CrossRef CAS PubMed.
- N. Venkatesan, D. Punithavathi and V. Arumugam, Br. J. Pharmacol., 2000, 129, 231–234 CrossRef CAS PubMed.
- R. Srivastava, M. Dikshit, R. C. Srimal and B. N. Dhawan, Thromb. Res., 1985, 40, 413–417 CrossRef CAS PubMed.
- M. Dikshit, L. Rastogi, R. Shukla and R. C. Srimal, Indian J. Med. Res., 1995, 101, 31–35 CAS.
- C. Nirmala and R. Puvanakrishnan, Mol. Cell. Biochem., 1996, 159, 85–93 CrossRef CAS PubMed.
- C. Nirmala and R. Puvanakrishnan, Biochem. Pharmacol., 1996, 51, 47–51 CrossRef CAS PubMed.
- K. M. Nelson, J. L. Dahlin, J. Bisson, J. Graham, G. F. Pauli and M. A. Walters, J. Med. Chem., 2017, 60, 1620–1637 CrossRef CAS PubMed.
- K. M. Nelson, J. L. Dahlin, J. Bisson, J. Graham, G. F. Paulin and M. A. Walters, ACS Med. Chem. Lett., 2017, 8, 467–470 CrossRef CAS PubMed.
- C. D. Mock, B. C. Jordan and C. Selvam, RSC Adv., 2015, 5, 75575–75588 RSC.
- C. L. Tham, C. Y. Liew, K. W. Lam, A. S. Mohamad, M. K. Kim, Y. K. Cheah, Z. A. Zakaria, M. R. Sulaiman, N. H. Lajis and D. A. Israf, Eur. J. Pharmacol., 2010, 628, 247–254 CrossRef CAS PubMed.
- C. L. Tham, K. W. Lam, R. Rajajendram, Y. K. Cheah, M. R. Sulaiman, N. H. Lajis, M. K. Kim and D. A. Israf, Eur. J. Pharmacol., 2011, 652, 136–144 CrossRef CAS PubMed.
- L. Ming-Tatt, S. I. Khalivulla, M. N. Akhtar, A. S. Mohamad, E. K. Perimal, M. H. Khalid, A. Akira, N. Lajis, D. S. Israf and M. R. Sulaiman, Basic Clin. Pharmacol. Toxicol., 2012, 110, 275–282 CrossRef PubMed.
- D. Lv, Y. Zhang, H. J. Kim, L. Zhang and X. Ma, Cell. Mol. Immunol., 2013, 10, 303–310 CrossRef CAS PubMed.
- B. M. Markaverich, T. H. Schauweker, R. R. Gregory, M. Varma, F. S. Kittrell, D. Medina and R. S. Varma, Cancer Res., 1992, 52, 2482–2488 CAS.
- B. Yadav, S. Taurin, R. J. Rosengren, M. Schumacher, M. Diederich, T. J. Somers-Edgar and L. Larsen, Bioorg. Med. Chem. Lett., 2010, 18, 6701–6707 CrossRef CAS PubMed.
- M. Singh, I. Ramos, D. Asafu-Adjei, W. Quispe-Tintaya, D. Chandra, A. Jahangir, X. Zang, B. B. Aggarwal and C. Gravekamp, Cancer Med., 2013, 2, 571–582 CrossRef CAS PubMed.
- A. S. Mohamad, M. N. Akhtar, S. I. Khalivulla, E. K. Perimal, M. H. Khalid, H. M. Ong, S. Zareen, A. Akira, D. A. Israfl, N. Lajis and M. R. Sulaiman, Basic Clin. Pharmacol. Toxicol., 2011, 108, 400–405 CrossRef CAS PubMed.
- M. Arnedos, C. Bihan, S. Delaloge and F. Andre, Ther. Adv. Med. Oncol., 2012, 4, 195–210 CrossRef CAS PubMed.
- W. Wang, E. R. Rayburn, S. E. Velu, D. Chen, D. H. Nadkarni, S. Murugesan, D. Chen and R. Zhang, Breast Cancer Res. Treat., 2010, 123, 321–331 CrossRef CAS PubMed.
- J. Pradhan and A. Goyal, Int. J. Biol., Pharm. Allied Sci., 2015, 4, 1–18 Search PubMed.
- H. Yang, Z. Du, W. Wang, M. Song, K. Sanidad, E. Sukamtoh, J. Zheng, L. Tian, H. Xian, Z. Liu and G. Zhang, J. Agric. Food Chem., 2017, 65, 4509–4515 CrossRef CAS PubMed.
- M. Nakhjiri, M. Safavi, E. Alipour, S. Emami, A. F. Atash, M. Jafari-Zavareh, S. K. Ardestani, M. Khoshneviszadeh, A. Foroumadi and A. Shafiee, Eur. J. Med. Chem., 2012, 50, 113–123 CrossRef CAS PubMed.
- T. Jia, L. Zhang, Y. Duan, M. Zhang, G. Wang, J. Zhang and Z. Zhao, Cancer Cell Int., 2014, 14, 126 CrossRef PubMed.
- D. A. Dias, S. Urban and U. Roessner, Metabolites, 2012, 2, 303–336 CrossRef CAS PubMed.
- N. M. Ali, S. K. Yeap, N. Abu, K. L. Lim, H. Ky, A. Z. M. Pauzi, W. Y. Ho, S. W. Tan, H. K. Alan-Ong, S. Zareen, N. B. Alitheen and M. N. Akhtar, Cancer Cell Int., 2017, 17, 30 CrossRef PubMed.
- G. Ferrari-Amorotti, C. Chiodoni, F. Shen, S. Cattelani, A. R. Soliera, G. Manzotti, G. Grisendi, M. Dominici, F. Rivasi, M. P. Colombo, A. Fatatis and B. Calabretta, Neoplasia, 2014, 16, 1047–1058 CrossRef CAS PubMed.
- P. Kaur, G. M. Nagaraja, H. Zheng, D. Gizachew, M. Galukande, S. Krishnan and A. Asea, BMC Cancer, 2012, 12, 120 CrossRef PubMed.
- X. D. Sun, X. E. Liu and D. S. Huang, Mol. Med. Rep., 2012, 6, 1267–1270 CAS.
- J. Qiu, X. Xue, C. Hu, H. Xu, D. Kou, R. Li and M. Li, J. Cancer, 2016, 7, 167–173 CrossRef PubMed.
- L. Gan, Z. Qiu, J. Huang, Y. Li, H. Huang, T. Xiang, J. Wan, T. Hui, Y. Lin, H. Li and G. Ren, Int. J. Biol. Sci., 2016, 12, 1533–1543 CrossRef PubMed.
- J. M. Kim, E. M. Noh, K. B. Kwon, J. S. Kim, Y. O. You, J. K. Hwang, B. M. Hwang, B. S. Kim, S. H. Lee, S. J. Lee, S. H. Jung, H. J. Youn and Y. R. Lee, Phytomedicine, 2012, 19, 1085–1092 CrossRef CAS PubMed.
- T. L. Chiu and C. C. Su, Int. J. Mol. Med., 2009, 23, 469–475 CAS.
- B. Farhangi, A. M. Alizadeh, H. Khodayari, S. Khodayari, M. J. Dehghan, V. Khori, A. Heidarzadeh, M. Khaniki, M. Sadeghiezadeh and F. Najafi, Eur. J. Pharmacol., 2015, 758, 188–196 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2017 |