Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Pyridine-based functionalized graphene oxides as a new class of corrosion inhibitors for mild steel: an experimental and DFT approach†
Rajeev Kumar Guptaa,
Manisha Malviyaa,
Chandrabhan Vermaab,
Neeraj K. Guptaa and
M. A. Quraishi *ac
*ac
aDepartment of Chemistry, Indian Institute of Technology, Banaras Hindu University, Varanasi 221005, India. E-mail: maquraishi.apc@itbhu.ac.in; Fax: +91-542-2368428; Tel: +91-9307025126
bDepartment of Chemistry, Faculty of Agriculture, Science and Technology, North-West University, Mafikeng Campus, Private Bag X2046, Mmabatho 2735, South Africa
cCenter of Research Excellence in Corrosion, Research Institute, King Fahd University of Petroleum & Minerals, Dhahran 31261, Saudi Arabia
First published on 9th August 2017
Abstract
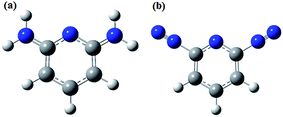
The aims of the present work were to synthesise two functionalized graphene oxides, namely, diazo pyridine functionalized graphene oxide (DAZP-GO) and diamino pyridine functionalized graphene oxide (DAMP-GO), and to evaluate them as corrosion inhibitors on mild steel in 1 M hydrochloric acid. Electrochemical studies reveal that the inhibition efficiencies of both of the tested functionalized graphene oxides were enhanced with increasing concentration and the maximum inhibition efficiencies of 95.08% and 96.73% were obtained for DAMP-GO and DAZP-GO, respectively, at a concentration as low as 25 mg L−1. A potentiodynamic polarization study suggests that both DAZP-GO and DAMP-GO act as mixed type inhibitors with a slight cathodic predominance. The formation of a protective film on the mild steel surface was confirmed using scanning electron microscope, energy dispersive X-ray spectroscopy, atomic force microscopy and X-ray photoelectron spectroscopy techniques. Dynamic functional theory parameters such as EHOMO, ELUMO, energy band gap (ΔE), electronegativity (χ), hardness (η), softness (σ) and a fraction of electron transfer (ΔN) were calculated to support the experimental results.
1. Introduction
Corrosion is a destructive phenomenon that results in huge economic losses and many safety issues.1–3 The global cost of corrosion is estimated to be $2.5 trillion (USD) which is approximately equivalent to 3.4% of the total Gross Domestic Product (GDP).4,5 A significant amount of this cost, approximately 15–35%, could be avoided by implementing suitable anti-corrosion technology. Among the available methods of corrosion control, the use of corrosion inhibitors is one of the most popular methods because of its cost-effectiveness and ease of application.6,7 Organic compounds with heteroatoms have been reported as potential corrosion inhibitors for metallic corrosion.8–10 However, because of their toxicity and thermal instability in an acid medium, they are not very useful for practical applications.11–13Recently graphene derivatives have been reported as anti-corrosive coating materials for marine and other corrosive environments because of their various unique properties such as high surface area, chemical resistance, thermal and electrical conductivity, enhanced mechanical strength, high level of impermeability and hydrophobicity.14–21 It is documented in the literature that the graphene is insoluble in an aqueous medium because of the lack of functional groups and therefore they are used as anti-coating materials only.15,16 However, graphene oxide (GO) and functionalized GOs could act as good corrosion inhibitors in the solution phase because of the presence of various oxygen (O)-containing functional groups such as hydroxyls, epoxides, carboxyl and carbonyl on their surfaces.17–19 In view of this observation, two functionalized GOs were synthesized, diazo pyridine functionalized graphene oxide (DAZP-GO) and diamino pyridine functionalized graphene oxide (DAMP-GO), to study their corrosion inhibition behavior on mild steel in 1 M hydrochloric acid (HCl) solution using experimental and dynamic functional theory (DFT) methods.
2. Experimental sections
2.1. Chemicals
The following chemicals were used in the present study: 2,6-diaminopyridine (DAP; Sigma-Aldrich), sodium hydroxide (NaOH; SD Fine Chemicals Ltd.), sodium nitrite (NaNO2; Merck), crystalline graphite powder (Alfa Aesar), sodium chloride (Merck), hydrogen peroxide (30%, Merck), concentrated sulfuric acid (Merck, 98% pure), potassium permanganate (Merck), methanol (99.9%, Merck), HCl (35%, Merck) and double distilled water.2.2. Procedure for synthesis of inhibitors
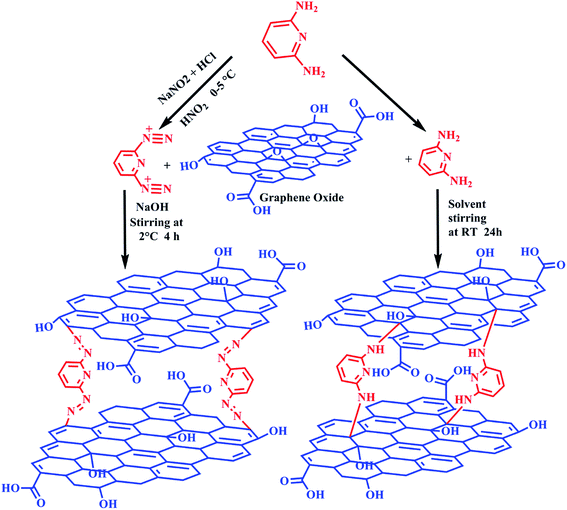
For the coupling of diazopyridine with GO, a homogenous solution of GO in 10 mL of water was made by mixing the solution ultrasonically for 1 h. To this solution, 25 mL of 2% NaOH was added dropwise and then stirred for 2 h. To this solution, diazonium salt solution was added dropwise at such a rate that the pH of the resulting solution remained constant at 9 and the temperature of the solution did not exceed 2 °C. After the reaction was complete, the pH of the reaction mixture was reduced to 7 and the crude product was then washed with double distilled water, methanol and dried at 80 °C under vacuum.24,25 Scheme 1 shows the synthesis of functionalized GOs.
2.3. Materials and measurements
 | (1) |
 | (2) |
| I = −EHOMO | (3) |
| A = ELUMO | (4) |
 | (5) |
 | (6) |
| ΔE = ELUMO − EHOMO | (7) |
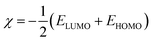 | (8) |
 | (9) |
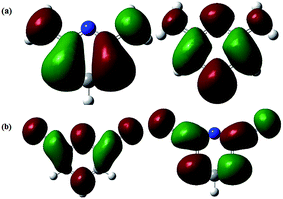
In the previous equations, EHOMO and ELUMO represent energies of highest occupied molecular orbital and lowest unoccupied molecular orbital, respectively, I is the ionization potential, A is the electron affinity, ΔE is the energy band gap, η is the hardness, σ is the softness, χ is the global electronegativity, and ΔN is the fraction of electron transfer. A value of 7.0 eV was used for the χFe, whereas ηFe was taken as 0 eV for bulk Fe atoms in accordance with the Pearson's electronegativity scale.
3. Results and discussion
3.1. Characterization of the inhibitors
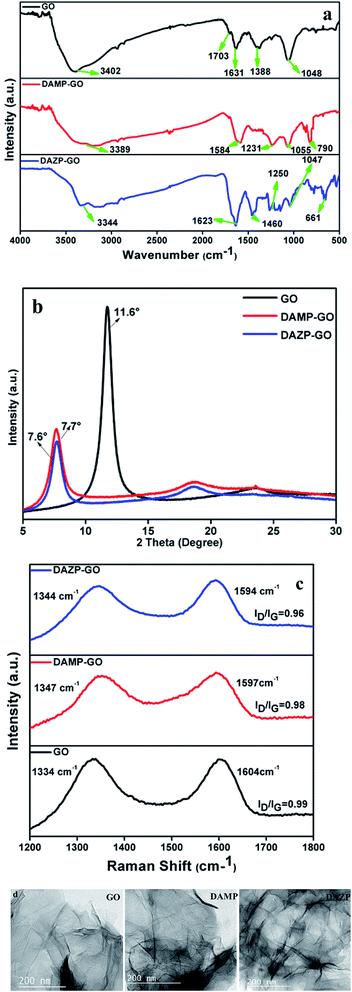
The synthesis of GO and its functionalization with diaminopyridine (DAMP-GO) and diazopyridine (DAZP-GO) was confirmed using FT-IR. Fig. 1(a) shows the FT-IR spectra for GO and DAMP-GO and DAZP-GO. For pure GO, the peaks at 3402, 1703, 1631, 1388, and 1048 cm−1 occur because of the νs(O–H), νs(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), νs(C
O), νs(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), νb(O–H), νs(C–O) of the epoxides νs(C–O–C), respectively.22,24 The broad peak in the region centered at 3500 cm−1 to 3000 cm−1 in DAMP-GO and DAZP-GO composites confirms the presence of –OH and –NH groups. The presence of the benzene ring is confirmed by the peak between 1633 and 1499 cm−1 occurring because of the νs(C
C), νb(O–H), νs(C–O) of the epoxides νs(C–O–C), respectively.22,24 The broad peak in the region centered at 3500 cm−1 to 3000 cm−1 in DAMP-GO and DAZP-GO composites confirms the presence of –OH and –NH groups. The presence of the benzene ring is confirmed by the peak between 1633 and 1499 cm−1 occurring because of the νs(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) in the benzene ring. In the DAZP-GO composites, the presence of the azo (–N
C) in the benzene ring. In the DAZP-GO composites, the presence of the azo (–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–) group is confirmed by a peak at 1460 cm−1 which occurs because of the νs(N
N–) group is confirmed by a peak at 1460 cm−1 which occurs because of the νs(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N).24,25 In both DAMP-GO and DAZP-GO composites, the νs(C–N) peaks between 1277 and 1210 cm−1 are observed. Also, the band at 1055 cm−1 represents the C–O group. The breaking of the epoxy bonds because of the addition of alkali is confirmed by the νs(C–O) of the epoxides (C–O–C).23 All these peaks and the other peaks in the region 800–650 cm−1 confirm the formation of the DAMP-GO and DAZP-GO composites.22–24
N).24,25 In both DAMP-GO and DAZP-GO composites, the νs(C–N) peaks between 1277 and 1210 cm−1 are observed. Also, the band at 1055 cm−1 represents the C–O group. The breaking of the epoxy bonds because of the addition of alkali is confirmed by the νs(C–O) of the epoxides (C–O–C).23 All these peaks and the other peaks in the region 800–650 cm−1 confirm the formation of the DAMP-GO and DAZP-GO composites.22–24
XRD results likewise confirm the formation of GO and its functionalized composite with diaminopyridine (DAMP-GO) and diazopyridine (DAZP-GO). The acquired XRD patterns of GO and DAMP, DAZP functionalized GO are presented in the Fig. 1(b). Diffraction peaks seen at a 2θ value of 11.6° in the XRD pattern of GO arise because of the diffraction from the different oxygen containing functional group on the sides of the sheets.22 After covalently bonding DAMP and DAZP with GO, the XRD peak of GO was observed to shift to the lower 2θ value of 7.6 in the DAMP-GO and DAZP-GO. The decrease in the XRD pattern occurs because of the expansion in the interlayer spacing in the presence of a ligand (DAMP and DAZP) in the GO sheets. A similar shift in the XRD pattern has been observed.22 The reduced intensity of peaks in pure GO and functionalized GO indicates that exfoliation occurs during the ultrasonic mixing.
The data obtained from Raman spectra shown in Fig. 1(c) further support the formation of DAMP-GO and DAZP-GO. The Raman spectra of GO shows the presence of a G peak (sp2) hybridized carbon atom at 1604 cm−1 and a D peak (sp3) hybridized carbon atom at 1334 cm−1. The functionalized GO shows shifting of these peaks which represents surface modification by incorporation of ligands (DAZP and DAMP) and the ratio of the intensity of the D-Raman peak and the G-Raman peak (ID/IG) ratio of DAMP-GO and DAZP-GO were found to be 0.98 and 0.96, respectively, which is smaller than that of GO (∼0.99). These observations support a percentage increment in sp2 carbon atoms in functionalized GOs which is caused by nucleophilic addition of the azo and amino groups of DAZP and DAMP to GO molecules and also supports the formation of covalent bonds. A similar explanation has been given for GO and functionalized GO by Gupta et al.22,24 and Singh et al.33
The transmission electron microscopy (TEM) images shown in Fig. 1(d) were used to characterize the GO, DAMP-GO, and DAZP-GO. The TEM images of GO indicate that there is typically two to three layers of GO. After the functionalization with DAMP and DAZP ligands, the GO layers are stacked together to form a composite structure, and the surface becomes very rough which is shown in Fig. 1(d) (DAMP-GO and DAZP-GO). This crosslinking of GO layers resulting from functionalization by ligands has also been reported by Gupta et al.22,24
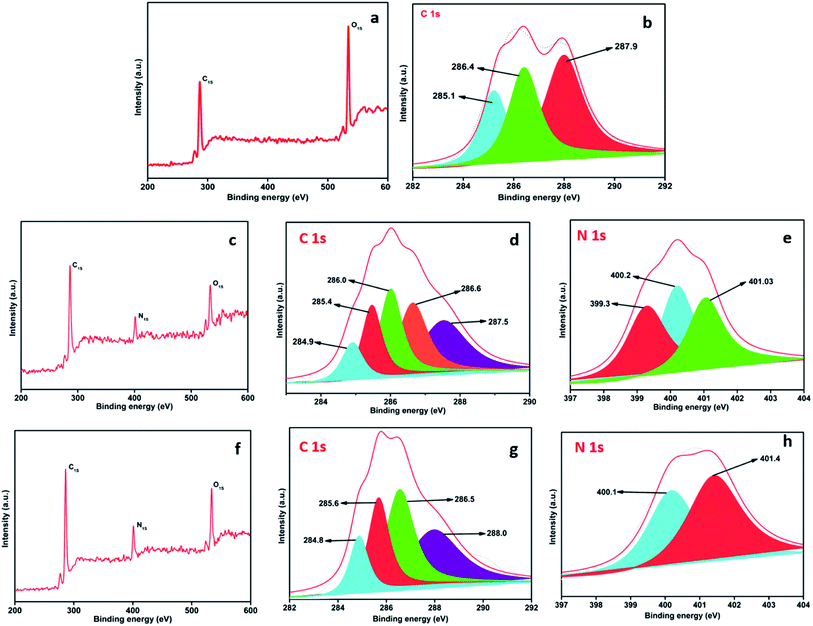
XPS was utilized to determine the binding energies of various functional groups in GO, DAMP-GO and DAZP-GO composites. The XPS pattern of GO, the core-level photoemission peaks [Fig. 2(a)] show the presence of only C and O 1s orbitals. The high-resolution carbon 1s XPS spectrum of GO [Fig. 2(b)] shows three peaks at 285.1, 286.4, and 287.9 eV, corresponding to C–C, C–O (hydroxyl and epoxide), and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (carboxyl) groups of GO, respectively. The XPS pattern of the DAMP-GO composite [Fig. 2(c)] indicates the characteristic C 1s, N 1s, and O 1s centered photoemission signals at ∼286, ∼401.5 and ∼534 eV, respectively.22 The high-resolution C 1s XPS spectrum of the DAMP-GO composite shown in Fig. 2(d) is conveniently fitted using five components. The first peak at 284.9 is ascribed to the C–C bond in graphene and its analogs. Likewise, the peaks at 285.4 and 286.0 arise from the C–N and –C
O (carboxyl) groups of GO, respectively. The XPS pattern of the DAMP-GO composite [Fig. 2(c)] indicates the characteristic C 1s, N 1s, and O 1s centered photoemission signals at ∼286, ∼401.5 and ∼534 eV, respectively.22 The high-resolution C 1s XPS spectrum of the DAMP-GO composite shown in Fig. 2(d) is conveniently fitted using five components. The first peak at 284.9 is ascribed to the C–C bond in graphene and its analogs. Likewise, the peaks at 285.4 and 286.0 arise from the C–N and –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds, respectively. Another peak at 286.6 represents the C–O bonds and the maximum binding energy peak at 287.5, occurs because of the carboxylic groups. In order to understand the chemical character of nitrogen (N) in the DAMP-GO, it is important to know the nature of the interaction between the GO sheet and the nitrogen containing functional group in DAMP-GO. Fig. 2(e) shows a high-resolution N 1s XPS spectrum, which was fitted with three peaks. The lower segment at 399.3 is ascribed to the –C
N bonds, respectively. Another peak at 286.6 represents the C–O bonds and the maximum binding energy peak at 287.5, occurs because of the carboxylic groups. In order to understand the chemical character of nitrogen (N) in the DAMP-GO, it is important to know the nature of the interaction between the GO sheet and the nitrogen containing functional group in DAMP-GO. Fig. 2(e) shows a high-resolution N 1s XPS spectrum, which was fitted with three peaks. The lower segment at 399.3 is ascribed to the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond of diaminopyridine and a middle segment comprises of the peak at 400.2 and indicates the presence of the amine group. This peak confirms the grafting of the amino group in DAMP with a GO skeleton. The third segment consists of the peak with the maximum binding energy at 401.03 and this occurs because of the protonated amino group in the DAMP-GO framework.22
N bond of diaminopyridine and a middle segment comprises of the peak at 400.2 and indicates the presence of the amine group. This peak confirms the grafting of the amino group in DAMP with a GO skeleton. The third segment consists of the peak with the maximum binding energy at 401.03 and this occurs because of the protonated amino group in the DAMP-GO framework.22
The XPS [Fig. 2(f)] of the DAZP-GO composite at ∼285, ∼401, and ∼534 eV emerges because of photoemission from the 1s orbital of C, N, and O, respectively.24 The highly resolved C 1s XPS composite represented in Fig. 2(g) consists of four parts. The minima and the maxima at 284.8 and 288.0 occur because of the presence of the C–C bond and a carboxyl group in the graphitic framework. The intermediate peaks at 285.6 and 286.5 occur as a result of the C–O and C–N bonds, respectively. Fig. 2(h) represents the high-resolution XPS originating from the 1s orbital of N. The peaks at 400.0 and 400.4 represent a composite band occurring because of the combined effect of C–N and the azo group in the DAZP-GO composite.24 The previous facts confirm the formation of DAZP-GO and DAMP-GO composite structure obtained by interacting GO with DAZP and DAMP atoms as proposed schematically in Scheme 1. The presence of a large number of epoxy and phenolic groups in the GO framework help in the interaction of aryl azo and amino cation with the GO and the formation of the C–N bond.
3.2. Corrosion inhibition studies
where Y0 is the CPE constant, commonly known as admittance, ω is the angular frequency, n is the phase shift, and j is the imaginary number. The value of n is a measure for surface roughness, in general, a lower value of n is associated with a higher surface roughness and vice versa. Careful observation of the results shown in Table 1 suggests that values of n are higher for inhibited metallic specimens (0.829 to 0.899) when compared to the uninhibited mild steel specimen and this suggests that mild steel surfaces are more smooth in the presence of DAMP-GO and DAZP-GO which is attributed to the formation of a protective film by DAMP-GO and DAZP-GO. The double layer capacitance (Cdl) value was calculated using following equation:
| Cdl = Y0(ωmax)n−1 |
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–) which enhance the adsorption tendency of the functionalized GOs.
N–) which enhance the adsorption tendency of the functionalized GOs.
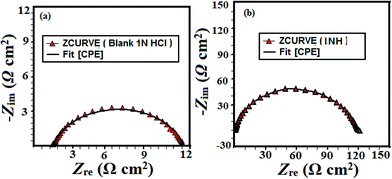 | ||
| Fig. 3 (a and b) Nyquist plots for mild steel corrosion in 1 M HCl in the absence and presence of different concentrations of composites, (c) equivalent circuit used for fitting the EIS data. | ||
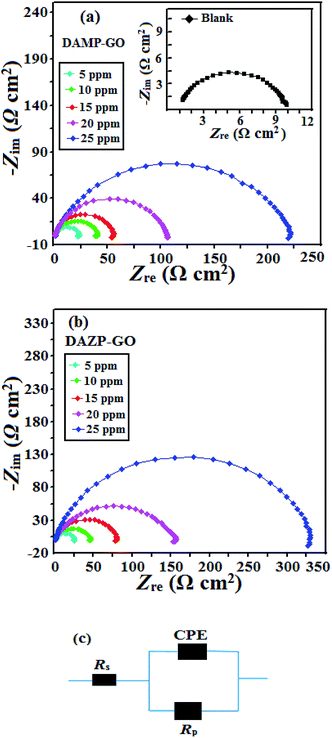 | ||
| Fig. 4 (a and b) Nyquist plots for mild steel corrosion in 1 M HCl in the absence and presence of different concentrations of composites, (c) equivalent circuit used for fitting the EIS data. | ||
| Inhibitor | Conc. (mg L−1) | Rs (Ω cm2) | Rp (Ω cm2) | n | Y0 | Cdl (μF cm−2) | η% | θ |
|---|---|---|---|---|---|---|---|---|
| Blank | — | 1.120 | 9.58 | 0.827 | 482.3 | 138.23 | — | — |
| DAMP-GO | 5 | 0.129 | 20.86 | 0.868 | 218.5 | 98.35 | 51.69 | 0.5169 |
| 10 | 0.880 | 40.52 | 0.829 | 136.5 | 48.98 | 73.59 | 0.7359 | |
| 15 | 1.097 | 54.07 | 0.899 | 80.29 | 44.44 | 80.21 | 0.8021 | |
| 20 | 0.822 | 111 | 0.879 | 85.8 | 43.58 | 90.36 | 0.9036 | |
| 25 | 1.104 | 217.6 | 0.806 | 102.3 | 41.99 | 95.08 | 0.9508 | |
| DAZP-GO | 5 | 0.881 | 25.18 | 0.805 | 315.2 | 112.55 | 57.50 | 0.5750 |
| 10 | 1.396 | 45.08 | 0.839 | 145.8 | 57.49 | 76.26 | 0.7626 | |
| 15 | 0.800 | 80.10 | 0.849 | 119.6 | 55.36 | 86.64 | 0.8664 | |
| 20 | 0.866 | 151.4 | 0.835 | 97.15 | 41.99 | 92.93 | 0.9293 | |
| 25 | 0.899 | 328.2 | 0.841 | 78.4 | 40.43 | 96.73 | 0.9673 |
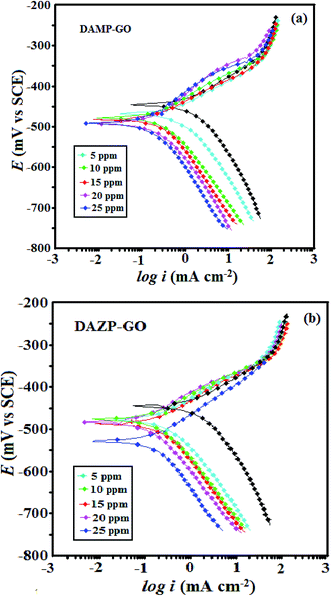 | ||
| Fig. 5 (a and b) Potentiodynamic polarization curves for mild steel corrosion in 1 M HCl in the presence and absence of various concentrations of composites studied. | ||
| Inhibitor | Conc. (mol L−1) | Ecorr (mV per SCE) | βa (μA cm−2) | βc (mV dec−1) | icorr (mV dec−1) | η% | θ |
|---|---|---|---|---|---|---|---|
| Blank | — | −445 | 70.5 | 114.6 | 1150 | — | — |
| DAMP-GO | 5 | −467 | 73.0 | 119.3 | 552.0 | 52.00 | 0.520 |
| 10 | −477 | 89.1 | 128.2 | 309.3 | 73.10 | 0.731 | |
| 15 | −482 | 51.9 | 73.6 | 212.7 | 81.50 | 0.815 | |
| 20 | −492 | 105.2 | 122.9 | 101.2 | 91.20 | 0.912 | |
| 25 | −491 | 97.4 | 121.4 | 59.8 | 94.80 | 0.948 | |
| DAZP-GO | 5 | −477 | 85.5 | 116.1 | 481.8 | 58.10 | 0.581 |
| 10 | −477 | 75.1 | 107.2 | 278.3 | 75.80 | 0.758 | |
| 15 | −487 | 58.4 | 77.5 | 147.2 | 87.20 | 0.872 | |
| 20 | −483 | 74.9 | 103.7 | 79.3 | 93.10 | 0.931 | |
| 25 | −528 | 88.1 | 158.0 | 35.6 | 96.90 | 0.969 |
3.2.3.1. SEM-EDX study. The SEM micrographs of the inhibited and uninhibited metallic specimens after 3 h immersion time are shown in Fig. 6. It can be seen that the surfaces of the inhibited specimens are smoother when compared to the uninhibited specimen. The corresponding EDX spectra of inhibited and uninhibited metallic specimens are shown in Fig. S1 (ESI).† Careful inspection of the EDX spectra reveals that the spectrum of the uninhibited mild steel surface shows signals for the presence of C, O and Fe. However, the EDX spectra of inhibited mild steel specimens showed an additional signal for N indicating the presence of DAMP-GO and DAZP-GO on the surface which could be possible because of their adsorption on the metallic surface (Table 3).
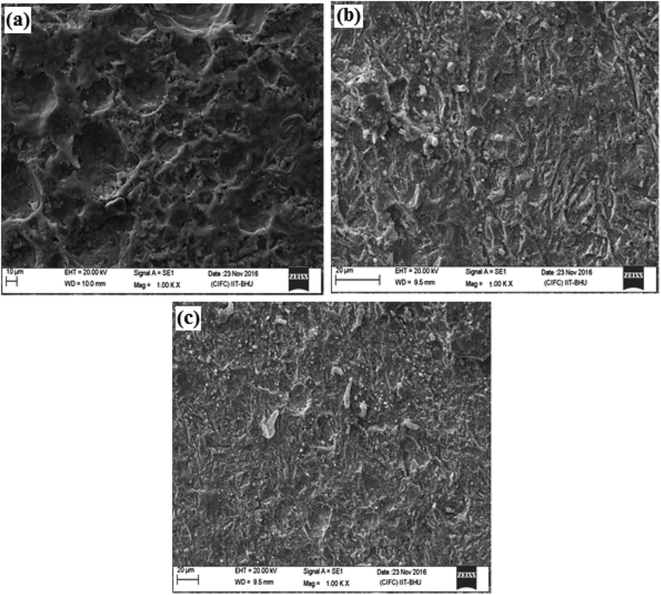 | ||
| Fig. 6 SEM micrographs in the absence (a) and the presence of DAMP-GO (b) and DAZP-GO (c) after 3 h immersion time. | ||
| Compounds | Parameters | ||||||
|---|---|---|---|---|---|---|---|
| EHOMO (eV) | ELUMO (eV) | ΔE (eV) | χ | η | σ | ΔN | |
| DAMP | −4.739 | 0.379 | 5.118 | 2.18 | 2.559 | 0.391 | 0.375 |
| DAZP | −3.036 | −2.432 | 0.604 | 2.73 | 0.302 | 3.311 | 4.100 |
3.2.3.2. AFM analysis. Fig. 7 shows the AFM micrographs of corroded mild steel surfaces in the presence and absence of 25 mg L−1 concentrations of the DAMP-GO and DAZP-GO. The average surface roughness of the uninhibited metallic specimen was 394 nm. However, in the presence of DAMP-GO and DAZP-GO, the surface roughness was reduced to 146 nm and 84 nm, respectively. Increased surface smoothness for inhibited specimens suggested that the inhibitors had been adsorbed by the metallic surface.
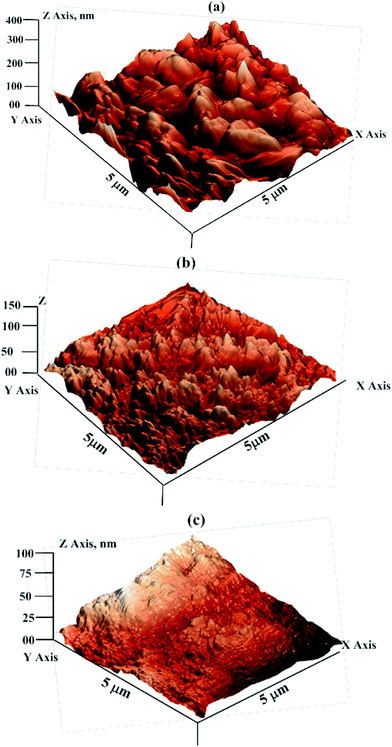 | ||
| Fig. 7 AFM micrographs in the absence (a) and presence of DAMP-GO (b) and DAZP-GO (c) after 3 h immersion time. | ||
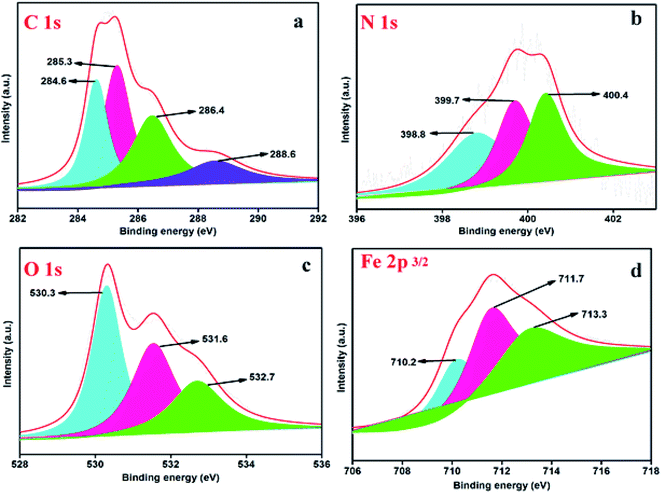
3.2.3.3. XPS analysis after inhibition. The XPS analysis after inhibition was used to study the adsorption of inhibitors on mild steel specimens in the presence of 25 mg L−1 of inhibitor and the results of this are shown in Fig. 8(a)–(d). The high-resolution C 1s XPS spectrum of mild steel specimens with concentrations of 25 mg L−1 of DAZP-GO consists of four peaks as shown in Fig. 8(a). The first signal at a binding energy of 284.6 eV is assigned to the C–C of graphene and its analogs. The second signal at 285.3 eV is attributed to the carbon atom bonded to nitrogen in DAZP-GO. The third and fourth signal are attributed to the C–O bond of the hydroxyl group (C–OH) and the presence of carboxylic group at 286.4 eV and 288.6, respectively.22,24 The high-resolution N 1s XPS spectrum of mild steel specimens with concentrations of 25 mg L−1 of DAZP-GO consists of three peaks as shown in Fig. 8(b). The first signal shows a peak at 398.8 eV from the nitrogen atoms of the DAZP ring bonded with the steel surface (N–Fe).46 The second signal at 399.7 eV in DAZP-GO results from the C–N bonds and unprotonated N atoms in DAZP-GO. The signal at 400.4 eV in DAZP-GO is from the protonated nitrogen atoms in the DAZP ring.22 The O 1s XPS spectra for DAZP-GO adsorbed mild steel specimens fitted the three signals as shown in Fig. 8(c). The first signal at 530.3 eV is attributed to O2− and this is attributed to an oxygen atom bonded to Fe3+ in the FeO, Fe2O3, and Fe3O4 oxides. The second signal observed at 531.6 eV indicates the presence of OH−, which can associate with the occurrence of hydrous iron oxides, such as FeOOH. The third signal at 532.7 eV indicates the presence of oxygen as an hydroxyl group (C–OH) in DAZP.46 The high-resolution Fe 2p XPS spectrum of mild steel specimens with concentrations of 25 mg L−1 of DAZP consists of three peaks which are shown in Fig. 8(d). The first signal at 710.2 eV indicates the presence of Fe3+ in ferric compounds such as Fe2O3 and FeOOH. The second signal at 711.7 eV indicates the presence of a small concentration of FeCl3 on the mild steel surface. The signal at a 713.6 eV indicates the presence of Fe(III).46 The results obtained from XPS analysis support the adsorption of inhibitors on the mild steel surface.
4. Conclusions
The present study involves the synthesis of two functionalized GOs. Namely, diazopyridine functionalized graphene oxide (DAZP-GO) and diaminopyridine functionalized graphene oxide (DAMP-GO) and their characterization using FT-IR, XRD, Raman, XPS and TEM methods. The inhibitive action of these functionalized GO was evaluated in 1 M HCl solution using several techniques. The following conclusions were drawn:(1) Both the functionalized GO act as superior corrosion inhibitors and their inhibition efficiency increases with their concentrations. The maximum inhibition efficiencies of 95.06% and 96.73% were obtained for DAMP-GO and DAZP-GO, respectively, at a concentration as low as 25 mg L−1.
(2) The EIS results showed that DAMP-GO and DAZP-GO inhibits corrosion by adsorption on the metallic surface, thus forming a protective barrier.
(3) The results of a potentiodynamic polarization study showed that both functionalized GOs acted as mixed type corrosion inhibitors.
(4) The results of the SEM and AFM studies suggest that surfaces of the inhibited metallic specimens become smoother when compared to the surface of the uninhibited metallic specimen which results in the adsorption and formation of the protective barrier by the functionalized GOs which protect the metal from corrosion. The formation of a protective film was further supported by the results of the EDX spectroscopic analysis where significant enhancement in the signal for nitrogen was observed with the inhibited metallic specimens.
(5) Adsorption of the tested functionalized GO on the metallic surface was further supported by the results of the XPS analysis.
(6) Experimental results were supported by DFT-based quantum chemical calculations. Several derived DFT-based parameters such as EHOMO, ELUMO, ΔE, χ, σ, η, and ΔN validated the experimentally determined order of inhibition efficiency.
Acknowledgements
RKG, NKG and CV, thankfully acknowledge the Government of India, Ministry of Human Resource Development (MHRD), New Delhi for providing funding.References
- R. Javaherdashti, Anti-Corros. Methods Mater., 2000, 47, 30–34 CrossRef.
- G. Koller, U. Fischer and K. Hungerbühler, Ind. Eng. Chem. Res., 2000, 39, 960–972 CrossRef CAS.
- G. Hamer, Biotechnol. Adv., 2003, 22, 71–79 CrossRef CAS PubMed.
- G. H. Koch, J. Varney, N. G. Thompson, O. Moghissi, M. Gould and J. Payer, International Measures of Prevention, Application, and Economics of Corrosion Technologies Study, NACE International, Houston, TX, 2016, pp. 2–6 Search PubMed.
- G. H. Koch, in Historic Congressional Study: Corrosion Costs And Preventive Strategies, The United States, 2002 Search PubMed.
- G. Gece, Corros. Sci., 2008, 50, 2981–2992 CrossRef CAS.
- F. Mansfeld, Electrochim. Acta, 1990, 35, 1533–1544 CrossRef CAS.
- M. Ormellese, L. Lazzari, S. Goidanich, G. Fumagalli and A. Brenna, Corros. Sci., 2009, 51, 2959–2968 CrossRef CAS.
- E. Sherif and S.-M. Park, Corros. Sci., 2006, 48, 4065–4079 CrossRef CAS.
- A. Yıldırım and M. Cetin, Corros. Sci., 2008, 50, 155–165 CrossRef.
- E. Oguzie, Y. Li and F. Wang, J. Colloid Interface Sci., 2007, 310, 90–98 CrossRef CAS PubMed.
- S. Umoren and E. Ebenso, Mater. Chem. Phys., 2007, 106, 387–393 CrossRef CAS.
- F. Bentiss, M. Traisnel and M. Lagrenee, J. Appl. Electrochem., 2001, 31, 41–48 CrossRef CAS.
- E. M. Fayyad, K. K. Sadasivuni, D. Ponnamma and M. A. Al-Maadeed, Carbohydr. Polym., 2016, 151, 871–878 CrossRef CAS PubMed.
- N. Kirkland, T. Schiller, N. Medhekar and N. Birbilis, Corros. Sci., 2012, 56, 1–4 CrossRef CAS.
- D. Prasai, J. C. Tuberquia, R. R. Harl, G. K. Jennings and K. I. Bolotin, ACS Nano, 2012, 6, 1102–1108 CrossRef CAS PubMed.
- J. Mondal, M. Marandi, J. Kozlova, M. Merisalu, A. Niilisk and V. Sammelselg, J. Chem. Chem. Eng., 2014, 8, 786–793 CAS.
- B. Ramezanzadeh, S. Niroumandrad, A. Ahmadi, M. Mahdavian and M. M. Moghadam, Corros. Sci., 2016, 103, 283–304 CrossRef CAS.
- A. B. Ikhe, A. B. Kale, J. Jeong, M. J. Reece, S.-H. Choi and M. Pyo, Corros. Sci., 2016, 109, 238–245 CrossRef CAS.
- J. Mondal, M. Kozlova and V. Sammelselg, J. Nanosci. Nanotechnol., 2015, 15, 6747–6750 CrossRef CAS PubMed.
- K. Chang, W. Ji, C. Li, C. Chang, Y. Peng, J. Yeh and W. Liu, eXPRESS Polym. Lett., 2014, 8, 908–919 CrossRef.
- A. Gupta, B. K. Shaw and S. K. Saha, RSC Adv., 2014, 4, 50542–50548 RSC.
- D. Dinda and S. K. Saha, J. Hazard. Mater., 2015, 291, 93–101 CrossRef CAS PubMed.
- A. Gupta, B. K. Shaw and S. K. Saha, J. Phys. Chem. C, 2014, 118, 6972–6979 CAS.
- A. Gupta and S. K. Saha, Nanoscale, 2012, 4, 6562–6567 RSC.
- C. Verma, M. Quraishi and A. Singh, J. Taiwan Inst. Chem. Eng., 2016, 58, 127–140 CrossRef CAS.
- C. Verma, M. Quraishi and E. Ebenso, Int. J. Electrochem. Sci., 2013, 8, 10851–10863 CAS.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS.
- C. Verma, L. Olasunkanmi, I. Obot, E. E. Ebenso and M. Quraishi, RSC Adv., 2016, 6, 15639–15654 RSC.
- A. D. Becke, Phys. Rev. A, 1988, 38, 3098 CrossRef CAS.
- C. Verma, M. Quraishi, L. Olasunkanmi and E. E. Ebenso, RSC Adv., 2015, 5, 85417–85430 RSC.
- C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785 CrossRef CAS.
- D. K. Singh, V. Kumar, V. K. Singh and S. H. Hasan, RSC Adv., 2016, 6, 56684–56697 RSC.
- P. Singh, E. E. Ebenso, L. O. Olasunkanmi, I. B. Obot and M. A. Quraishi, J. Phys. Chem. C, 2016, 120, 3408–3419 CAS.
- L. O. Olasunkanmi, I. B. Obot, M. M. Kabanda and E. E. Ebenso, J. Phys. Chem. C, 2015, 119, 16004–16019 CAS.
- C. Verma, E. E. Ebenso, L. O. Olasunkanmi, M. A. Quraishi and I. B. Obot, J. Phys. Chem. C, 2016, 120, 11598–11611 CAS.
- J. Haque, V. Srivastava, C. Verma and M. Quraishi, J. Mol. Liq., 2017, 225, 848–855 CrossRef CAS.
- R. Solmaz, G. Kardaş, M. Culha, B. Yazıcı and M. Erbil, Electrochim. Acta, 2008, 53, 5941–5952 CrossRef CAS.
- M. Erbil, Chim. Acta Turc., 1988, 1, 59–70 Search PubMed.
- A. Yousefi, S. Javadian, N. Dalir, J. Kakemam and J. Akbari, RSC Adv., 2015, 5, 11697–11713 RSC.
- C. Verma, A. Singh, G. Pallikonda, M. Chakravarty, M. Quraishi, I. Bahadur and E. Ebenso, J. Mol. Liq., 2015, 209, 306–319 CrossRef CAS.
- A. B. Ikhe, A. B. Kale, J. Jeong, M. J. Reece, S.-H. Choi and M. Pyo, Corros. Sci., 2016, 109, 238–245 CrossRef CAS.
- R. Yıldız, T. Doğan and I. Dehri, Corros. Sci., 2014, 85, 215–221 CrossRef.
- P. Mourya, S. Banerjee and M. Singh, Corros. Sci., 2014, 85, 352–363 CrossRef CAS.
- P. Singh and M. Quraishi, Measurement, 2016, 86, 114–124 CrossRef.
- P. Singh, V. Srivastava and M. Quraishi, J. Mol. Liq., 2016, 216, 164–173 CrossRef CAS.
- T. Arslan, F. Kandemirli, E. E. Ebenso, I. Love and H. Alemu, Corros. Sci., 2009, 51, 35–47 CrossRef CAS.
- I. Obot and Z. Gasem, Corros. Sci., 2014, 83, 359–366 CrossRef CAS.
- I. Obot and N. Obi-Egbedi, Corros. Sci., 2010, 52, 657–660 CrossRef CAS.
- S. Yesudass, L. O. Olasunkanmi, I. Bahadur, M. M. Kabanda, I. Obot and E. E. Ebenso, J. Taiwan Inst. Chem. Eng., 2016, 64, 252–268 CrossRef CAS.
- M. ElBelghiti, Y. Karzazi, A. Dafali, B. Hammouti, F. Bentiss, I. Obot, I. Bahadur and E. Ebenso, J. Mol. Liq., 2016, 218, 281–293 CrossRef CAS.
- L. C. Murulana, M. M. Kabanda and E. E. Ebenso, J. Mol. Liq., 2016, 215, 763–779 CrossRef CAS.
- L. O. Olasunkanmi, M. M. Kabanda and E. E. Ebenso, Phys. E, 2016, 76, 109–126 CrossRef CAS.
- A. Khadiri, R. Saddik, K. Bekkouche, A. Aouniti, B. Hammouti, N. Benchat, M. Bouachrine and R. Solmaz, J. Taiwan Inst. Chem. Eng., 2016, 58, 552–564 CrossRef CAS.
- A. Döner, R. Solmaz, M. Özcan and G. Kardaş, Corros. Sci., 2011, 53, 2902–2913 CrossRef.
- I. Obot, D. Macdonald and Z. Gasem, Corros. Sci., 2015, 99, 1–30 CrossRef CAS.
- N. O. Eddy, H. Momoh-Yahaya and E. E. Oguzie, J. Adv. Res., 2015, 6, 203–217 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra05825j |
| This journal is © The Royal Society of Chemistry 2017 |