Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
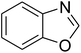
Synthesis of triphenylamines via ligand-free selective ring-opening of benzoxazoles or benzothiazoles under superparamagnetic nanoparticle catalysis†
Oanh T. K. Nguyen,
Long T. Nguyen,
Nhu K. Truong,
Viet D. Nguyen,
Anh T. Nguyen,
Nhan T. H. Le,
Dung T. Le* and
Nam T. S. Phan *
*
Faculty of Chemical Engineering, HCMC University of Technology, VNU-HCM, 268 Ly Thuong Kiet, District 10, Ho Chi Minh City, Vietnam. E-mail: ltdung@hcmut.edu.vn; ptsnam@hcmut.edu.vn; Fax: +84 8 38637504; Tel: +84 8 38647256 ext. 5681
First published on 22nd August 2017
Abstract
CuFe2O4 superparamagnetic nanoparticles were utilized as an effective recyclable heterogeneous catalyst for the synthesis of triphenylamines via the ligand-free selective ring-opening reaction of benzoxazoles or benzothiazoles with iodoarenes. The nano CuFe2O4 demonstrated noticeably higher catalytic efficiency than a series of homogeneous catalysts and heterogeneous catalysts. It was possible to separate the nano CuFe2O4 by using a magnet, and the recovered catalyst was reused many times while its activity could be maintained. To the best of our knowledge, this is the first example of heterogeneous catalysis for the transformation of benzoxazoles, and the transformation of benzothiazoles to triphenylamines has not been previously reported in the literature.
1. Introduction
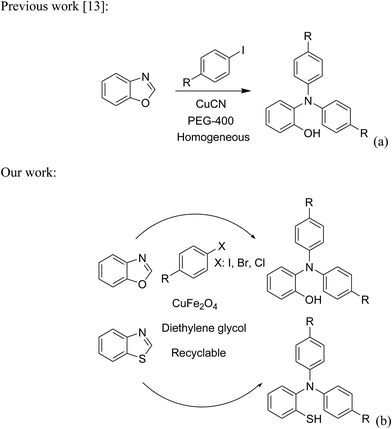
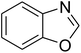
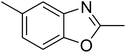
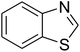
Triphenylamines and their derivatives represent a notable family of aromatic compounds that provides a broad range of practical applications in pharmaceuticals, agrochemicals, dye-sensitized solar cells, fluorescence sensors, photoelectrochemical sensors, and photofunctional polymers.1–5 These skeletons are conventionally generated by utilizing Ullmann-type coupling reactions.6–9 Several efforts have been devoted to improve the formation of triphenylamines, thanks to their splendid contribution to many fields. Safaei-Ghomi et al. previously described a CuI-catalyzed synthesis of triphenylamines via the coupling of anilines with iodobenzenes in the presence of 1,10-phenanthroline as ligand.10 Shaikh et al. expressed the production of triphenylamine derivatives by using a Pd2(dba)3-catalyzed C–N bond forming amination protocol.11 Nevertheless, efficient routes towards the synthesis of these structures still remain challenging. Recently, Kim et al. demonstrated for the first time a CuCN-catalyzed selective ring-opening of benzoxazoles with iodobenzenes to generate triphenylamines in pivalonitrile as solvent and triphenylphosphine as ligand.12 He et al. subsequently modified the method by performing the reaction in polyethylene glycol under ligand-free conditions (Scheme 1a).13 Indeed, reports on this transformation has been very limited in the literature. Although interesting results were achieved, the homogeneous copper catalyst could not be recycled and reused. To develop more environmentally benign approaches, the synthesis of triphenylamines should be conducted with solid catalysts.The last decade has witnessed an tremendous breakthrough in ‘‘nanocatalysis’’.14–17 Active nanoparticles have attracted considerable attention as they could shorten the gap between heterogeneous and homogeneous catalysts.18–20 At nanometer dimension, the external surface of the catalyst could be maximized, making most of active sites accessible to reactants, and therefore catalytic activity would be extraordinarily improved.21–23 Nevertheless, the separation and reusing of the nanoparticles still remains really bothersome, and the issue should be worked out.24 Superparamagnetic nanoparticles could incorporate benefits of high catalytic activity with straightforward isolation via magnetic separation approaches.25,26 Certainly, unfunctionalized superparamagnetic nanoparticles have recently explored as productive heterogeneous catalysts for the synthesis of oxindoles and their derivatives,27 the borylation of aryliodides or β-bromostyrenes to produce arylboronates,28 the synthesis of vinylboronates via regioselective hydroboration of internal as well as terminal alkynes,29 the one-pot multicomponent preparation of quinolinones,30 the photocatalytic degradation of organic pollutants,31 the transamidation,32 the synthesis of tetrahydropyridines and pyrrole derivatives,33 the synthesis of 2,4,5-trisubstituted imidazoles,34 the synthesis of azoles,35,36 the oxidation of alcohols to aldehydes,37 and synthesis of phenols.38 Herein, we would like to present the synthesis of triphenylamines via ligand-free selective ring-opening of benzoxazoles or benzothiazoles with aryl halides under CuFe2O4 superparamagnetic nanoparticle catalysis (Scheme 1b). To our best knowledge, this is the first heterogeneous catalysis for the transformation of benzoxazoles, and the transformation of benzothiazoles to triphenylamines was not previously reported. Moreover, using aryl bromides and aryl chlorides for this reaction was not previously mentioned in the literature.
2. Experimental
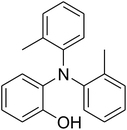
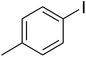
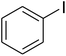
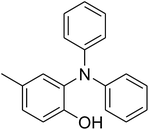
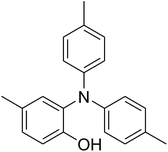
CuFe2O4 superparamagnetic nanoparticles were supplied by Sigma-Aldrich. The nanoparticles was consequently characterized by utilizing several analysis approaches (see Fig. S1–S6 in ESI†). In an exemplary experiment, a mixture of benzoxazole (0.0357 g, 0.3 mmol), iodobenzene (0.1836 g, 0.9 mmol), Cs2CO3 (0.2443 g, 0.75 mmol) and diphenyl ether (0.0511 g, 0.3 mmol) as an internal standard in diethylene glycol (2 ml) was added into an 8 ml screw cap vial containing the catalyst. The catalyst quantity was determined with regard to the copper/benzoxazole mole fraction. The reaction mixture was stirred at 120 °C for 2 h under an argon atmosphere. Samples were withdrawn, and quenched with water (1 ml). Organic constituents were extracted into ethyl acetate (4 ml), shaken with anhydrous Na2SO4 to remove any water residue, and analyzed by GC concerning diphenyl ether. The major product, 2-(diphenylamino)phenol, was purified on silica gel by column chromatography (petroleum ether: ethylacetate = 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). The product identity was additionally authenticated by GC-MS, 1H NMR, and 13C NMR. For the catalyst reusing investigation, ethanol was added to reduce the viscosity of the mixture, and the superparamagnetic nanoparticles were subsequently isolated by decantation using a permanent magnet. The recovered catalyst was washed thoroughly with water, ethanol, ethyl acetate, and ether, subsequently heated at 150 °C under vacuum on a Shlenk line for 6 h, and then reutilized for new catalytic transformation.
1, v/v). The product identity was additionally authenticated by GC-MS, 1H NMR, and 13C NMR. For the catalyst reusing investigation, ethanol was added to reduce the viscosity of the mixture, and the superparamagnetic nanoparticles were subsequently isolated by decantation using a permanent magnet. The recovered catalyst was washed thoroughly with water, ethanol, ethyl acetate, and ether, subsequently heated at 150 °C under vacuum on a Shlenk line for 6 h, and then reutilized for new catalytic transformation.
3. Results and discussion
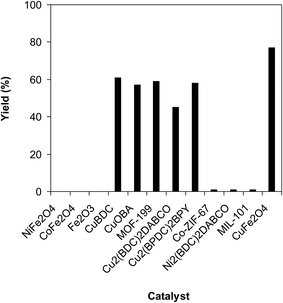
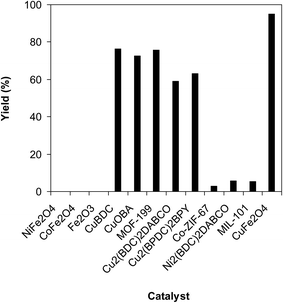
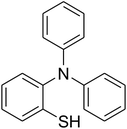
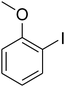
A series of solid catalysts were screened for the ring-opening reaction of benzoxazole with iodobenzene to generate 2-(diphenylamino)phenol as the major product (Scheme 2), including nano CuFe2O4, nano NiFe2O4, nano CoFe2O4, nano Fe2O3, and a number of metal–organic frameworks including Cu(BDC), Cu(OBA), MOF-199, Cu2(BDC)2(DABCO), Cu2(BPDC)2(BPY), Co-ZIF-67, Ni2(BDC)2(DABCO), and MIL-101. Following this approach, triphenylamines could be formed from benzoxazoles as alternative starting materials to anilines under heterogeneous catalysis conditions. The reaction was conducted in diethylene glycol at 120 °C under argon for 2 h, employing 2.5 equivalents of Cs2CO3, in the presence of 10 mol% catalyst, with 3 equivalents of iodobenzene, at benzoxazole concentration of 0.15 M. Nano NiFe2O4, nano CoFe2O4, and nano Fe2O3 were found to be totally inactive for this transformation, with no trace amount of 2-(diphenylamino)phenol being recorded after 2 h. Cu-MOFs offered reasonable catalytic activity, while cobalt-, nickel-, and iron-based MOFs were not appropriate for the ring-opening reaction. Among these catalysts, the CuFe2O4 superparamagnetic nanoparticles exhibited the best performance, affording the desired product in 77% yield after 2 h (Fig. 1). It was therefore decided to employ the nano CuFe2O4 as catalyst for the synthesis of triphenylamines via the ring-opening reaction.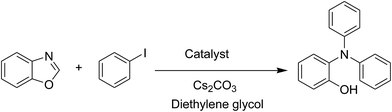 | ||
| Scheme 2 The ring-opening reaction of benzoxazole with iodobenzene to generate 2-(diphenylamino)phenol. | ||
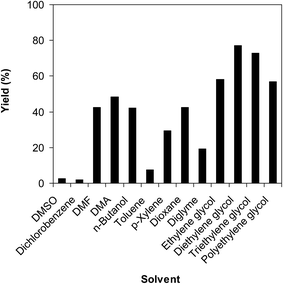
Kim,12 He13 et al. previously pointed out that the solvent displayed a notable impact on the generation of the amine. We then conducted the transformation in a variety of solvents at 120 °C under argon for 2 h, employing 2.5 equivalents of Cs2CO3, in the presence of 10 mol% catalyst, with 3 equivalents of iodobenzene, at benzoxazole concentration of 0.15 M. It was noted that DMSO, toluene, dichlorobenzene, and diglyme should not be used for the reaction. DMF, n-butanol, and dioxane offered better performance, affording 2-(diphenylamino)phenol in 42% yield after 2 h. Performing the reaction in DMA, 48% yield of the triphenylamine was obtained after 2 h. He et al. previously reported that this transformation using CuCN catalyst proceeded readily in polyethylene glycol.13 However, in this work, it was observed that only 57% yield of the triphenylamine was generated in polyethylene glycol after 2 h. Ethylene glycol as solvent led to the formation of 2-(diphenylamino)phenol in 58% yield after 2 h. Interestingly, this value could be amended considerably to 77% after 2 h for the case of diethylene glycol. However, conducting the reaction in triethylene glycol caused a deterioration in the yield of the expected product (Fig. 2). This could be explicated based on the high viscosity of longer polyols, resulting in difficulty for the dispersion of the solid catalyst in the reaction mixture.
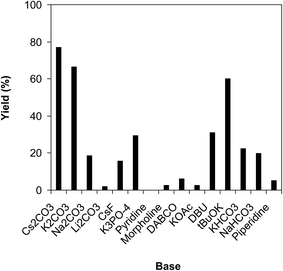
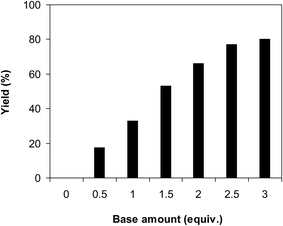
It was previously reported that a base was required for the ring-opening reaction of benzazoles.39 The influence of base on the reaction of benzoxazole with iodobenzene to generate 2-(diphenylamino)phenol was then explored. The reaction was performed in diethylene glycol at 120 °C under argon for 2 h, employing 2.5 equivalents of base, in the presence of 10 mol% catalyst, with 3 equivalents of iodobenzene, at benzoxazole concentration of 0.15 M. Experimental data revealed that many carbonates as base could initiate the formation of the triphenylamine. However, Li2CO3 and Na2CO3 offered low reactivity, affording the desired product in 2% and 19% after 2 h. The yield could be boosted to 66% for the case of K2CO3. Among various bases, Cs2CO3 expressed the best functioning, with 77% yield of the triphenylamine being recorded after 2 h. The reaction utilizing tBuOK as base could also provide 60% yield after 2 h. K3PO4, KHCO3, and NaHCO3 were not the base of choice for this reaction, producing the triphenylamine in 29%, 22%, and 20% yields, respectively, after 2 h. Organic bases such as pyridine, morpholine, piperidine, DABCO, and DBU were not suitable for this reaction (Fig. 3). The quantity of base also expressed a notable impact on the generation of the triphenylamine. The reaction could not proceed in the absence of the base, with the product being recorded in trace amount after 2 h. The presence of Cs2CO3 in the reaction mixture considerably accelerated the conversion. However, utilizing more than 2.5 equivalents of the base was inessential as the yield of 2-(diphenylamino)phenol was not ameliorated remarkably (Fig. 4).
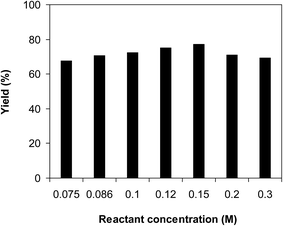
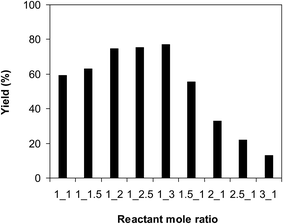
As the ring-opening reaction of benzoxazole with iodobenzene to generate 2-(diphenylamino)phenol was conducted in solution phase, the rate of the transformation could be immensely altered by the reactant concentration as a consequence of the mass transfer issue. It was consequently determined to probe the influence of benzoxazole concentration on the yield of 2-(diphenylamino)phenol. The reaction was implemented in diethylene glycol at 120 °C under argon for 2 h, employing 2.5 equivalents of Cs2CO3, in the presence of 10 mol% catalyst, with 3 equivalents of iodobenzene, at various benzoxazole concentrations. It was noted that the reaction yield could be amended by changing the concentration of benzoxazole. Extending the concentration from 0.075 M to 0.15 M, the yield of 2-(diphenylamino)phenol was elevated from 68% to 77% after 2 h. However, increasing the amount of benzoxazole in the reaction mixture caused a drop in the yield of the triphenylamine (Fig. 5). Furthermore, experimental results disclosed that the reactant molar ratio also displayed an impact on the transformation. The reaction utilizing 1 equivalent of iodobenzene produced the expected product in 59% yield after 2 h. This value was boosted to 75% for the reaction with 2 equivalents of benzoxazole. Employing more benzoxazole for the reaction did not amend the yield substantially. It was also noted that utilizing a large excess amount of benzoxazole led to considerable low yield of 2-(diphenylamino)phenol (Fig. 6). Moreover, 2-(phenylamino)phenol, the diphenylamine product, was produced noticeably in the presence of excess benzoxazole.
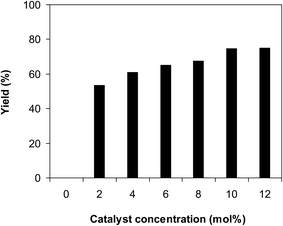
Another feature that must be scrutinized for the ring-opening reaction of benzoxazole with iodobenzene to generate 2-(diphenylamino)phenol is the required catalyst quantity. In the first demonstration of this transformation in pivalonitrile as solvent and triphenylphosphine as ligand, Kim et al. employed 10 mol% CuCN catalyst.12 He et al. subsequently performed the reaction in polyethylene glycol under ligand-free conditions at 10 mol% CuCN catalyst.13 We then probed the influence of catalyst quantity on the generation of the triphenylamine. The reaction was conducted in diethylene glycol at 120 °C under argon for 2 h, employing 2.5 equivalents of base, with 2 equivalents of iodobenzene, at benzoxazole concentration of 0.15 M, in the presence of different catalyst amounts. The reaction could not progress to produce the triphenylamine, with no trace quantity of 2-(diphenylamino)phenol being noted after 2 h. This observation supported the requirement of the catalyst for the conversion. Utilizing 2 mol% catalyst, the yield of the desired product was considerably improved to 53% after 2 h. As expected, increasing the catalyst quantity facilitated the production of the triphenylamine via the ring-opening transformation. Expanding the catalyst amount to 4 mol% resulted in 61% yield being probed after 2 h. The reaction yield could be upgraded to 65% after 2 h in the presence of 6 mol% catalyst. Raising the catalyst concentration to 8 mol% offered 68% yield after 2 h. As noted earlier, the reaction employing 10 mol% catalyst could progress to 75% yield after 2 h. Boosting the catalyst amount to more than 10 mol% was noticed to be superfluous as the yield of the triphenylamine was not intensified exceptionally (Fig. 7).
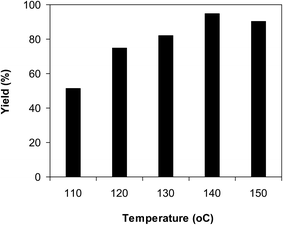
With these data on hand, we subsequently tried to improve the yield of 2-(diphenylamino)phenol by boosting the reaction temperature. He et al. previously conducted the reaction in polyethylene glycol with CuCN catalyst under ligand-free condition at 130 °C for 18 h.13 In the first demonstration of this reaction with CuCN catalyst in pivalonitrile as solvent and triphenylphosphine as ligand, Kim et al. achieved high yields of triphenylamines at 120 °C after 24 h.12 The reaction was implemented in diethylene glycol under argon at benzoxazole concentration of 0.15 M for 2 h, employing 2.5 equivalents of Cs2CO3, in the presence of 10 mol% catalyst, with 2 equivalents of iodobenzene, at 110 °C, 120 °C, 130 °C, 140 °C, and 150 °C, respectively. As mentioned earlier, the reaction carried out at 120 °C provided 2-(diphenylamino)phenol with 75% yield after 2 h. Expanding the reaction time to 5 h at 120 °C did not intensify the yield of the triphenylamine significantly. Decreasing the temperature to 110 °C caused a drop in the yield of the triphenylamine. It was noticed that higher yields were achieved when the transformation was conducted at higher temperature. Indeed, boosting the temperature to 130 °C led to a considerable improvement, with 82% yield of the desired product being recorded after 2 h. This value could be enhanced to 95% when the reaction was conducted at 140 °C. Extending the reaction temperature to 150 °C was noticed to be worthless, as the yield of 2-(diphenylamino)phenol was not upgraded considerably (Fig. 8).
Since the ring-opening reaction of benzoxazole with iodobenzene to generate 2-(diphenylamino)phenol was performed in solution phase, it is vital to carry out the leaching test. In several examples, the reaction might progress under partly homogeneous catalysis owing to the fact that some active sites were dissolved into the reaction solvent. In order to work out if homogeneous catalysis donated to the yield of 2-(diphenylamino)phenol in this transformation, a control experiment was consequently conducted. The reaction was performed in diethylene glycol at 140 °C under argon for 2 h, employing 2.5 equivalents of Cs2CO3, with 2 equivalents of iodobenzene, at benzoxazole concentration of 0.15 M, in the presence of 10 mol% catalyst. After the experiment, ethyl acetate was added to reduce the viscosity of the reaction mixture, and the CuFe2O4 superparamagnetic nanoparticles were isolated by magnetic decantation. The liquid phase was transferred to a new flask, and ethyl acetate was removed under vacuum, leaving the diethylene glycol solution. Fresh reagents were subsequently added to this solution, and the mixture was heated with magnetic stirring at 140 °C under argon for 2 h, with samples being taken during the experiment. It was noticed that no more 2-(diphenylamino)phenol was produced in the absence of the catalyst (Fig. 9). These observations verified that the ring-opening of benzoxazole with iodobenzene to generate 2-(diphenylamino)phenol could only progress in the presence of the solid CuFe2O4 superparamagnetic nanoparticles.
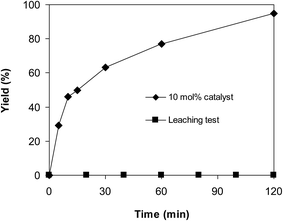 | ||
| Fig. 9 Leaching assessment verified that 2-(diphenylamino)phenol was not generated in the absence of CuFe2O4 superparamagnetic nanoparticles. | ||
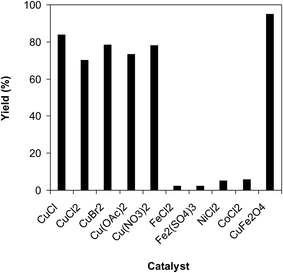
To spotlight the superiority of utilizing the CuFe2O4 superparamagnetic nanoparticles for the ring-opening reaction of benzoxazole with iodobenzene to generate 2-(diphenylamino)phenol, the activity of this catalyst was compared with many popular homogeneous catalysts such as CuCl, CuCl2, CuBr2, Cu(OAc)2, Cu(NO3)2, FeCl2, Fe2(SO4)3, NiCl2, and CoCl2. The reaction was carried out in diethylene glycol at 140 °C under argon for 2 h, employing 2.5 equivalents of Cs2CO3, with 2 equivalents of iodobenzene, at benzoxazole concentration of 0.15 M, in the presence of 10 mol% catalyst. Experimental data showed that FeCl2, Fe2(SO4)3, NiCl2, and CoCl2 were almost inactive for this transformation, with less than 5% yield of 2-(diphenylamino)phenol being recorded after 2 h. Several common copper salts expressed high catalytic activity for the generation of 2-(diphenylamino)phenol via the ring-opening reaction. The reaction utilizing CuCl catalyst progressed to 84% yield after 2 h, while 70% yield was observed for that using CuCl2 catalyst. This value could be upgraded to 78% in the presence of CuBr2 catalyst. Similarly, the Cu(NO3)2-catalyzed ring-opening reaction of benzoxazole with iodobenzene afforded 78% yield of 2-(diphenylamino)phenol after 2 h. Cu(OAc)2 emerged to be less active for the transformation than Cu(NO3)2, though 73% yield of the expected product was detected after 2 h (Fig. 10). It should be noted that the reaction utilizing CuFe2O4 superparamagnetic nanoparticles as catalyst proceeded to 95% yield after 2 h. Once again, under the optimized reaction conditions, the CuFe2O4 superparamagnetic nanoparticles offered better performance than other solid catalysts, with 95% yield of 2-(diphenylamino)phenol being recorded after 2 h (Fig. 11).
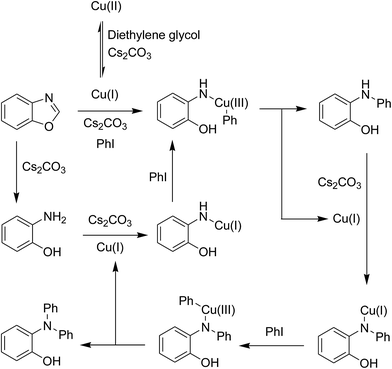
As previously mentioned, the ring-opening reaction proceeded readily in the presence of several copper salts as catalyst. However, replacing these copper-based catalysts by iron salts, less than 5% yield of 2-(diphenylamino)phenol was detected. Moving to heterogeneous catalysts, nano Fe2O3, nano CoFe2O4, nano NiFe2O4, and Fe-MOFs exhibited no activity for the transformation, while the reaction using nano CuFe2O4 afforded 95% yield. These data would verify that copper species on the nano CuFe2O4 were active for the ring-opening reaction. To receive perception into the pathway of the reaction between benzoxazole and iodobenzene to generate 2-(diphenylamino)phenol, some control experiments were then performed. In the first experiment, benzoxazole was heated with 2.5 equivalents of Cs2CO3 in diethylene glycol at 140 °C under argon for 2 h, in the presence of 10 mol% catalyst. It was noted that 95% of benzoxazole was converted to 2-aminophenol under these conditions. After that, 2 equivalents of iodobenzene were added, and the reaction mixture was heated at 140 °C under argon for additional 2 h. The experiment afforded 39% yield of 2-(diphenylamino)phenol. In the second experiment, the reaction between 2-aminophenol and iodobenzene was conducted under similar conditions. Interestingly, 41% yield of 2-(diphenylamino)phenol was recorded for this transformation. These data suggested that the nano CuFe2O4-catalyzed reaction between benzoxazole and iodobenzene to generate 2-(diphenylamino)phenol would proceed via both pathways: (1) the ring-opening of benzoxazole to form 2-aminophenol, following by Ullmann-type coupling with iodobenzene, and (2) concerted ring-opening of benzoxazole/Ullmann-type coupling in one step (Scheme 3). However, further studies would be necessary to elucidate the mechanism of this transformation.
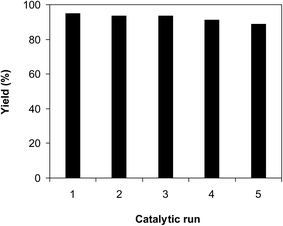
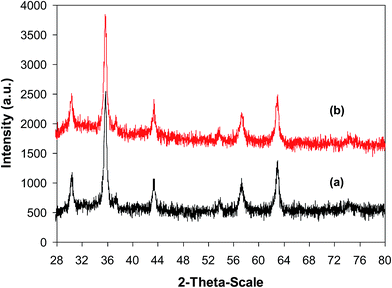
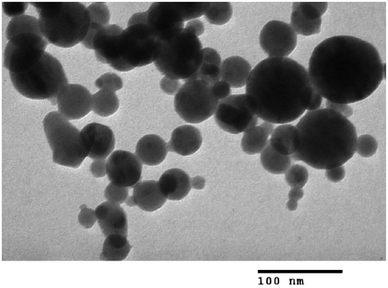
As previously mentioned, the CuFe2O4 superparamagnetic nanoparticles displayed better catalytic efficiency than a series of homogeneous and heterogeneous catalysts. To spotlight the superiority of utilizing these nanoparticles as catalyst for the ring-opening reaction of benzoxazole with iodobenzene to generate 2-(diphenylamino)phenol, the straightforwardness of catalyst recycling was then studied. The superparamagnetic nanoparticles were consequently investigated for reusability for the transformation over 5 consecutive runs. The reaction was conducted in diethylene glycol at 140 °C under argon for 2 h, employing 2.5 equivalents of Cs2CO3, with 2 equivalents of iodobenzene, at benzoxazole concentration of 0.15 M, in the presence of 10 mol% catalyst. After the first run, ethanol was added to reduce the viscosity of the mixture, and the catalyst was subsequently isolated by decantation utilizing a permanent magnet. The recovered catalyst was washed thoroughly with water, ethanol, ethyl acetate, and ether to eliminate any physisorbed compounds, and then heated at 150 °C under vacuum on a Shlenk line for 6 h. New catalytic transformation was afterwards conducted utilizing the recovered catalyst. Experimental data revealed that it was possible to reutilize the catalyst for the ring-opening reaction of benzoxazole with iodobenzene without a remarkable decline in catalytic activity. Certainly, 89% yield of 2-(diphenylamino)phenol was still obtained in the 5th run (Fig. 12). In addition, XRD analysis of the recovered CuFe2O4 superparamagnetic nanoparticles displayed that the catalyst structure was preserved during the course of the experiment (Fig. 13). TEM (Fig. 14) micrograph of the recovered catalyst indicated that the particles were still in nanosize range, though a slight agglomeration was recorded. Elemental analysis with AAS verified that the Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe molar ratio in the recovered catalyst was approximately 1
Fe molar ratio in the recovered catalyst was approximately 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, corresponding to CuFe2O4 structure.
2, corresponding to CuFe2O4 structure.
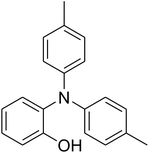
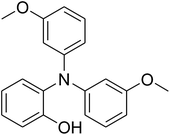
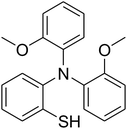
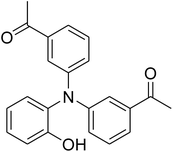
The research scope was afterwards expanded to the synthesis of different triphenylamines via ligand-free selective ring-opening reaction utilizing the CuFe2O4 catalyst (Table 1). The reaction was performed in diethylene glycol at 140 °C under argon for 2 h, employing 2.5 equivalents of Cs2CO3, with 2 equivalents of iodobenzene, at benzoxazole concentration of 0.15 M, in the presence of 10 mol% catalyst. After the experiment, triphenylamines were purified by column chromatography utilizing silica gel. Following this procedure, 2-(diphenylamino)phenol was achieved in 92% isolated yield via the ring-opening reaction of benzoxazole with iodobenzene (Entry 1). Moreover, to demonstrate the potential of industrial application, a gram-scale reaction was conducted with 0.71 g of benzoxazole and 3.67 g of iodobenzene. Under optimized reaction conditions, 1.36 g of 2-(diphenylamino)phenol was obtained, affording 87% isolated yield. Iodoarenes bearing a substituent were noticed to be less reactive in the reaction with benzoxazole. The ring-opening of benzoxazole with 1-iodo-2-methylbenzene progressed to 87% yield of 2-(dio-tolylamino)phenol (Entry 2), while 91% of 2-(dip-tolylamino)phenol yield was obtained for the case of 1-iodo-4-methylbenzene (Entry 3). 1-Iodo-4-methoxybenzene exhibited lower reactivity than 1-iodo-4-methylbenzene, though 65% yield of 2-(bis(4-methoxyphenyl)amino)phenol (Entry 4) was still obtained. Similarly, 2-(bis(2-methoxyphenyl)amino)phenol (Entry 5) was produced in 63% yield, while 68% yield of 2-(bis(3-methoxyphenyl)amino)phenol (Entry 6) was achieved via the reaction between benzoxazole and 1-iodo-3-methoxybenzene. 2,5-Dimethylbenzo[d]oxazole was also reactive towards this transformation, producing 2-(diphenylamino)-4-methylphenol (Entry 7), 2-(dip-tolylamino)-4-methylphenol (Entry 8), and 2-(bis(3-methoxyphenyl)amino)-4-methylphenol (Entry 9) in 86%, 88%, and 72% yields, respectively.
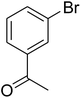
Inspired by these results, we then investigated the ring-opening reaction of benzothiazole with iodobenzene in the presence of the CuFe2O4 catalyst. Indeed, the conversion of benzothiazole to corresponding triphenylamines was not previously reported in the literature. It was noticed that benzothiazole was less reactive than benzoxazole, and the reaction time had to be extended to 16 h. The reaction between benzothiazole and iodobenzene produced 2-(diphenylamino)benzenethiol in 79% yield (Entry 10). Similarly, 2-(bis(2-methoxyphenyl)amino)benzenethiol (Entry 11) was generated in 85% yield, while 2-(bis(4-methoxyphenyl)amino)benzenethiol (Entry 12) was formed in 84% yield. The research scope was also expanded to the ring-opening reaction using aryl bromides and aryl chlorides. Certainly, these halides were not previously employed for this transformation. Aryl bromides were found to be reactive towards the ring-opening transformation. Utilizing the CuFe2O4 catalyst, 1,1′-(3,3′-(2-hydroxyphenylazanediyl)bis(3,1-phenylene))diethanone (Entry 13) was formed in 58% yield from the reaction of benzoxazole and 1-(3-bromophenyl)ethanone. Similarly, the reaction between benzothiazole and 1-bromo-3-methoxybenzene produced 2-(bis(3-methoxyphenyl)amino)benzenethiol (Entry 14) in 68% yield, while 77% yield of 2-(bis(2-methoxyphenyl)amino)benzenethiol (Entry 15) was achieved for the case of 1-bromo-2-methoxybenzene. Interestingly, 1-chloro-4-methoxybenzene was reactive towards the reaction, forming 2-(bis(4-methoxyphenyl)amino)benzenethiol (Entry 16) in 61% yield.
4. Conclusions
CuFe2O4 superparamagnetic nanoparticles were utilized as an effective recyclable heterogeneous catalyst for the synthesis of triphenylamines via the ligand-free selective ring-opening of benzoxazoles or benzothiazoles with iodoarenes. The nature of the base and the solvent expressed a notable impact on the transformation, and the combination of Cs2CO3 as the base and diethylene glycol as the solvent should be the best option. The CuFe2O4 nano catalyst was more active towards the ring-opening reaction than nano NiFe2O4, nano CoFe2O4, and nano Fe2O3, as well as several solid catalysts such as Cu(BDC), Cu(OBA), MOF-199, Cu2(BDC)2(DABCO), Cu2(BPDC)2(BPY), Co-ZIF-67, Ni2(BDC)2(DABCO), and MIL-101. The CuFe2O4 nano catalyst also displayed better performance than numerous homogeneous catalysts. After each catalytic run, it was possible to separate the nano CuFe2O4 by utilizing a magnet, and the recovered catalyst was reutilized many times while its efficiency could be maintained. The fact that the synthesis of triphenylamines has been very limited in the literature, and that these structures could be generated using a recyclable heterogeneous catalyst would attract attention from the chemical industry.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We would like to thank the Viet Nam National Foundation for Science and Technology Development (NAFOSTED) for financial support under Project code 104.01-2015.35 (Contract number 14/2016/104/HĐTN).References
- A. Chowdhury and P. S. Mukherjee, J. Org. Chem., 2015, 80, 4064–4075 CrossRef CAS PubMed.
- G. Wu, F. Kong, Y. Zhang, X. Zhang, J. Li, W. Chen, W. Liu, Y. Ding, C. Zhang, B. Zhang, J. Yao and S. Dai, J. Org. Chem., 2014, 118, 8756–8765 CAS.
- C. Fan, C. Ye, X. Wang, Z. Chen, Y. Zhou, Z. Liang and X. Tao, Macromolecules, 2015, 48, 6465–6473 CrossRef CAS.
- Z. Fan, H. Zhao, N. Li, Y. Quan, Q. Chen, S. Ye, S. Li, Y. Wang, Q. Fan and W. Huang, ACS Appl. Mater. Interfaces, 2015, 7, 9445–9452 CAS.
- N. Kaneza, J. Zhang, H. Liu, P. S. Archana, Z. Shan, M. Vasiliu, S. H. Polansky, D. A. Dixon, R. E. Adams, R. H. Schmehl, A. Gupta and S. Pan, J. Phys. Chem. C, 2016, 120, 9068–9080 CAS.
- H. B. Goodbrand and N.-X. Hu, J. Org. Chem., 1999, 64, 670–674 CrossRef CAS.
- J. P. Wolfe, H. Tomori, J. P. Sadighi, J. J. Yin and S. L. Buchwald, J. Org. Chem., 2000, 65, 1158–1174 CrossRef CAS PubMed.
- J. Hassan, M. Sevignon, C. Gozzi, E. Schulz and M. Lemaire, Chem. Rev., 2002, 102, 1359–1469 CrossRef CAS PubMed.
- I. P. Beletskaya and A. V. Cheprakov, Coord. Chem. Rev., 2004, 248, 2337–2364 CrossRef CAS.
- J. Safaei-Ghomi, Z. Akbarzadeh and A. Ziarati, RSC Adv., 2014, 4, 16385–16390 RSC.
- A. M. Shaikh, B. K. Sharma, S. Chacko and R. M. Kamble, RSC Adv., 2016, 6, 60084–60093 RSC.
- D. Kim, K. Yoo, S. E. Kim, H. J. Cho, J. Lee, Y. Kim and M. Kim, J. Org. Chem., 2015, 80, 3670–3676 CrossRef CAS PubMed.
- Y. He, J. Mao, G. Rong, H. Yan and G. Zhang, Chem.–Asian J., 2016, 11, 1672–1676 CrossRef CAS PubMed.
- W. J. Stark, P. R. Stoessel, W. Wohlleben and A. Hafner, Chem. Soc. Rev., 2015, 44, 5793–5805 RSC.
- R. Purbia and S. Paria, Nanoscale, 2015, 7, 19789–19873 RSC.
- R. K. Sharma, S. Sharma, S. Dutta, R. Zboril and M. B. Gawande, Green Chem., 2015, 17, 3207–3230 RSC.
- D. Wang, Y. Sun, Y. Sun, J. Huang, Z. Liang, S. Li and L. Jiang, Nanoscale, 2017, 9, 7727–7733 RSC.
- C. Ray and T. Pal, J. Mater. Chem. A, 2017, 5, 9465–9487 CAS.
- A. Ghorbani-Choghamarani, A. A. Derakhshan, M. Hajjami and L. Rajabi, RSC Adv., 2016, 6, 94314–94324 RSC.
- W. Yang, L. Wei, F. Yi and M. Cai, Catal. Sci. Technol., 2016, 6, 4554–4564 CAS.
- R. K. Rai, K. Gupta, D. Tyagi, A. Mahata, S. Behrens, X. Yang, Q. Xu, B. Pathak and S. K. Singh, Catal. Sci. Technol., 2016, 6, 5567–5579 CAS.
- S. Cao, F. F. Tao, Y. Tang, Y. Li and J. Yu, Chem. Soc. Rev., 2016, 45, 4747–4765 RSC.
- J. Lemus, J. Bedia, L. Calvo, I. L. Simakova, D. Y. Murzin, B. J. M. Etzold, J. J. Rodriguez and M. A. Gilarranz, Catal. Sci. Technol., 2016, 6, 5196–5206 CAS.
- L. Hu, R. Zhang and Q. Chen, Nanoscale, 2014, 6, 14064–14105 RSC.
- N. T. S. Phan, C. S. Gill, J. V. Nguyen, Z. J. Zhang and C. W. Jones, Angew. Chem., Int. Ed., 2006, 45, 2209–2212 CrossRef CAS PubMed.
- K. V. S. Ranganath and F. Glorius, Catal. Sci. Technol., 2011, 1, 13–22 CAS.
- F. M. Moghaddam, G. Tavakoli, F. Latifi and B. Saeednia, Catal. Commun., 2016, 75, 37–41 CrossRef CAS.
- B. Mohan, H. Kang and K. H. Park, Catal. Commun., 2016, 85, 61–65 CrossRef CAS.
- B. Mohan and K. H. Park, Appl. Catal., A, 2016, 519, 78–84 CrossRef CAS.
- M. A. E. A. A. A. El-Remaily and H. A. Hamad, J. Mol. Catal. A: Chem., 2015, 404–405, 148–155 CrossRef.
- R. Rameshbabu, N. Kumar, A. Karthigeyan and B. Neppolian, Mater. Chem. Phys., 2016, 181, 106–115 CrossRef CAS.
- P. B. Thale and P. N. B. G. S. Shankarling, RSC Adv., 2016, 6, 52724–52728 RSC.
- M. A. E. A. A. El-Remaily, A. M. Abu-Dief and R. M. El-Khatib, Appl. Organomet. Chem., 2016, 30, 1022–1029 CrossRef.
- E. Eidi, M. Z. Kassaee and Z. Nasresfahani, Appl. Organomet. Chem., 2016, 30, 561–565 CrossRef CAS.
- D. Yang, X. Zhu, W. Wei, N. Sun, L. Yuan, M. Jiang, J. You and H. Wang, RSC Adv., 2014, 4, 17832–17839 RSC.
- D. Yang, X. Zhu, W. Wei, M. Jiang, N. Zhang, D. Ren, J. You and H. Wang, Synlett, 2014, 25, 0729–0735 CrossRef CAS.
- X. Zhu, D. Yang, W. Wei, M. Jiang, L. Li, X. Zhu, J. You and H. Wang, RSC Adv., 2014, 4, 64930–64935 RSC.
- D. Yang, B. An, W. Wei, M. Jiang, J. You and H. Wang, Tetrahedron, 2014, 70, 3630–3634 CrossRef CAS.
- L. Yao, Q. Zhou, W. Han and S. Wei, Eur. J. Org. Chem., 2012, 2012, 6856–6860 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra06168d |
| This journal is © The Royal Society of Chemistry 2017 |