Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Remarkable lignin degradation in paper wastewaters over Fe2O3/γ-Al2O3 catalysts using the catalytic wet peroxide oxidation method†
Di Wua,
Zhen Hua,
Xuqi Zhanga,
Cuiran Zhanga,
Kai Sunb and
Shuxiang Lu *a
*a
aCollege of Chemical Engineering and Materials Science, Tianjin University of Science and Technology, Tianjin 300457, People's Republic of China. E-mail: lshx@tust.edu.cn; Fax: +86 22 60600030; Tel: +86 22 60601157
bSchool of Environment and Energy, South China University of Technology, Guangzhou 510641, People's Republic of China
First published on 28th July 2017
Abstract
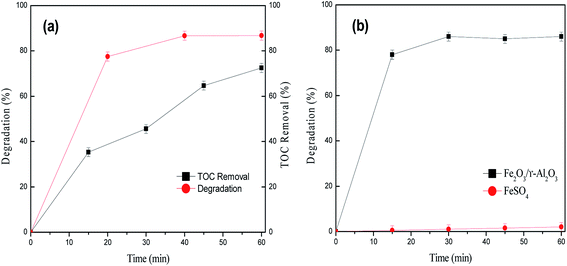
Heterogeneous catalytic wet peroxide oxidation (CWPO) has been found to be one of the most effective approaches for the degradation of toxic and paper factory wastewater. Fe2O3/γ-Al2O3 catalysts were prepared by an impregnation method and characterized by X-ray diffraction (XRD), temperature-programmed desorption (TPD), transmission electron microscopy (TEM) and scanning electron microscopy (SEM). The catalytic activity of Fe2O3/γ-Al2O3 for the CWPO of lignin was assessed. The effects of initial solution pH, catalyst dosage, initial concentration of hydrogen peroxide, reaction temperature and initial concentration of lignin on the reactivity of the system were investigated. Higher lignin degradation can be achieved under not only under acidic but also neutral and slightly alkaline conditions. Under initial conditions of pH = 9, T = 348 K, 6 g L−1 catalyst, 6.396 mM hydrogen peroxide and 100 mg L−1 lignin, 87% lignin degradation and 86% total organic content (TOC) removal were achieved in 60 min. The negligible amount of leaching Fe ions found in solution was measured by inductively coupling plasma-atomic emission spectra (ICP-AES).
1. Introduction
Recently, environmental problems have attracted much attention, and many stringent regulations have been published to restrict harmful industry effluents at a low level. The paper manufacturing process produces much wastewater, which incorporates quantities of lignin, a phenolic derivative with high colority and total organic content (TOC).1,2 Lignin is an irregular tri-dimensional polymer composed of aromatic/phenolic units and is resistant to microbial attack3,4, although some methods for the degradation of lignin have previously been reported.5 After secondary treatment using a conventional microbial method, most TOC is removed; however, stubborn paper factory lignin and its derivatives still remain, resulting in severe environmental pollution.6,7 In recent years, advanced oxidation processes (AOPs)8 with Fenton's reagent (Fe2+/Fe3+/H2O2) as an oxidatant9 have been used for the degradation of a great variety of organic compounds.10 However, homogenous Fenton systems have some well-known drawbacks, including limited pH range, catalyst deactivation and the production of iron-containing sludge that requires further disposal.11 Many researchers have recently reported on the use of heterogeneous catalysts containing hydrogen, including the heterogeneous catalytic wet peroxide oxidation (CWPO) process, as an alternative. Heterogeneous catalytic oxidation systems provide easy separation and recovery of the catalyst from the treated wastewater and avoid the drawbacks of the Fenton's reagent, primarily difficulties in recycling. Many materials predominantly containing iron as the precursor, and supported or intercalated on or in oxides, clays, zeolite and polymers, have been proposed for the removal of various organic compounds, such as phenols,12,13 dyes,14,15 2-chlorophenol,16 atrazine,17 methyl t-butyl ether (MTBE)18 and hydrogen peroxide. No papers have been published in respect of the catalytic oxidation of high-molecular lignin obtained from the Kraft digestion of wheat straw in such heterogeneous catalytic oxidation systems. The main objective of this research was to evaluate the catalytic activity of Fe2O3/γ-Al2O3 catalysts for the CWPO of lignin in an aqueous solution. The influencing factors, such as temperature, catalyst dosage, H2O2 concentration and pH of the solution, were investigated. The reactivity of this process was monitored in terms of lignin degradation and TOC removal.2. Experimental
2.1. Materials
γ-Al2O3 (296.8 m2 g−1) was purchased from Tianjin Chemical Research and Design Institute, China. Sulfuric acid was used in the lignin precipitation, and then the precipitate was washed with deionized water and dried at room temperature.19 Lignin aqueous solutions were prepared by dissolving the obtained lignin in 0.05 M NaOH solution. 30 wt% hydrogen peroxide aqueous solutions were used as an oxidant.2.2. Catalyst preparation
Fe2O3/γ-Al2O3 catalysts were prepared by the impregnation method with Fe(NO3)2 as the precursor and γ-Al2O3 as the carrier. The mean diameter of the alumina particle was in the range 124–150 μm. Alumina was impregnated with the Fe(NO3)2 solution via the wet procedure for 12 h under room conditions. Next, the particles were dried at 353 K for 10 h, and then calcined in a furnace at 673 K for 4 h to obtain Fe2O3/γ-Al2O3 catalyst containing 15 wt% Fe component.2.3. Characterization of the catalyst
Characterization of the samples was performed using various techniques. X-ray powder diffraction (XRD) data were acquired on a Bruker AXS D8-Focus diffractometer using Cu-Kα radiation. The data were collected in a 2θ range of 10° to 80° with a scanning speed of 4° min−1. The morphology and microstructure were examined on Transmission 100 Electron Microscope (TEM) operating at 200 kV coupled with energy-dispersive X-ray spectroscopy (EDS), and scanning electron microscopy (SEM) was performed using a Hitachi Limited US-1510 system. The acidity of the catalyst samples was measured in the temperature range 323–823 K by measuring the temperature-programmed desorption of ammonia (NH3-TPD).2.4. Procedures and analysis
For the catalytic oxidation of lignin, a defined concentration of simulated lignin wastewater was poured into a 500 mL three-necked round-bottomed glass reactor which was equipped with continuous mechanical stirring for each test. In each trial, 250 mL lignin solution was added to the reactor. During the reaction process, 5 mL samples were taken out for analysis every 15 min. The samples were centrifuged to remove the solids. Lignin concentration was determined using an ultraviolet (UV)/visible (Vis) spectrophotometer (Shanghai Spectrum, SP-2102UV) (Fig. S1†). Hydrogen peroxide conversion was determined by iodometric titration. A Beer-Lambert law was established to correlate the absorbency at 280 nm to lignin concentration.16 TOC was determined using a Sievers Innovox Laboratory TOC analyzer (General Electric Analytical Instruments).The degradation of lignin was determined in terms of the change in concentration of lignin and the reduction in TOC. The percentage degradation was calculated as follows (eqn (1)):
| Degradation ratio (%) = (C0 − C)/C0 × 100 | (1) |
TOC removal was calculated as follows (eqn (2)):
| TOC removal (%) = (TOC0 − TOC)/TOC0 × 100 | (2) |
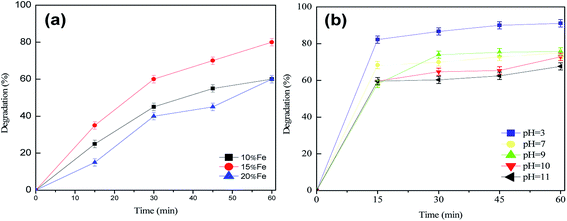
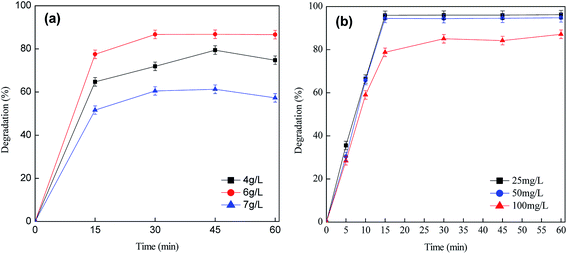
The effect of different Fe2O3 loadings (10%, 15%, 20%), initial pH value (pH 3–11), H2O2 concentration (0.799 mmol L−1, 1.599 mmol L−1, 6.396 mmol L−1, 9.594 mmol L−1), catalyst dosage/catalyst loading (10–60 mg) and initial lignin concentration (25–100 mg L−1) on the catalytic degradation reaction were investigated.
3. Results and discussion
3.1. Catalyst characterization
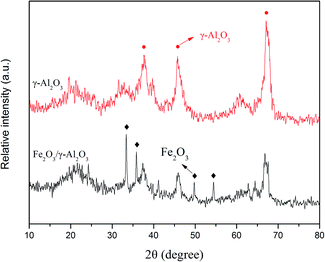
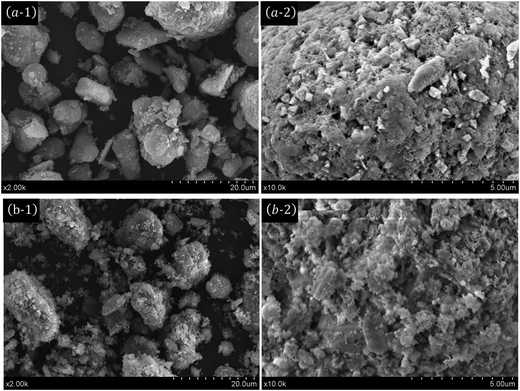
The crystallographic structures of the γ-Al2O3 support and Fe2O3/γ-Al2O3 catalyst samples were investigated by XRD patterns. Two typical diffraction peaks corresponding to γ-Al2O3 are observed in Fig. 1. Both of the XRD patterns show diffraction peaks at 2θ values of 37.54°, 45.67° and 66.60°, which were indexed to the diffraction peaks corresponding to the (311), (400) and (440) crystal planes, respectively, matching well with the standard phase of the γ-Al2O3 support. For the Fe2O3-loaded γ-Al2O3 catalyst, four strong diffraction peaks at 2θ values of 33.28°, 35.74°, 49.50° and 54.23° were indexed to the (104), (110), (024) and (116) crystal planes, respectively. The structure of the γ-Al2O3 support remained intact after the modification treatment, whereas a decrease in the intensity of the γ-Al2O3 diffraction peaks was observed compared with the γ-Al2O3 support, which could result from the higher X-ray absorption coefficient of the iron compounds.The morphology and structure of the synthesized materials were investigated using an SEM micrograph. Fig. 2 shows the SEM images of the γ-Al2O3 and Fe2O3/γ-Al2O3 catalyst. The micrograph of γ-Al2O3 exhibits a spherical shape ascribed to the γ-Al2O3 support. As can be seen in Fig. 2(b-1) and (b-2), Fe2O3 particles were loaded on the surface of γ-Al2O3. Moreover, the sphericity, which is compared with that of γ-Al2O3, hardly changes. In addition, the SEM images indicate that crystalline Fe2O3 was uniformly dispersed on the γ-Al2O3 support. The SEM images are consistent with the results of the XRD analysis.
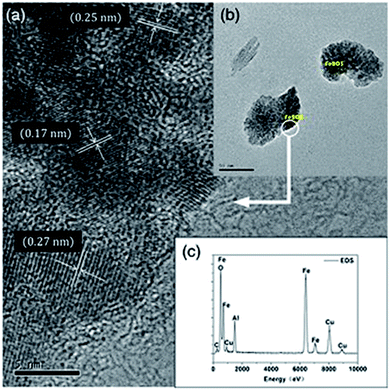
Fig. 3 shows the micrographs obtained by TEM for the Fe2O3/γ-Al2O3 catalyst. From the HR-TEM image (Fig. 3(a)), the lattice fringes of Fe2O3/γ-Al2O3 were clear. The d-spacing of 0.25 nm is attributed to the α-Fe2O3 (110) crystallite plane,20 and the d-spacing of 0.27 nm corresponds to the α-Fe2O3 (104) crystallite plane.21 These observations from HR-TEM were consistent with the results of the XRD analysis. The TEM image (Fig. 3(b)) indicated that Fe2O3/γ-Al2O3 exhibited a certain degree of agglomeration. In addition, the EDS confirmed the existence of Fe and Al, and the results provide evidence of the presence of Cu (Fig. 3(c)).
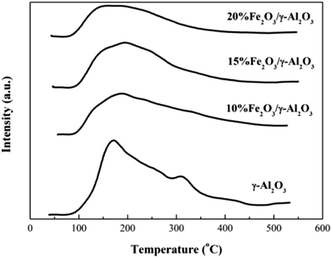
The NH3-TPD profiles are displayed in Fig. 4. In general, the overall NH3-desorption peak area decreases with increasing Fe2O3 loading. This is mainly due to the fact that Fe2O3 can cover some of the acid sites on the support surface. However, in comparison with the Al2O3 support, the peak which was attributed to the weak acid site is moving in the direction of higher temperature for the Fe2O3/Al2O3 catalyst. The weak acid sites continue increasing with the addition of 10, 15 and 20% wt% Fe2O3. When the loading reaches 15 wt%, the desorption peak area corresponding to the weak acid center is largest. Moreover, the peak which was attributed to the weak acid site is moving in the direction of higher temperature. In comparison with other catalysts, when the load is up to 15 wt%, the total acid amount and the acidic strength are highest. Therefore, it is clear that the loading of Fe2O3 not only reduces the surface area of the Al2O3 support, but also modifies the surface acid properties, since Fe2O3 interacts differently with the acid sites with different strengths. The modification of Al2O3 acidity is due to the covalent bond formation between the –OH surface group and the Fe-intermediate during preparation. Therefore, the introduction of the metal/metal oxides results in modulating the surface acidity.
3.2. Lignin oxidation test
| H2O2 + ˙OH → HO2˙ + H2O | (3) |
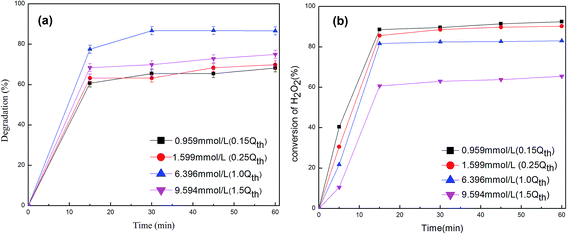
Generally, HO2˙ radicals are less active than ˙OH radicals and do not play any important role in the degradation of organic compounds compared with ˙OH radicals. Fig. 6(a) shows that lignin degradation rates increase with H2O2 concentration from 0.799 mM to 6.396 mM, then decrease with further increases in H2O2 concentration. This means the critical concentration of H2O2 is 6.396 mM for the degradation of 100 mg L−1 lignin solution in the presence of Fe2O3/γ-Al2O3 catalyst.
The effective utilization (i.e., the useful fraction of H2O2 that contributes to degrade organic contaminates) is a key factor in the cost of the CWPO process.23 For this reason, high degradation of organic contaminates with as little H2O2 as possible is preferred. The selectivity of H2O2 is given by eqn (4):
 | (4) |
The theoretical H2O2 consumption needed for the removal of obtained TOC conversion can be estimated17 according to the following reaction (eqn (5)):
| C + 2H2O2 → CO2 + 2H2O | (5) |
Selectivity was used to measure the effective utilization of H2O2. The selectivity is always below 100%, which means that there is some hydrogen peroxide decomposition useless to oxygen. Fig. 6(b) shows conversion of different concentrations of H2O2 on the process degradation of lignin. The conversion of H2O2 is almost complete in 15 min, and conversion decreases with increasing hydrogen peroxide concentration. The highest selectivity of H2O2 achieves 87% in 60 min with 6.396 mol L−1 of initial H2O2 concentration. This shows that higher initial H2O2 concentrations may not benefit degradation of organic contaminates and also increase the economic cost.
3.3. Influence of Fe2O3 leached out from the catalysts
The biggest problem with this process is Fe2O3 leached out from the catalysts into solution during heterogeneous CWPO processes.29 We measured the concentration of dissolved Fe in the solution after a 2 h catalytic reaction using the ICP method. The maximum concentration of Fe ions was 0.085 mg L−1 in the solution at various pHs. It is known that the leaching of metal from heterogeneous catalysts depends on the pH; when the pH is above 5, the leaching metal concentration in the solution is negligible.15,22,23 Since leaching of the Fe ion could play an important role in the CWPO of lignin, further catalytic studies were carried out. The activity of FeSO4 as a homogenous catalyst was compared with that of the Fe2O3/γ-Al2O3 heterogeneous catalyst for the catalytic oxidation of lignin under same reaction conditions. The concentration of the Fe ion in the solution was 0.085 mg L−1, which was equivalent to the concentration of the leaching Fe ion from Fe2O3/γ-Al2O3. It can be observed from Fig. 8(b) that only 0.8% lignin degradation is achieved in 1 h, whereas 87% lignin degradation is reached in the presence of Fe2O3/γ-Al2O3. A comparison of these two experiments shows that the homogeneous catalytic reaction formed by leaching of the Fe ion from Fe2O3/γ-Al2O3 has a negligible effect as compared with the heterogeneous catalytic process. This also proves that the oxidation of lignin took place on the catalyst surface via a heterogeneous mechanism.3.4. Mechanism of reaction system
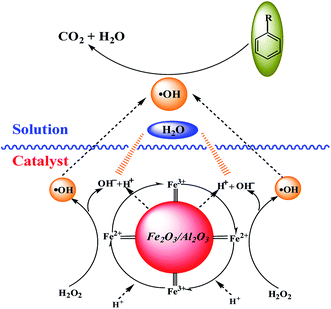
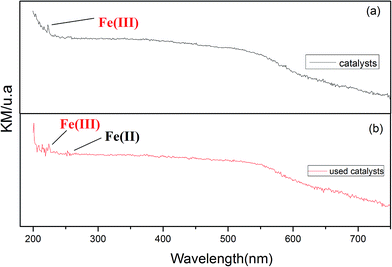
The mechanism of the heterogeneous Fenton-like system has previously been described,24,25 but not the oxidation of lignin. In this mechanism (Fig. 9), ˙OH radicals may be formed in situ on the active sites of the catalyst rather than generating ˙OH radicals in the solution. In addition, the enhanced surface acidity of the catalyst can promote the degradation of lignin in a slightly alkaline environment. Combined with the NH3-TPD analysis, the following reason may be offered for this phenomenon. Because of the surface acidity of the catalyst, which for the alkali lignin solution is adsorbed on the catalyst surface providing an acidic environment, lignin can be degraded under acidic conditions. Further, almost the same degradation rate is achieved whether it is acidic or slightly alkaline lignin wastewater. Therefore, it can be concluded that for Fe2O3/γ-Al2O3, the acid–base property of the solution can not affect the activity. Without pH-range limitation, Fe2O3/γ-Al2O3 is a promising catalyst for catalytic oxidation pulp and paper effluents, because almost all initial effluents are in slightly alkaline conditions.We tested fresh catalyst and catalyst after being subjected to UV-vis, and showed that an extra Fe(II) ion emerges in addition to the Fe(III) ion. This further confirms the conversion between Fe(II) and Fe(III) ions. This further proves the mechanism shown in Fig. 10, and shows that Fe2O3/γ-Al2O3 catalysts can also degrade lignin effectively from neutral up to slightly alkaline environments (pH range 3–11) because of the presence of a few hydrogen ions.
4. Conclusion
Fe2O3/γ-Al2O3 was prepared and used as the catalyst in the heterogeneous CWPO of lignin under mild conditions (at atmospheric pressure and a temperature of 348 K). From the results of catalyst characterization, crystalline hematite (α-Fe2O3) particles have been formed on the surface of γ-Al2O3 with better dispersion. In addition, from the NH3-TPD covers, when the loading is up to 15 wt%, the total acid amount and acidic strength are highest. There are critical concentrations of catalyst dosage and initial concentration of H2O2 for the degradation of lignin in this system. Above this critical concentration, the degradation of lignin will decrease due to a scavenging effect. Fe2O3/γ-Al2O3 has shown better catalytic activity for the CWPO of lignin in a wider pH range (from pH = 3 to pH = 11), suggesting that this is a promising catalyst for the catalytic oxidation of wastewater containing toxic and paper factory organic compounds in neutral and alkaline environments.Acknowledgements
We would like to acknowledge the financial support of key Project of Tianjin Research Program of Application Foundation and Advanced Technology (No. 14JCZDJC40600) and National Undergraduate Innovation and Entrepreneurship Training Project (No. 201710057010).References
- S. K. Kansal, M. Singh and D. Sud, J. Hazard. Mater., 2008, 153, 412–417 CrossRef CAS PubMed.
- A. Raj, M. M. K. Reddy, R. Chandra, H. J. Purohit and A. Kapley, Biodegradation, 2007, 18, 783–792 CrossRef CAS PubMed.
- A. M. Elfadly, I. F. Zeid, F. Z. Yehia, M. M. Abouelela and A. M. Rabie, Fuel Process. Technol., 2017, 163, 1–7 CrossRef CAS.
- M. Ksibi, S. Ben Amor, S. Cherif, E. Elaloui, A. Houas and M. Elaloui, J. Photochem. Photobiol., A, 2003, 154, 211–218 CrossRef CAS.
- M. Niu, Y. Hou, W. Wu and R. Yang, Fuel Process. Technol., 2017, 161, 295–303 CrossRef CAS.
- J. L. Tambosi, M. Di Domenico, W. N. Schirmer, H. J. José and R. de F. Moreira, J. Chem. Technol. Biotechnol., 2006, 81, 1426–1432 CrossRef CAS.
- O. A. Makhotkina, S. V. Preis and E. V. Parkhomchuk, Appl. Catal., B, 2008, 84, 821–826 CrossRef CAS.
- M. Kitis and S. S. Kaplan, Chemosphere, 2007, 68, 1846–1853 CrossRef CAS PubMed.
- J. Li, L. Zhao, L. Qin, X. Tian, A. Wang, Y. Zhou, L. Meng and Y. Chen, Chemosphere, 2016, 146, 442–449 CrossRef CAS PubMed.
- P. Bautista, A. F. Mohedano, J. A. Casas, J. A. Zazo and J. J. Rodriguez, J. Chem. Technol. Biotechnol., 2008, 83, 1323–1338 CrossRef CAS.
- M. Tekbaş, H. C. Yatmaz and N. Bektaş, Microporous Mesoporous Mater., 2008, 115, 594–602 CrossRef.
- K. Fajerwerg and H. Dehellefontaine, Appl. Catal., B, 1996, 10, 229–235 CrossRef.
- J. A. Zazo, J. A. Casas, A. F. Mohedano and J. J. Rodriguez, Appl. Catal., B, 2006, 65, 261–268 CrossRef CAS.
- P. Baldrian, V. Merhautova, J. Gabriel, F. Nerud, P. Stopka, M. Hruby and M. J. Benes, Appl. Catal., B, 2006, 66, 258–264 CrossRef CAS.
- T. L. P. Dantas, V. P. Mendonca, H. J. Jose, A. E. Rodrigues and R. F. P. M. Moreira, Chem. Eng. J., 2006, 118, 77–82 CrossRef CAS.
- H.-H. Huang, M.-C. Lu and J.-N. Chen, Water Res., 2001, 35, 2291–2299 CrossRef CAS PubMed.
- J. A. Botas, J. A. Melero, F. Maeinez and M. I. Pariente, Catal. Today, 2010, 149, 334–340 CrossRef CAS.
- O. A. Makhotkina, S. V. Preis and E. V. Parkhomchuk, Appl. Catal., B, 2008, 84, 821–826 CrossRef CAS.
- A. García, A. Toledano, L. Serrano, I. Egüés, M. González, F. Marín and J. Labidi, Sep. Purif. Technol., 2009, 68, 193–198 CrossRef.
- W.-J. Liu, F.-X. Zeng, H. Jiang, X.-S. Zhang and W.-W. Li, Chem. Eng. J., 2012, 180, 9–18 CrossRef CAS.
- H. Liu, G. Wang, J. Park, J. Wang, H. Liu and C. Zhang, Electrochim. Acta, 2009, 54, 1733–1736 CrossRef CAS.
- C. L. Hsueh, Y. H. Huang, C. C. Wang and C. Y. Chen, Chemosphere, 2005, 58, 1409–1414 CrossRef CAS PubMed.
- K. Maduna Valkaj, A. Katovic, V. Tomašic and S. Zrncevic, Chem. Eng. Technol., 2008, 31, 398–403 CrossRef.
- W. P. Kwan and B. M. Voelker, Environ. Sci. Technol., 2003, 37, 1150–1158 CrossRef CAS PubMed.
- R. L. Valentine and H. C. A. Wang, J. Environ. Eng., 1998, 124, 31–36 CrossRef CAS.
- M. Hermanek, R. Zboril, I. Medrik, J. Pechousek and C. Gregor, J. Am. Chem. Soc., 2007, 129, 10929–10936 CrossRef CAS PubMed.
- K. Huang, Y. Xu, L.-G. Wang and D.-F. Wu, RSC Adv., 2015, 5, 32795–32803 RSC.
- K. Huang, J.-J. Wang, D. Wu and S. Lin, RSC Adv., 2015, 11, 8455–8462 RSC.
- Y. Yan, X.-W. Wu and H.-P. Zhang, Sep. Purif. Technol., 2016, 171, 52–61 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra05696f |
| This journal is © The Royal Society of Chemistry 2017 |