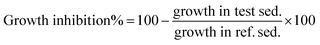Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis and ecotoxicological impact of ferrocene-derived amino-phosphonates using a battery of bioassays†
J. Lewkowski *a,
R. Karpowicza,
M. Morawskaa,
P. Rychter*b,
D. Rogaczb,
K. Lewickab and
P. Dobrzyński
*a,
R. Karpowicza,
M. Morawskaa,
P. Rychter*b,
D. Rogaczb,
K. Lewickab and
P. Dobrzyński bc
bc
aDepartment of Organic Chemistry, Faculty of Chemistry, University of Łódź, Tamka 12, 91-403 Łódź, Poland. E-mail: jlewkow@uni.lodz.pl
bInstitute of Chemistry, Environmental Protection and Biotechnology, Jan Długosz University in Częstochowa, 13/15 Armii Krajowej Av., 42-200 Częstochowa, Poland. E-mail: p.rychter@ajd.czest.pl
cCentre of Polymer and Carbon Materials, Polish Academy of Sciences, M. Curie-Skłodowskiej 34, 41-819 Zabrze, Poland
First published on 4th August 2017
Abstract
Due to the fact that α-aminophosphonates are structurally analogous to α-amino acids, the search for new biologically active agents based on the chemistry of these compounds has stimulated growing interest. Although there is increasing attention focused on the various applications of these compounds, there is poor information about their ecotoxicological impact on the environment. In this study, ferrocene-derived aminophosphonates have been synthesized and their environmental impact on selected plants, namely Avena sativa and Raphanus sativus as well as on the bacteria Vibrio fischeri, and the crustacean Heterocypris inconguens, has been assessed and their ecotoxicological profile determined.
Introduction
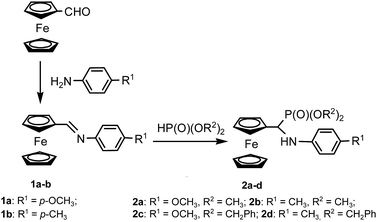
Aminophosphonic systems have found application in the area of environmental protection, and have been successfully applied as herbicides for over 20 years (e.g. glyphosate), being especially known as the inhibitors of various plant enzymes.1 New examples are still being investigated with respect to their potential application in agrochemistry and their synthesis and the assessment of their bioactivity in plants have been reported quite recently.2–4 Ferrocene derivatives have attracted attention since the discovery of their fascinating properties,5 providing prospects for their application as redox controlled molecular switches. Their biological properties are also very promising. Jaouen et al.6 published the first report of a tamoxifen-based ferrocene derivative, which was found to be an effective estrogen receptor blocker. Studies on the potential applications of ferrocene derivatives as anticancer hormonal agents were described later on.7,8 The antimalarial activity of ferrocene-derived artemisinin, artemisitene or quinine were also reported.9For us, the most interesting reports were on some attempts to apply ferrocene derivatives in agriculture,10,11 describing their herbicidal,10 antifungal,11 larvicidal11 or plant regulatory11 properties. The idea of synthesizing aminophosphonic derivatives of ferrocene arose almost twenty years ago12a and since that time, we have synthesized a series of ferrocene-derived aminophosphonates and aminophosphonates12 and tried to investigate their molecular properties.12e,f
In view of the above, nobody can deny the potential usefulness of aminophosphonates as biological agents. According to REACH regulations (Registration, Evaluation, Authorization and Restriction of Chemicals), there is an urgent need to follow the environmental behaviour of new compounds to avoid unexpected, harmful effects on the environment. Proposed by European Chemicals Agency (ECHA), ecotoxicological testing is an essential tool for evaluating the effects of chemicals on the environment, and even more, it is required for REACH registration of new chemical compounds. It has to be taken into consideration that newly synthesized compounds, especially potentially bioactive α-aminophosphonic derivatives, may cause environmental risk. Therefore, the objective of this study is to evaluate the phytotoxicity of four novel, ferrocene-derived aminophosphonates, namely: dimethyl N-(4-methoxyphenyl)amino(ferrocenyl)methylphosphonate (2a), dimethyl N-(4-methylphenyl)amino(ferrocenyl)-methylphosphonate (2b), dibenzyl N-(4-methoxy-phenyl)-amino(ferrocenyl)methylphosphonate (2c) and dibenzyl N-(4-methylphenyl)amino(ferrocenyl)methylphosphonate (2d) against dicotyledonous radish (Raphanus sativus) and monocotyledonous oat (Avena sativa). Ecotoxicological assessment was also performed using Vibrio fischeri bacteria and Heterocypris incongruens crustaceans.
Experimental
Chemistry – general
All solvents (POCh, Gliwice, Poland) were routinely distilled and dried prior to use. Amines: p-toluidine and p-anisidine, dimethyl and dibenzyl phosphites, and ferrocenecarboxaldehyde (Aldrich, Poznań, Poland) were used as received. NMR spectra were recorded on a Bruker Avance III 600 MHz, operated at 600 MHz (1H NMR), 150 MHz (13C NMR) and 243 MHz (31P NMR). Phosphoric acid was used as the external standard for 31P NMR, while 1H and 13C NMR spectra were set on deuterated solvents. Elemental analyses were carried out at the Centre for Molecular and Macromolecular Science of the Polish Academy of Science in Łódź, Poland (2a, 2b, 2d) and in the Laboratory of Molecular Spectroscopy, Faculty of Chemistry, University of Łódź (2c).Synthesis of Schiff bases 1a–b
Ferrocenecarboxaldehyde (2.14 g, 10 mmol) was dissolved in methanol dried over CaH2 (20–30 mL), and to this solution, amine (10 mmol, 1.07 g of p-toluidine, or 1.23 g of p-anisidine) was added. The mixture was stirred at room temperature for 24 h, then the solvent was evaporated and the residue dried under vacuum to obtain the crude Schiff bases 1a–b, which were crystallized from hexane–ethyl acetate 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,12h and were used as soon as possible for further conversions.
1,12h and were used as soon as possible for further conversions.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, 1H), 7.14 (approx. d, J = 8.6 Hz, p-C6H4, 2H), 6.91 (approx. d, J = 8.6 Hz, p-C6H4, 2H), 4.79 (s, Hferr, 2H), 4.46 (s, Hferr, 2H), 4.24 (s, Cp, 5H); 3.83 (s, OCH3, 3H).
N, 1H), 7.14 (approx. d, J = 8.6 Hz, p-C6H4, 2H), 6.91 (approx. d, J = 8.6 Hz, p-C6H4, 2H), 4.79 (s, Hferr, 2H), 4.46 (s, Hferr, 2H), 4.24 (s, Cp, 5H); 3.83 (s, OCH3, 3H).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, 1H), 7.18 (approx. d, J = 7.5 Hz, p-C6H4, 2H), 6.91 (approx. d, J = 7.5 Hz, p-C6H4, 2H), 4.80 (s, Hferr, 2H), 4.47 (s, Hferr, 2H), 4.24 (s, Cp, 5H); 2.36 (s, CH3, 3H).
N, 1H), 7.18 (approx. d, J = 7.5 Hz, p-C6H4, 2H), 6.91 (approx. d, J = 7.5 Hz, p-C6H4, 2H), 4.80 (s, Hferr, 2H), 4.47 (s, Hferr, 2H), 4.24 (s, Cp, 5H); 2.36 (s, CH3, 3H).General procedure for the preparation of aminophosphonates 2a–d
To a hot solution of an imine (10 mmol) in acetonitrile (30 mL), a solution of dialkyl phosphite (10 mmol) in acetonitrile (10 mL) was added, followed by 3–5 drops of trifluoroacetic acid. The obtained solution was heated at 78–80 °C for 48 h, then evaporated and the residue was chromatographed on silica gel (ethyl acetate–hexane 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The obtained products were finally purified by crystallization from ethyl acetate–hexane (the suspension of the product in hexane was heated and then hot ethyl acetate was added until dissolution, then filtered, cooled and kept in a freezer for a couple of days). Crystals were collected by suction to give products 2a–d as dark yellow crystals.
1). The obtained products were finally purified by crystallization from ethyl acetate–hexane (the suspension of the product in hexane was heated and then hot ethyl acetate was added until dissolution, then filtered, cooled and kept in a freezer for a couple of days). Crystals were collected by suction to give products 2a–d as dark yellow crystals.
Plant growth test of new synthesized compounds
The plant growth test of aminophosphonates 2a–d was performed under laboratory conditions, following the OECD 208 Guideline for Terrestrial Plants Growth Test for oat (Avena sativa) as a monocotyledonous plant and radish (Raphanus sativus L. subvar. radicula Pers.), a dicotyledonous plant.13According to the mentioned OECD 208 standard, the plant growth tests of compounds 2a–d were carried out in sandy soil having the following parameters: granulometric composition of soil—77% sand, 16% dust and loam, organic carbon content of approx. 1.6%, pH (KCl) equal to 6.6.
Tests were carried out in polypropylene pots (diameter of 90 mm and capacity of 300 cm3), which were filled with the control soil or with the soil mixed with the tested compounds 2a–d added at the following concentrations: 1, 10, 100, 200, 400, 800 and 1000 mg kg−1 of soil dry weight. Each concentration was done in triplicate (3 pots for oat, 3 pots for common radish).
Twenty seeds of each of the selected plant species were sown into the soil. Seeds originated from the same source. Plants were grown for 14 days under controlled conditions: a constant humidity content at the level required for the plants (70% field water capacity), temperature (20 ± 2 °C), constant light intensity (7000 lux), were maintained in the system for 16 h per day and 8 h per night.
The evaluation of the phytotoxicity of the studied aminophosphonates 2a–d at applied concentrations was made by comparing the germination and dry weight of the control plants sprouts (seedlings) with the germination and dry and fresh plant sprouts grown in the soil with an admixture of given amounts of the tested compounds. In order to determine the growth inhibition of the roots and shoots of selected plants, the height of the shoot and length of root were measured as described by Wang et al.14a The length of plants was defined as the length from the tip of the longest leaf to the base of the culms, while root length was measured from the tip of the longest root to the root–shoot junction.
The inhibition ratio was calculated according to the method given by Pawlowska and Biczak,14b namely, length in the control group − length in the treatment group × 100%/length of the control group.
Effective concentration EC50 for fresh plant matter was calculated using GraphPad Prism software (Version 7, GraphPad Software, Inc., La Jolla, CA 92037, USA).
The dry weights of tested plants were measured after drying at 75 °C until constant weight.
Values of the no observed effect concentration (NOEC) and the lowest observed effect concentration (LOEC) of the compounds under study, 2a–d, were determined. The visual evaluation of the phytotoxicity of aminophosphonates 2a–d at applied concentrations was performed by digital photography. Obtained pictures were analyzed in terms of any type of damage to the tested plants, such as their growth inhibition, chlorosis, and necrosis. Tests were carried out three times for each sample.
Pigment assay
The photosynthetic pigments content was determined according to the method reported by Oren et al.15 Briefly, 200 mg of fresh leaves were thoroughly homogenized in 20 mL of 80% acetone by means of a cooled mortar, and centrifuged. The content of chlorophyll a, chlorophyll b and carotenoids was calculated based on the absorbance at wavelength 470, 647 and 664 nm. The contents of photosynthetic pigments were expressed in mg g−1 of dry weight.Microtox® toxicity assay
The detailed procedure for the Microtox Toxicity Assay has been described previously by Lewkowski et al.16 The method is based on the analysis of the light emission reduction of luminescent bacteria (Vibrio fischeri) under toxic stress. The tests were carried out in a Microtox® M500 analyzer, according to the 1992 Microtox® Manual. The Microtox® Solid-Phase Test (MSPT) was adapted from the report of Doe et al.17The MSPT procedure allowed the test organisms to come into direct contact with the solid sample in an aqueous suspension of the test sample. Thus, it was possible to detect the toxicity due to the insoluble solids that were not in the solution. All materials and reagents were purchased from MODERNWATER (New Castle, DE, USA). Toxicity was determined by using the marine luminescent bacterium, Vibrio fischeri, naturally adapted to a saline environment. Briefly, the bacteria were regenerated with 1 mL of reconstitution solution (0.01%) and were placed in the reagent well of the Microtox®. A suspension of 7 g of the sediment was prepared in 35 mL of a Solid Phase Diluent (3.5% NaCl) and was magnetically stirred for 10 min. Then, a series of dilutions were made and bacteria (approx. 1 × 106 cell per mL per assay) were exposed to these dilutions and to a blank (3.5% NaCl solution) for 20 min. After filtration, the light output of the supernatant containing exposed bacteria was measured after 5 min with a Microtox® Analyzer 500. Inhibition was calculated as the concentration of compound loaded to sediment (mg kg−1) that caused a 50% reduction in the light emitted by the bacteria, and EC50 along with 95% confidence limit were determined by the software provided with the Analyzer.
Ostracod test kit
The ecotoxicity evaluation of the synthesized compounds was performed in a short term contact test using the Ostracodtoxkit F™ provided by MicroBiotests Inc. (Gent, Belgium). This direct sediment contact bioassay was performed in multiwell test plates using neonates of the benthic ostracod crustacean Heterocypris incongruens hatched from cysts.18After 6 days of contact with the sediment (or soil), the percentage mortality and the growth of the crustaceans were determined and compared to the results obtained in a non-treated reference sediment (soil). Briefly, according to the manual of the Ostracodtoxkit test, the cysts (Heterocypris incongruens) were transferred into a Petri dish filled with 10 mL of standard fresh water (reconstituted water) and were incubated at 25 °C for 52 h under continuous illumination (approx. 3000–4000 lux). After 48 h of cyst incubation, pre-feeding of the freshly hatched ostracods was performed with algae (spirulina-powder) provided in the test kit. After hatching, before feeding with algal food suspension, the length measurements of the ostracod neonates were done. Algae (Selenastrum capricornutum) used as feed in the test plate was reconstituted according to the manufacturer's procedures. Each well of a test plate was filled in the following order: 2 mL standard freshwater, 2500 μL of sediment (soil) treated and non-treated for comparison (blank), 2 mL already prepared algal suspension, 10 ostracods. The test plates were covered with Parafilm® and closed with a lid. Then multi wall plates were incubated at 25 °C in darkness for 6 days. After 6 days of exposure, the ostracods were recovered from the multiwells to determine the percentage mortality. To calculate the growth inhibition of surviving organisms, their length measurements were determined. The mortality of test organisms was determined in six replicates. The measurement of length was carried out by means of a micrometric strip placed on the bottom of a glass microscope plate. Growth inhibition (GI) of H. incongruens in the test sediment was calculated as follows:
Statistical analysis
The significance of the obtained results was evaluated using the analysis of variance (ANOVA). The least significant difference (LSD) values at a confidence level of 95% were computed using the Tukey test. Moreover, the mean standard deviations were determined and plotted in diagrams.Results and discussion
Synthesis of studied Schiff bases 1a–b and aminophosphonates 2a–d
Schiff bases 1a–b were synthesized following the previously published procedure, by simply mixing ferrocenecarboxaldehyde with the appropriate amine in dry methanol and stirring them at room temperature for 24 hours.12h This procedure gave imines 1a–b in over 90% yield. Their identities were confirmed by melting point measurement and 1H NMR spectral data, which were compared with the previously obtained data.12h The 1H NMR spectra showed the diagnostic singlet at around 8.3 ppm, corresponding to a proton of the azomethine group (–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–) and the characteristic set of signals of mono-substituted ferrocene – two singlets at 4.7 and 4.5 ppm of a substituted ring and the third, at ca. 4.2 ppm, corresponding to unsubstituted cyclopentadiene.
N–) and the characteristic set of signals of mono-substituted ferrocene – two singlets at 4.7 and 4.5 ppm of a substituted ring and the third, at ca. 4.2 ppm, corresponding to unsubstituted cyclopentadiene.
Ferrocene-derived aminophosphonates 2a–d were synthesized by the addition of methyl or benzyl phosphite to the azomethine bond of the corresponding Schiff bases 1a–b in acetonitrile. The conversion was monitored by means of TLC and 31P NMR, and after 48 hours the reaction was complete. The resulting crude aminophosphonates 2a–d were purified by column chromatography followed by crystallization. They were obtained in 50–70% yields. (Scheme 1). The aminophosphonate 2b has already been described by us while testing the Kabachnik–Fields reaction in non-classical conditions;12j the others, i.e. 2a and 2c–d, to our best knowledge, have never been described. Their identity has been verified by means of 1H, 13C and 31P NMR spectroscopy. The 1H NMR spectra of aminophosphonates 2a–d showed diagnostic signals typical of aminophosphonates, i.e. a doublet of doublets attributable to a proton from a HCP group, which couples with a phosphorus nucleus and with a proton of a NH group. Sets of signals were also observed, which are typical for monosubstituted ferrocenes, i.e. two multiples corresponding to protons of a substituted ring between 4 and 4.5 ppm and a singlet of 5 protons of an unsubstituted ring. Two doublets of OMe groups linked to phosphorus (2a–b) and two ABX systems of benzyloxy groups (2c–d) were also observed.
13C NMR spectra of aminophosphonates 2a–d showed a doublet at around 50 ppm having a coupling constant around 150 Hz corresponding to a CP bond, which is characteristic of aminophosphonates. Ferrocenyl rings also gave characteristic signals of their carbons: a signal at ca. 85 ppm of a quaternary carbon coupled with phosphorus, a sharp signal of a Cp ring as well as doublets attributed to carbons neighboring an aminomethylphosphonic group (at around 70–65 ppm). Spectra of aminophosphonates 2a–d are shown in ESI, Fig. S1–S14.†
The purity of prepared compounds 2a–d was confirmed by the elemental analysis and melting point measurements.
Ecotoxicological activity
It is commonly known that natural amino acid analogues, substituted α-aminophosphonate derivatives, are crucial intermediates in the synthesis of various biologically active compounds, which have found wide range applications mostly in medicinal chemistry. Besides the diverse biological activities like enzyme inhibitors, antibiotics, or anticancer agents, substituted α-aminophosphonate derivatives have stimulated great interest as antiviral and antifungal agents and they have been extensively used as insecticides and herbicides.19–23 Therefore, considering the biological values and possibilities in the chemical modification of α-amino-phosphonates, the growing concern and the development of new organophosphorus compounds are still of great interest.24–30Plant growth test – germination, growth inhibition, dry and fresh matter
To evaluate the impact of the tested substances on selected plants, the Plant Growth Test according to OECD 208 Standard has been used as a representative, reliable and well accepted method. It has already been successfully used by the authors to evaluate the toxicity effect of such substances like ionic liquids, thiophene-derived aminophosphonic derivatives or furan-derived aminophosphonic acids, N-aryl furaldimines and 5-nitrofuraldimine.31–33The percentage growth inhibition (GI%) of the shoot and root of examined plants treated with samples of ferrocene-derived aminophosphonates 2a–d are presented in Table 1. The percentage growth inhibition (GI%) of shoots for both radish and oat seedlings increased with growing concentration of examined samples 2a–d in soil. Comparing the percentage growth inhibition of oat seedlings for all tested compounds, it was observed that dimethyl aminophosphonates 2a and 2b much more negatively affect the growth of this plant, when compared to their dibenzyl analogues 2c and 2d (Table 1). The growth inhibition values of compounds 2a and 2b were 40 and 55% respectively, and the higher impact was revealed for the aminophosphonate 2b. Compound 2c at the highest concentration, i.e. 1000 mg per kg of soil dry weight, caused the inhibition effect at the level of 19%. The percentage growth inhibition of oat for dibenzyl N-(4-methylphenyl)amino-(ferrocenyl)methylphosphonate 2d, even at the highest applied concentration, did not exceed 1%; therefore, this compound is considered non-toxic to the upper, green part of the oat seedlings. It is worth mentioning that N-(4-methylphenyl)amino-(ferrocenyl)methylphosphonate 2b is over 40 times more toxic to oat shoots when compared to the dibenzyl analogue 2d (for 400, 800 and 1000 mg kg−1). Comparing the samples 2a and 2c, it was noticed that the dimethyl ester 2a was almost two times more toxic (at the highest concentration, 1000 mg kg−1).
| Concentration [mg kg−1 of soil d.w.] | Inhibition biomarkers [%] | |||||||
|---|---|---|---|---|---|---|---|---|
| Oat | Radish | |||||||
| 2a | 2b | 2c | 2d | 2a | 2b | 2c | 2d | |
| Shoot height | ||||||||
| Control | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 0.47 ± 0.15 | 0.04 ± 0.10 | 0.29 ± 2.28 | 0.05 ± 1.28 | 2.06 ± 1.14 | 2.69 ± 0.94 | 0.65 ± 2.96 | 0.62 ± 2.88 |
| 10 | 0.97 ± 0.30 | 0.85 ± 0.23 | 0.97 ± 0.39 | 0.07 ± 0.99 | 3.44 ± 0.23 | 3.06 ± 0.23 | 1.29 ± 0.40 | 1.75 ± 0.18 |
| 100 | 1.57 ± 0.56 | 1.01 ± 2.01 | 1.05 ± 0.32 | 0.20 ± 0.92 | 6.18 ± 0.12 | 10.43 ± 0.27 | 1.35 ± 0.17 | 2.13 ± 0.24 |
| 200 | 1.88 ± 0.58 | 5.39 ± 3.51 | 1.12 ± 0.57 | 0.22 ± 0.97 | 20.17 ± 0.11 | 31.56 ± 0.12 | 1.42 ± 0.29 | 2.87 ± 0.25 |
| 400 | 2.09 ± 3.88 | 19.02 ± 2.15 | 7.08 ± 1.85 | 0.36 ± 1.85 | 42.54 ± 0.57 | 64.65 ± 1.92 | 6.45 ± 2.96 | 10.88 ± 0.12 |
| 800 | 6.62 ± 3.72 | 34.12 ± 3.18 | 13.65 ± 2.82 | 0.53 ± 1.82 | 63.44 ± 1.53 | 67.15 ± 0.78 | 23.87 ± 2.90 | 11.50 ± 1.56 |
| 1000 | 40.21 ± 3.21 | 55.37 ± 3.54 | 18.88 ± 2.42 | 0.86 ± 1.42 | x | x | 25.81 ± 1.12 | 16.38 ± 1.95 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| Root length | ||||||||
| Control | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 0.04 ± 0.08 | 7.07 ± 0.24 | 0.97 ± 0.57 | 0.04 ± 2.84 | 0.35 ± 0.71 | 2.09 ± 0.57 | 1.46 ± 0.72 | 0.68 ± 0.48 |
| 10 | 0.85 ± 0.33 | 11.92 ± 1.37 | 1.43 ± 0.21 | 0.09 ± 0.12 | 5.57 ± 1.11 | 6.26 ± 0.89 | 1.80 ± 1.00 | 1.69 ± 1.01 |
| 100 | 1.015 ± 2.80 | 23.07 ± 0.83 | 5.71 ± 1.68 | 0.17 ± 0.56 | 12.00 ± 1.87 | 9.22 ± 1.94 | 1.63 ± 1.27 | 1.89 ± 1.05 |
| 200 | 5.39 ± 2.38 | 24.13 ± 0.09 | 8.57 ± 2.87 | 0.19 ± 1.24 | 18.26 ± 2.13 | 11.30 ± 2.00 | 1.72 ± 1.50 | 2.70 ± 2.04 |
| 400 | 19.02 ± 2.51 | 24.63 ± 1.12 | 11.39 ± 1.37 | 0.22 ± 1.64 | 22.09 ± 2.62 | 18.61 ± 2.57 | 3.66 ± 2.06 | 9.19 ± 2.68 |
| 800 | 34.12 ± 3.47 | 25.53 ± 1.41 | 17.14 ± 1.44 | 0.45 ± 2.50 | 37.04 ± 2.42 | 75.65 ± 1.64 | 5.01 ± 2.00 | 29.05 ± 2.00 |
| 1000 | 55.37 ± 3.53 | 53.69 ± 1.43 | 22.43 ± 2.57 | 0.56 ± 1.67 | 84.35 ± 2.72 | 78.09 ± 2.00 | 9.24 ± 2.01 | 39.19 ± 2.50 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| Fresh matter | ||||||||
| Control | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | −11.41 ± 5.01 | 5.94 ± 6.51 | 0.36 ± 2.30 | −0.90 ± 8.52 | 4.96 ± 3.90 | 6.78 ± 3.56 | −4.10 ± 4.02 | 5.37 ± 4.61 |
| 10 | −12.01 ± 3.52 | 2.13 ± 0.52 | 6.00 ± 0.13 | −6.80 ± 0.42 | 6.98 ± 0.51 | 9.12 ± 0.56 | −5.47 ± 2.45 | 4.53 ± 0.05 |
| 100 | −7.39 ± 6.56 | 2.57 ± 0.81 | 8.38 ± 0.17 | 5.34 ± 0.73 | 7.61 ± 0.40 | 10.39 ± 0.42 | −7.94 ± 3.32 | 7.19 ± 3.22 |
| 200 | −14.27 ± 5.13 | 3.78 ± 0.11 | 9.52 ± 0.12 | 5.45 ± 0.10 | 46.43 ± 0.20 | 57.91 ± 0.23 | −5.49 ± 2.56 | 10.28 ± 4.21 |
| 400 | −16.82 ± 9.7 | 19.04 ± 7.84 | 13.85 ± 7.33 | 5.01 ± 6.74 | 64.62 ± 7.47 | 70.73 ± 3.04 | 12.45 ± 1.65 | 32.59 ± 1.69 |
| 800 | 5.73 ± 7.64 | 20.99 ± 1.63 | 20.52 ± 5.89 | 5.54 ± 6.61 | 70.69 ± 4.42 | 73.28 ± 6.00 | 40.59 ± 1.26 | 43.49 ± 8.76 |
| 1000 | 63.85 ± 4.48 | 56.20 ± 2.50 | 30.83 ± 2.54 | 7.54 ± 3.50 | x | x | 59.27 ± 3.47 | 47.19 ± 7.76 |
The above findings suggest that ferrocene-derived dimethyl aminophosphonates are much more harmful to oat than dibenzyl aminophosphonates, regardless of the methyl or methoxy substitution of the phenyl ring.
The tested compounds have shown a considerably higher negative impact on the shoot of the radish, as compared to that of oat. The most harmful compounds were dimethyl aminophosphonates 2a and 2b, as in the case of the oat. At the concentration of 1000 mg per kg of soil, total inhibition of the radish shoot was noticed. At the concentration of 800 mg kg−1 of soil, the percentage growth inhibition was measurable and reached 63 and 67% for samples 2a and 2b, respectively.
It is worth noting that these values were higher when compared to the GI% of the oat shoot. At the concentration of 800 mg kg−1 of soil, the aminophosphonate 2a was almost 10 times more toxic for radish than for oat seedlings, while the toxicity value of the compound 2b was only two times higher for radish than for oat.
The percentage growth inhibition values of sample 2c for both plants were comparable, and sample 2d exhibited a much stronger negative impact on the green parts of the radish to finally reach 16% of the GI value at the highest concentration.
The percentage growth inhibition of the oat and radish root increased with the increasing concentration of the examined aminophosphonates in soil, similar to the case of the GI% of the shoot. Dimethyl esters 2a and 2b caused much higher inhibition when compared to their dibenzyl analogues. The values of GI% of both mentioned substances for oat at a concentration 1000 mg kg−1 of soil were almost the same and reached ca. 55%. At the same concentration, compounds 2a and 2b caused more harmful effects on the growth of the radish root and were 84 and 78%, respectively.
Aminophosphonates 2c and 2d were less toxic for root growth when compared to 2a and 2b. The compound 2d was almost non-toxic for the oat root, and this observation coincides with the results of GI% of shoots, where only 1% of inhibition was noticed. However, its toxic effect against radish roots is significant as its GI% value reached 39%. Based on the obtained results concerning the growth inhibition of the oat root and shoot for the aminophosphonate 2d, it can be concluded that this compound is totally harmless to this plant. In contrast, the radish was revealed as the more sensitive plant to compound 2d, when compared to oat seedlings, where the roots were especially less resistant.
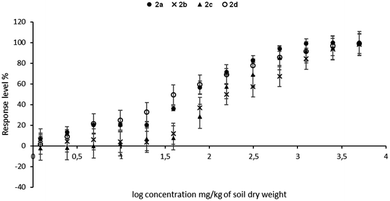
The percentage growth inhibition (GI%) values of dibenzyl N-(4-methoxyphenyl)-amino(ferrocenyl)methylphosphonate 2c for roots were slightly surprising, as the value for oat reached 22%, whereas for radish roots, this value was only 9%. This shows that the aminophosphonate 2c is more toxic to oat roots than to radish roots. Percentage growth inhibition coincides with the EC50 values of oat shoots (Fig. 1).
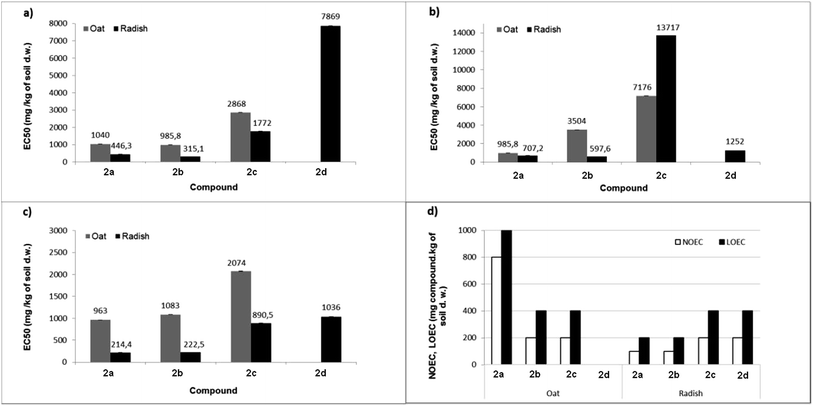 | ||
| Fig. 1 The EC50 values of the growth inhibition of shoot (a), root (b) and fresh matter (c), as well as NOEC and LOEC values related to fresh matter (d) following exposure to tested compounds. | ||
The toxicity of chemicals is usually expressed in terms of the dosage that gives a 50% effect in the response (such as soil respiration or growth of a plant e.g. root length), compared to the control. The effect can be either an increase or a decrease in response, where the decrease is the effective concentration of the tested sample, and the increase is demonstrated toxicity. Generally, values of effective concentration EC50 for each tested compound was lower for radish, compared to oat seedlings.
Since compound 2d shows the total lack of toxicity toward oat, the value of EC50 was immeasurable. For comparison, the lowest EC50 value given by dimethyl aminophosphonate 2b (Fig. 1a) corresponds to a high value of the GI% of oat shoot. The same relationship has been noticed between values of EC50 and the growth inhibition of the root length (Fig. 1b). EC50 values of compounds 2a and 2b were much lower for both shoots and roots of both plants, compared to EC50 values of aminophosphonates 2c–d. Moreover, the aminophosphonate 2b is slightly more toxic for the shoots of both plants. Certainly, aminophosphonates 2a–b are generally more harmful to the radish.
EC50 values calculated for fresh matter of both plants was in the following order: 2a < 2b < 2c < 2d. Dimethyl aminophosphonates 2a–b have shown the strongest inhibition effect on the content of fresh matter; the same tendency was observed in the case of the growth inhibition of shoot or root.
The dibenzyl analogues 2c and 2d exhibited much less harmful effects on the tested plants. Considering the obtained results, it has been concluded that all investigated compounds have a much stronger influence on the fresh matter of the radish as compared to oat seedlings. The lowest effects of concentration values were noticed for compound 2a, namely 963 and 214 mg kg−1 of soil dry weight for oat and radish, respectively. For comparison, the lack of toxicity against oat was observed for dibenzyl N-(4-methylphenyl)amino-(ferrocenyl)methylphosphonate 2d (EC50 was not measured) and the same compound showed relatively low toxicity for radish, i.e. 1036 mg kg−1 of soil.
Values of no observed effect concentration (NOEC) and lowest observed effect concentration (LOEC), determined for fresh matter of both plants (Fig. 1d) correspond to values of EC50. In the case of oat, a low EC50 value for sample 2a in comparison to high values of NOEC and LOEC was caused by the fact that when no changes in fresh matter content were observed in the concentration range 100–800 mg kg−1 of soil dry weight, the highest concentration significantly decreased the level of fresh matter in oat seedlings and the inhibition growth reached 63%. The calculation made by GraphPad Prism software essentially decreased the value of EC50 calculated for the fresh matter of oat. For the compound 2d, the NOEC/LOEC values distinctly demonstrate the difference between the action of this compound on oat and radish (no effect for oat up to 1000 mg kg−1 of soil vs. LOEC = 400 mg for radish).
The percentages of oat sprouts germination for dimethyl aminophosphonates 2a–d at their highest concentration (1000 mg kg−1 of soil dry weight) when compared to control plants were 79, 83, 93 and 97%, respectively (Table 2). In the case of the radish, all tested substances exhibited stronger negative impact against the germination of the common radish. At the highest applied concentration, the percent of germination decrease observed for compounds 2a and 2b reached 100%, referring to untreated plants, while for aminophosphonates 2c and 2d, these values were 71 and 85%, respectively. It is worth noting that respectively, dimethyl derivatives 2a and 2b exhibited 50 and 77% of inhibition in the germination of sprouts, already at the concentration of 800 mg per kg of soil. As has been revealed in the case of shoot, root and fresh matter inhibition, the examination of radish germination has also proved that the radish is much more sensitive to all tested compounds, and that dimethyl aminophosphonates 2a–b are much more harmful to the tested plants than their dibenzyl analogues 2c–d.
| Sample concentration in soil (mg kg−1 of soil d.w.) | Number of emerged seedlings | Germination% | |||
|---|---|---|---|---|---|
| Oat | Radish | Oat | Radish | ||
| a LSDS = 1 LSDC = 2. | |||||
| 2a | Control | 18 | 15 | 100 | 100 |
| 1 | 19 | 15 | 106 | 102 | |
| 10 | 19 | 13 | 109 | 91 | |
| 100 | 19 | 13 | 106 | 86 | |
| 200 | 19 | 11 | 108 | 77 | |
| 400 | 18 | 9 | 104 | 64 | |
| 800 | 17 | 7 | 94 | 50 | |
| 1000 | 14 | x | 79 | x | |
| 2b | Control | 19 | 15 | 100 | 100 |
| 1 | 18 | 14 | 93 | 100 | |
| 10 | 19 | 14 | 97 | 98 | |
| 100 | 19 | 14 | 95 | 100 | |
| 200 | 19 | 11 | 98 | 79 | |
| 400 | 19 | 12 | 97 | 81 | |
| 800 | 19 | 11 | 98 | 77 | |
| 1000 | 16 | x | 83 | x | |
| 2c | Control | 20 | 18 | 100 | 100 |
| 1 | 20 | 19 | 102 | 102 | |
| 10 | 20 | 16 | 100 | 85 | |
| 100 | 19 | 17 | 98 | 95 | |
| 200 | 19 | 17 | 98 | 91 | |
| 400 | 19 | 16 | 98 | 85 | |
| 800 | 18 | 14 | 93 | 78 | |
| 1000 | 18 | 13 | 93 | 71 | |
| 2d | Control | 19 | 15 | 100 | 100 |
| 1 | 19 | 14 | 100 | 91 | |
| 10 | 19 | 14 | 100 | 91 | |
| 100 | 19 | 14 | 98 | 93 | |
| 200 | 19 | 14 | 100 | 91 | |
| 400 | 19 | 14 | 98 | 91 | |
| 800 | 19 | 13 | 98 | 87 | |
| 1000 | 19 | 13 | 97 | 85 | |
Digital photographs of the emerged seedlings and roots of the oat and common radish plants treated with the aminophosphonate 2a one day before the determination of phytotoxicity are presented in Fig. 2, while similar pictures for compounds 2b–d are presented in ESI as Fig. S15–17.† The photographs exhibit morphological changes like necrosis, chlorosis or any other damage.
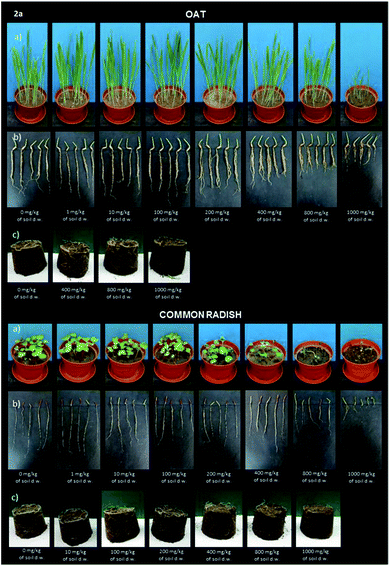 | ||
| Fig. 2 Digital photographs of oat and radish seedlings treated with sample 2a. (a) The green parts of plants (shoots), (b) roots, (c) the extent of the branching of roots in soil. | ||
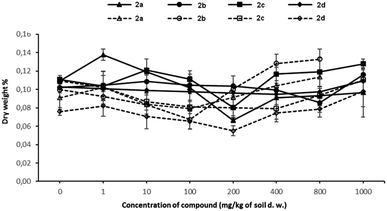
Changes in the dry weight of plants treated with growing concentrations of tested compounds are presented in Fig. 3. As expected, the dry weights of both tested plants have increased at the highest concentrations of investigated compounds 2a–d.
Since plants take in nutrients and water through their roots, their growth and development to a large extent depend on the composition and concentration of mineral nutrients available in the soil. At the beginning of the plant's growth, the plant absorbs the nutrients or contaminants very intensively. When the concentration of xenobiotics in the plant is high enough, one of the defence mechanisms against toxic compounds is activated, i.e. the plants absorb large amounts of water in order to start the detoxification process. Unfortunately, after a longer exposure time to contaminants, dysfunction of the roots makes absorbing water and soluble nutrients impossible, therefore, the amount of dry matter in the plant increases.
The changes in the water content in plants seem to be the typical behaviour of plants defending against tested xenobiotics. It is worth noting that oat behaves differently in contact with compound 2d, which is totally harmless to this plant. In this case, the dry weight% values remain almost unchanged in increasing concentration of the compound 2d.
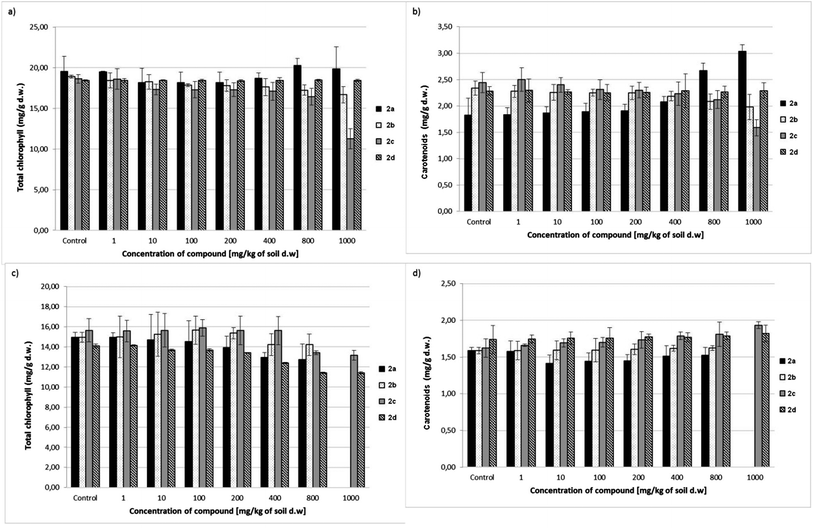
Results related to the total chlorophyll and carotenoid level in oat and radish seedlings treated with aminophosphonates 2a–d are presented in Fig. 4. Dibenzyl aminophosphonate 2d did not cause any changes in chlorophyll and carotenoid levels in oat seedlings, as in the case of the shoot and root. For comparison, the growing concentration of this compound (2d) in soil caused a significant decrease in the chlorophyll levels in radish seedlings.
Dimethyl ester 2a, considered until now to be the most toxic, caused a significant increase in carotenoid content in oat seedlings with growing concentration in soil, while its slightly negative impact on the chlorophyll level was observed only in radish seedlings.
The aminophosphonate 2b was totally inert on the level of pigments in both tested plants. Only small, statistically unimportant changes were observed when compared to the untreated, control plants. The highest applied concentration of dibenzylaminophosphonate 2c (1000 mg kg−1 of soil dry weight) caused a decrease in both pigments in oat seedlings. In the case of radish, the drop in chlorophyll content was observed for the compound 2c at concentrations of 800 and 1000 mg kg−1 of soil dry weight, while the carotenoid level increased slightly at the highest concentration. The growing carotenoid content indicates the first symptoms of chlorosis of oat seedlings (Fig. 4b).
Microtox assay
Values of EC50 calculated by the Microtox Analyzer software are plotted in Fig. 5 and listed in Table 3.| Compounds | EC50 (lower limit; upper limit) | Coefficient of determination (R2) |
|---|---|---|
| 2a | 48.3 (34.7; 67.1) | 0.8954 |
| 2b | 183.9 (124.2; 272.3) | 0.8895 |
| 2c | 234.9 (203.1; 271.8) | 0.9786 |
| 2d | 59.2 (48.0; 73.0) | 0.9657 |
Based on the obtained results of EC50 values related to the ecotoxicological impact against Vibrio fischeri bacteria, the compound 2a was revealed as the most toxic (48.3 mg kg−1 of soil dry weight). Surprisingly, aminophosphonate 2d exhibited a similar EC50 value at the level of 59.2 mg kg−1 of soil dry weight. In contrast, compounds 2b and 2c expressed 3–4 times higher values of EC50, which correspond to much lower ecotoxicity when compared to samples 2a and 2d.
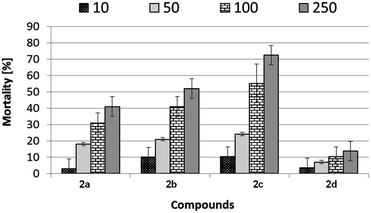
Ostracod test kit
Mortality evaluation using Heterocypris incongruens has shown various effects of tested substances 2a–d. Generally, as expected, the higher the concentration of samples in soil, the higher the mortality (Fig. 6).Among the tested samples, the highest mortality of Heterocypris incongruens was revealed for sample 2c. At the highest concentration (250 mg kg−1 of soil dry weight), the percent of mortality reached more than 70%. In contrast, the lowest dead impact against tested organisms was observed for sample 2d. At the highest applied concentration, the percentage value of mortality was about 14, which is five times lower, compared to sample 2c at the same concentration.
Apart from mortality, growth inhibition is the second criterion of the toxic effect indicated by the Ostracodtoxkit F™ microbiotest. This criterion allows the evaluation of the sub-lethal toxicity of sediments. The growth inhibition was determined by comparing the size of the surviving ostracods living in the test sediment with the size of ostracods living in the reference sediment at the end of the test. Determination of the sub-lethal impact of sediment toxicants was justified only for sediments that do not cause a high ostracod mortality. According to the manual of the Ostracodtoxkit test,34 the growth inhibition should only be determined for sediments, where the mortality was found to be less than 30%. Therefore, the growth inhibition was chosen as a criterion of sub-lethal effects to determine toxicity not inducing substantial mortality in tested organisms, therefore, measurements of the length of organisms were only performed for compounds causing mortality less than 30%.
Percentage growth inhibition reports for ostracods treated with increasing concentration of samples 2a–d coincide with the mortality of these crustaceans. The lowest growth inhibition of ostracods was noticed for sample 2d, which reached the highest concentration at only 22%, compared to untreated crustaceans (Table 4). The results of the growth inhibition of ostracods coincide with their mortality. The highest growth inhibition was noticed for samples 2b and 2c and was reached with the concentration of 50 mg kg−1 of soil dry weight, ca. 14%. At the higher concentration level (100 and 250 mg kg−1 of soil dry weight), the growth inhibition, according to recommendations in the Ostracodtoxit test manual34 was not measured. Sample 2a was slightly less toxic and its highest measurable inhibition was 19% for the concentration of 250 mg kg−1 of soil.
| Concentration [mg kg−1 of soil d.w.] | Growth inhibition [%] | |||
|---|---|---|---|---|
| 2a | 2b | 2c | 2d | |
| Control | 0 | 0 | 0 | 0 |
| 10 | 0 ± 1 | 2 ± 1 | 2 ± 1 | 0 ± 1 |
| 50 | 10 ± 1 | 13 ± 1 | 14 ± 2 | 7 ± 1 |
| 100 | 19 ± 2 | NM | NM | 11 ± 1 |
| 250 | NM | NM | NM | 22 ± 2 |
Conclusions
Based on the ecotoxicological assessment of the newly synthesized N-arylamino(ferrocenyl)methylphosphonates, a few conclusion can be drawn from the work:Plant responses to tested compounds are dose dependent. EC50 values, which correspond to the growth inhibition of the shoot, root and fresh matter have proved that the dimethyl esters 2a and 2b are the most toxic against tested plants, and the dicotyledonous radish has been revealed as the more sensitive plant, compared to the monocotyledonous oat. Dibenzyl N-(4-methoxyphenyl)amino(ferrocenyl)methylphosphonate (2c) showed moderate, but non-selective toxicity against both plants. All three compounds, 2a–c, were toxic for crustaceans Heterocypris incongruens, but paradoxically, aminophosphonates 2b–c were found to be harmless for bacteria Vibrio fischeri; dimethyl N-(4-methoxyphenyl)amino-(ferrocenyl)methylphosphonate (2a) was effectively toxic for this microbe.
Dibenzyl N-(4-methylphenyl)amino(ferrocenyl)methyl-phosphonate (2d) was almost non-toxic for all of the tested parameters of oat seedlings, while its impact on radish seedlings showed the important symptoms of its moderate toxicity against this plant. It also presented the lowest negative impact against Heterocypris incongruens. Results of the action of the aminophosphonate 2d on the Vibrio fischeri bacteria showed its important toxicity, thus spoiling the ideality of this compound with respect to its potential herbicidal applications. Nevertheless, compound 2d seems to be a good candidate for further investigation in terms of its probable application as herbicide: it is completely non-toxic to monocotyledonous oat, practically harmless for crustaceans and although moderately toxic for dicotyledonous radish, and it is able to negatively influence radish roots.
The ecotoxicological impact of dimethyl N-(4-methylphenyl)amino-(ferrocenyl)methylphosphonate (2b) is equally interesting. Its phytotoxicity is also significantly selective, as its EC50 values for radish are several times smaller, when compared to the corresponding values for oat. The compound 2b is relatively harmless to Vibrio fischeri but, unfortunately, it is toxic to Heterocypris incongruens. Although ecotoxicologically ambiguous, these two compounds have shown their potential; therefore, their phytotoxicity will be investigated more profoundly, e.g. their impact on persistent weeds.
Conflict of interest
There are no conflicts of interest to declare.Acknowledgements
Studies were founded by the National Centre of Science of Polish State (NCN), grant no. 2014/13/B/NZ9/02418. Costs of publishing in the open access system are covered by the Faculty of Chemistry, University of Łódź.Notes and references
- P. Kafarski and B. Lejczak, Phosphorus, Sulfur Silicon Relat. Elem., 1991, 63, 193 CrossRef CAS.
- G. Forlani, Ł. Berlicki, M. Duò, G. Dziędzioła, S. Giberti, M. Bertazzini and P. Kafarski, J. Agric. Food Chem., 2013, 61, 6792 CrossRef CAS PubMed.
- A. Occhipinti, Ł. Berlicki, S. Giberti, G. Dziędzioła, P. Kafarski and G. Forlani, Pest Manage. Sci., 2010, 66, 51 CrossRef CAS PubMed.
- S. Giberti, M. Bertazzini, M. Liboni, Ł. Berlicki, P. Kafarski and G. Forlani, Pest Manage. Sci., 2017, 73, 435 CrossRef CAS PubMed.
- E. C. Constable, Angew. Chem., Int. Ed. Engl., 1991, 30, 407 CrossRef.
- S. Top, A. Vessières, C. Cabestaing, I. Laios, G. Leclercq, C. Provot and G. Jaouen, J. Organomet. Chem., 2001, 637–639, 500 CrossRef CAS.
- E. A. Hillard, A. Vessières, S. Top, P. Pigeon, K. Kowalski, M. Huche and G. Jaouen, J. Organomet. Chem., 2007, 692, 1315 CrossRef CAS.
- A. Nguyen, S. Top, A. Vessières, P. Pigeon, M. Huché, E. A. Hillard and G. Jaouen, J. Organomet. Chem., 2007, 692, 1219 CrossRef CAS.
- C. Roux and C. Biot, Future Med. Chem., 2012, 4, 783 CrossRef CAS PubMed.
- N. Metzler-Nolte and M. Salmain, in Ferrocenes. Ligands, Materials and Biomolecules, ed. P. Stepnicka, J. Wiley&Sons Ltd, Chichester, 2008, p. 619 Search PubMed.
- B. Floris, Chem. Biol. Technol. Agric., 2015, 2, 15 CrossRef.
- (a) J. Lewkowski, M. Rzeźniczak, R. Skowroński and J. Zakrzewski, J. Organomet. Chem., 2001, 631, 105 CrossRef CAS; (b) J. Lewkowski, M. Rzeźniczak and R. Skowroński, Heteroat. Chem., 2003, 14, 144 CrossRef CAS; (c) J. Lewkowski, J. Organomet. Chem., 2003, 681, 225 CrossRef CAS; (d) J. Lewkowski, M. Rzeźniczak and R. Skowroński, J. Organomet. Chem., 2004, 689, 1265 CrossRef CAS; (e) J. Lewkowski, M. Rzeźniczak and R. Skowroński, J. Organomet. Chem., 2004, 689, 1684 CrossRef CAS; (f) J. Lewkowski and R. Skowroński, Pol. J. Chem., 2005, 79, 525 CAS; (g) J. Lewkowski, R. Skowroński, D. Krasowska and R. Karpowicz, Tetrahedron Lett., 2006, 47, 1589 CrossRef CAS; (h) J. Lewkowski and R. Karpowicz, Pol. J. Chem., 2007, 81, 2097 CAS; (i) J. Lewkowski, R. Karpowicz and R. Skowroński, Phosphorus, Sulfur Silicon Relat. Elem., 2009, 184, 815 CrossRef CAS; (j) J. Lewkowski, R. Karpowicz, E. Miękoś and M. Zieliński, Heteroat. Chem., 2014, 25, 163 CrossRef.
- Terrestrial plant test: 208: Seedling emergence and seedling growth test, in OECD Guideline for the testing of chemicals, Organization For Economic Co-Operation and Development, Paris, France, 1984 Search PubMed.
- (a) L.-S. Wang, L. Wang, L. Wang, G. Wang, Z.-H. Li and J.-J. Wang, Environ. Toxicol., 2009, 24, 296 CrossRef CAS PubMed; (b) B. Pawlowska and R. Biczak, Chemosphere, 2016, 149, 24 CrossRef CAS PubMed.
- A. Oren, M. Kuehl and U. Karsten, Mar. Ecol.: Prog. Ser., 1995, 128, 151 CrossRef.
- J. Lewkowski, M. Rodriguez Moya, M. Chmielak, D. Rogacz, K. Lewicka and P. Rychter, Molecules, 2016, 21, 936 CrossRef PubMed.
- K. Doe, R. Scroggins, D. Mcleay and G. Wohlgeschaffen, in Small-Scale Freshwater Toxicity Investigations, ed. C. Blaise and J. F. Férard, Springer, Dordrecht, 2005, vol. 1, pp. 107–136 Search PubMed.
- M. J. Martínez-Sánchez, C. Pérez-Sirvent, M. L. García-Lorenzo, S. Martínez-López, J. Bech, R. García-Tenorio and J. P. Bolívar, J. Geochem. Explor., 2014, 147, 130 CrossRef.
- H. R. Hudson, in Aminophosphonic and Aminophosphinic Acids. Chemistry and Biological Activity, ed. V. P. Kukhar and H. R. Hudson, John Wiley & Sons, New York, 2000, p. 443 Search PubMed.
- H. Kleszczynska, J. Sarapuk and D. Bonarska, Cell. Mol. Biol. Lett., 2001, 6, 271 CAS.
- S. Sobhani and Z. Tashrifi, Tetrahedron, 2010, 66, 1429 CrossRef CAS.
- C. S. Sundari, N. B. Reddy, S. S. Prasad, K. U. M. Rao, S. H. J. Prakash and C. S. Reddy, Phosphorus, Sulfur Silicon Relat. Elem., 2014, 180, 551 CrossRef.
- P. P. Onys’ko, K. A. Zamulko, O. I. Kyselyova, I. P. Yelenich and Y. V. Rassukana, J. Fluorine Chem., 2016, 185, 191 CrossRef.
- Z. M. Jászay, G. Németh, T. S. Pham, I. Petneházy, A. Grün and L. Tőke, Tetrahedron: Asymmetry, 2005, 16, 3837 CrossRef.
- W. W. Smith and P. A. Bartlett, J. Am. Chem. Soc., 1998, 120, 4622 CrossRef CAS.
- T. E. Ali and S. M. El-Edfawy, Res. Chem. Intermed., 2016, 42, 1329 CrossRef CAS.
- G. Yang, Z. Liu, J. Liu and H. Yang, Heteroat. Chem., 2000, 11, 313 CrossRef CAS.
- K. M. Yager, C. M. Taylor and A. B. Smith, J. Am. Chem. Soc., 1994, 116, 9377–9378 CrossRef CAS.
- I. M. Lefebvre and S. A. J. Evans, J. Org. Chem., 1997, 62, 7532 CrossRef CAS.
- Z. Rezaei, S. Khabnadideh, K. Zomorodian, K. Pakshir, S. Nadali, N. Mohtashami and E. F. Mirzaei, Int. J. Med. Chem., 2011, 678101 Search PubMed.
- J. Lewkowski, Z. Malinowski, A. Matusiak, M. Morawska, D. Rogacz and P. Rychter, Molecules, 2016, 21, 694 CrossRef PubMed.
- A. Matusiak, J. Lewkowski, P. Rychter and R. Biczak, J. Agric. Food Chem., 2013, 61, 7673 CrossRef CAS PubMed.
- R. Biczak, B. Pawłowska, P. Bałczewski and P. Rychter, J. Hazard. Mater., 2014, 274, 181 CrossRef CAS PubMed.
- MicroBioTests, Toxkit Advantages/Assets, available online: http://www.microbiotests.be/information/toxkit-advantagesassets/, accessed on 19 July 2016.
Footnote |
| † Electronic supplementary information (ESI) available: NMR spectra of imines 1a–b, NMR spectra of aminophosphonates 2a–d and digital photographs of studied plants. See DOI: 10.1039/c7ra06079c |
| This journal is © The Royal Society of Chemistry 2017 |