Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
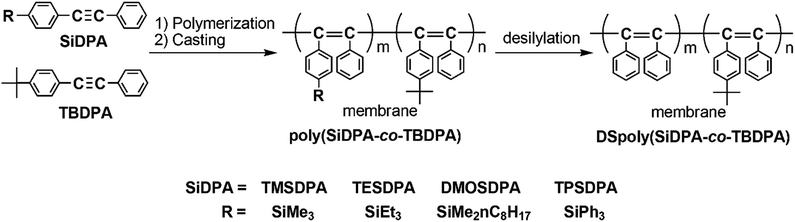
Enhancement of oxygen permeability by copolymerization of silyl group-containing diphenylacetylenes with tert-butyl group-containing diphenylacetylene and desilylation of copolymer membranes
T. Sakaguchi *,
Y. Lin and
T. Hashimoto
*,
Y. Lin and
T. Hashimoto
Department of Materials Science and Engineering, Graduate School of Engineering, University of Fukui, Bunkyo, Fukui 910-8507, Japan. E-mail: sakaguchi@matse.u-fukui.ac.jp
First published on 15th June 2017
Abstract
Diphenylacetylenes having various silyl groups [PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CC6H4-R; R = p-SiMe3 (TMSDPA), p-SiEt3 (TESDPA), p-SiMe2-n-C8H17 (DMOSDPA), and p-SiPh3 (TPSDPA)] were copolymerized with diphenylacetylene having a tert-butyl group (PhC
CC6H4-R; R = p-SiMe3 (TMSDPA), p-SiEt3 (TESDPA), p-SiMe2-n-C8H17 (DMOSDPA), and p-SiPh3 (TPSDPA)] were copolymerized with diphenylacetylene having a tert-butyl group (PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CC6H4-tertBu; TBDPA) using a TaCl5–n-Bu4Sn catalyst in various monomer feed ratios to provide high-molecular-weight copolymers in high yields. The free-standing membranes were fabricated by solution-casting, except poly(TPSDPA-co-TBDPA). Interestingly, the gas permeability of poly(TMSDPA-co-TBDPA) was higher than those of the homopolymers, poly(TMSDPA) and poly(TBDPA). The permeability of the copolymers became lower as the silyl groups became bulkier. The desilylation of membranes was carried out using a mixture of trifluoroacetic acid/hexane. When bulkier silyl groups were removed, the oxygen permeability increased to larger extents. The oxygen permeability coefficients of the copolymers and desilylated copolymers increased with increasing composition ratio of TBDPA. The gas diffusivity and gas solubility were also increased upon desilylation.
CC6H4-tertBu; TBDPA) using a TaCl5–n-Bu4Sn catalyst in various monomer feed ratios to provide high-molecular-weight copolymers in high yields. The free-standing membranes were fabricated by solution-casting, except poly(TPSDPA-co-TBDPA). Interestingly, the gas permeability of poly(TMSDPA-co-TBDPA) was higher than those of the homopolymers, poly(TMSDPA) and poly(TBDPA). The permeability of the copolymers became lower as the silyl groups became bulkier. The desilylation of membranes was carried out using a mixture of trifluoroacetic acid/hexane. When bulkier silyl groups were removed, the oxygen permeability increased to larger extents. The oxygen permeability coefficients of the copolymers and desilylated copolymers increased with increasing composition ratio of TBDPA. The gas diffusivity and gas solubility were also increased upon desilylation.
Introduction
Membrane separation for mixed gases has become important because of the low costs and energy consumption compared with traditional separation techniques such as adsorption and absorption, and a variety of polymers with high permeability or high selectivity have been studied.1–5 Membranes of disubstituted acetylenic polymers with bulky spherical substituents have a large free volume and exhibit pretty high gas permeability. This feature originates from their stiff main chain composed of alternating double bonds and the steric repulsion of the substituents.6–8 For instance, the oxygen permeability coefficient (PO2) of poly[1-phenyl-2-(p-trimethylsilyl)phenylacetylene], poly(TMSDPA), was as large as 1100–1500 barrer.9,10 Poly[1-phenyl-2-(p-tert-butyl)phenylacetylene], poly(TBDPA), also shows high gas permeability, and its PO2 value was 1100 barrer.11 These poly(substituted acetylene)s are promising materials for gas separation membranes, and further high gas-permeable polymer membranes are desired as oxygen-enriched membranes. Teraguchi et al. reported that the desilylation of a membrane of poly(TMSDPA) afforded a membrane of poly(diphenylacetylene),12 which cannot be obtained by solution-casting and hot-press methods because it is insoluble and infusible. In the desilylation reaction of polymer membrane, it has been predicted that the spaces occupied by silyl groups are maintained in some level as micro-scale voids since the mobility of polymer chain is restrained in a solid state. Contrary to prediction, the desilylation of membrane of poly(TMSDPA), however, decreased the gas permeability.10 This is because the chain packing is induced by the elimination of bulky spherical trimethylsilyl groups. If the chain packing during the desilylation is prevented, the gas permeability of the membrane should increase.In the present study, we synthesized copolymers of diphenylacetylenes having silyl and tert-butyl groups at para position of phenyl groups (Scheme 1), and examined the desilylation of the membranes. The desilylated copolymers have still bulky tert-butyl groups, and therefore the spaces occupied by silyl groups are possibly maintained after desilylation by the steric repulsion of tert-butyl groups.
Experimental
Materials
Toluene which is polymerization solvent was purified by two times of distillation in the presence of CaH2. Tantalum(V) chloride (TaCl5, 99.999%, Aldrich) as main catalyst was used without further purification, and tetra-n-butyltin (n-Bu4Sn, Wako) as cocatalyst was used after distillation under reduced pressure. Phenylacetylene (Aldrich), triethylamine, triphenylphosphine, copper(I) iodide, dichlorobis(triphenylphosphine)palladium(II), p-bromo-tert-butylbenzene, 1,4-dibromobenzene, trifluoroacetic acid (TFA), n-butyllithium hexane solution (1.6 M), chlorotrimethylsilane, chlorotriethylsilane, chlorodimethyl-n-octylsilane, chlorotriphenylsilane, and common solvents (Wako Pure Chemical) were used without further purification. 1-Phenyl-2-(p-trimethylsilyl)phenylacetylene (TMSDPA),9 1-phenyl-2-(p-triethylsilyl)phenylacetylene (TESDPA),13 1-phenyl-2-(p-dimethyl-n-octylsilyl)phenylacetylene (DMOSDPA),10 1-phenyl-2-(p-triphenylsilyl)phenylacetylene (TPSDPA),14 and 1-phenyl-2-(p-tert-butyl)phenylacetylene (TBDPA)11 were synthesized according to the literatures.Measurements
The molecular weight distribution (MWD) of polymers were measured by gel permeation chromatography (GPC) in CHCl3 (at a 1.0 mL min−1 flow rate) at 40 °C on a Shimadzu LC-10AD chromatograph equipped with three polystyrene gel columns (Shodex K-804L, K-805L, and K-807L) and a Shimadzu RID-6A refractive index detector. The weight-average molecular weight (Mw) and polydispersity ratio [weight-average molecular weight/number-average molecular weight (Mw/Mn)] were calculated from chromatograms based on a polystyrene calibration. 1H (500 MHz) and 13C (125 MHz) NMR spectra were recorded on Jeol ECX-500 instrument in CDCl3 at room temperature. IR spectra were recorded on a Nicolet iS5 560 spectrometer. A membrane thickness was measured using a micrometer, and it was estimated as an average amount of ten points on each membrane.Gas permeability coefficients of polymer membranes were measured with a Tsukubarikaseiki K-315-N gas permeability apparatus equipped with an MKS Baratron detector at 25 °C. The downstream side of the membrane was evacuated at ca. 0.3 Pa, while the upstream side was filled with a gas at ca. 1 atm (105 Pa), and the increase of pressure in a downstream receiving vessel was measured. The permeability coefficient (P) expressed in barrer unit [1 barrer = 1 × 10−10 cm3 (STP) cm cm−2 s−1 cmHg−1] was calculated from the slopes of time–pressure curves in the steady state where Fick's law held. The diffusion coefficient (D) expressed in cm2 s−1 units was determined by the time lag method using the following equation:
| D = l2/6θ |
| S = P/D. |
Copolymerization
Copolymerizations were performed in a glass tube equipped with a three-way stopcock at 80 °C for 24 h under dry nitrogen at the following reagent concentrations: [M]0 + [TBDPA]0 = 0.20 M, [TaCl5] = 20 mM, [n-Bu4Sn] = 80 mM. A detailed procedure of polymerization is as follows: the monomer solution was prepared in a glass tube by mixing TMSDPA (0.25 g), TBDPA (0.23 g) and dry toluene (5.0 mL). Another glass tube was charged with TaCl5 (71 mg), 0.8 M of n-Bu4Sn in toluene (1.0 mL), and dry toluene (4.0 mL); this catalyst solution was aged at 80 °C for 10 min. Then the monomer solution was added to the catalyst solution. Polymerization was carried out at 80 °C for 24 h, which was quenched with a small amount of methanol. The resulting polymer was isolated by precipitation into a large excess of methanol and its yield was determined by gravimetry. Poly(TPSDPA-co-TBDPA) was isolated by precipitation into a large excess of acetone.Membrane fabrication and desilylation
The membranes (thickness ca. 140–200 μm) of copolymers were fabricated by casting from toluene solution of the copolymers (concentration ca. 0.20–0.60 wt%) into Petri dishes at room temperature. The dish was covered with a glass vessel to slow solvent evaporation (3–5 days). After a membrane was formed, the membrane was peeled off, and it was immersed in methanol for 24 h and dried to constant weight at room temperature. With reference to the method described in the literature,12 the desilylation of membranes was carried out using a mixture of trifluoroacetic acid and hexane. A detailed procedure is as follows: the copolymer membrane was immersed in a mixture of trifluoroacetic acid and hexane (volume ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at room temperature for 24 h. To remove residual impurities in the polymer matrix, the membrane was immersed in acetone followed by methanol at room temperature for 24 h. It was dried at room temperature under atmospheric pressure for 24 h. The completion of desilylation was confirmed by the comparison between IR spectra of the polymer membranes before and after the reaction.
1) at room temperature for 24 h. To remove residual impurities in the polymer matrix, the membrane was immersed in acetone followed by methanol at room temperature for 24 h. It was dried at room temperature under atmospheric pressure for 24 h. The completion of desilylation was confirmed by the comparison between IR spectra of the polymer membranes before and after the reaction.
Results and discussion
Copolymerization
The copolymerizations were carried out using TaCl5/n-Bu4Sn catalyst at 80 °C in toluene for 24 h under nitrogen, whose conditions are known to produce high-molecular-weight poly(diphenylacetylene) derivatives in high yields.15–17 The results of copolymerizations are summarized in Table 1.| M | Feed ratio | Polymerb | ||
|---|---|---|---|---|
(M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TBDPA) TBDPA) |
Yield, % | Mwc | Mw/Mnc | |
| a In toluene at 80 °C for 24 h; [M]0 + [TBDPA]0 = 0.20 M, [TaCl5] = 20 mM, [n-Bu4Sn] = 80 mM.b Methanol-insoluble product.c Measured by GPC.d Acetone-insoluble product.e CHCl3-soluble part. | ||||
| TMSDPA | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
84 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 320 320![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
5.05 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
83 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 230 230![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
4.61 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
83 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 160 160![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
4.86 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
86 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
4.88 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
93 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 080 080![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
5.43 | |
| TESDPA | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
64 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 120 120![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2.69 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
66 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 440 440![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
4.21 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
73 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
5.17 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
74 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 170 170![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
4.27 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
91 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 630 630![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
5.29 | |
| DMOSDPA | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
67 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 080 080![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
4.24 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
80 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 560 560![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
3.90 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
81 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 010 010![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
4.06 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
88 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 360 360![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
5.01 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
87 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
3.57 | |
| TPSDPA | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
70d | 378![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000e 000e |
2.79 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
76d | 376![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000e 000e |
2.51 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
80d | 233![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000e 000e |
2.75 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
74d | 335![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000e 000e |
2.27 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
79d | 397![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000e 000e |
1.79 | |
The copolymerization of TMSDPA having trimethylsilyl group with TBDPA in feed ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 produced poly(TMSDPA-co-TBDPA) with Mw of 3
1 produced poly(TMSDPA-co-TBDPA) with Mw of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 320
320![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 in 84% yield. The copolymerizations in feed ratios of 2
000 in 84% yield. The copolymerizations in feed ratios of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, and 1
2, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 also proceeded in the same manner and copolymers were obtained with Mw of 3
4 also proceeded in the same manner and copolymers were obtained with Mw of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 230
230![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 3
000, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 160
160![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 3
000, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300
300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, and 2
000, and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 080
080![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, respectively. The copolymerizations of TESDPA having triethylsilyl group with TBDPA under the same conditions gave poly(TESDPA-co-TBDPA)s (Mw = 1
000, respectively. The copolymerizations of TESDPA having triethylsilyl group with TBDPA under the same conditions gave poly(TESDPA-co-TBDPA)s (Mw = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 120
120![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–2
000–2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 170
170![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000). DMOSDPA having dimethyl-n-octylsilyl group also copolymerized with TBDPA to afford poly(DMOSDPA-co-TBDPA)s with high molecular weight (Mw = 4
000). DMOSDPA having dimethyl-n-octylsilyl group also copolymerized with TBDPA to afford poly(DMOSDPA-co-TBDPA)s with high molecular weight (Mw = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 080
080![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–5
000–5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 010
010![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000). The copolymerizations of TPSDPA having triphenylsilyl group with TBDPA provided poly(TPSDPA-co-TBDPA)s in high yields, but the obtained poly(TPSDPA-co-TBDPA)s showed poor solubility. Poly(TPSDPA-co-TBDPA)s were partially soluble in chloroform, and the Mw's of CHCl3-soluble parts were one order of magnitude lower than those of the other copolymers.
000). The copolymerizations of TPSDPA having triphenylsilyl group with TBDPA provided poly(TPSDPA-co-TBDPA)s in high yields, but the obtained poly(TPSDPA-co-TBDPA)s showed poor solubility. Poly(TPSDPA-co-TBDPA)s were partially soluble in chloroform, and the Mw's of CHCl3-soluble parts were one order of magnitude lower than those of the other copolymers.
Fabrication and desilylation of membranes
The solubility of copolymers is summarized in Table 2. Regardless of the feed ratios, poly(TMSDPA-co-TBDPA)s, poly(TESDPA-co-TBDPA)s, and poly(DMOSDPA-co-TBDPA)s showed good solubility in relatively low polarity solvents including toluene, chloroform, and tetrahydrofuran, while poly(TPSDPA-co-TBDPA)s showed poor solubility. Poly(TPSDPA-co-TBDPA) was slightly soluble in toluene, chloroform and tetrahydrofuran. All the present copolymers were insoluble in polar solvents such as N,N-dimethylformamide, dimethyl sulfoxide, and methanol.| Feed ratio | Toluene | CHCl3 | THF | DMF | DMSO | Methanol |
|---|---|---|---|---|---|---|
(RDPA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TBDPA) TBDPA) |
||||||
| a Symbols: (+) soluble, (±) partly soluble, (−) insoluble. | ||||||
| Poly(TMSDPA-co-TBDPA), poly(TESDPA-co-TBDPA), poly(DMOSDPA-co-TBDPA) | ||||||
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
+ | + | + | − | − | − |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
+ | + | + | − | − | − |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
+ | + | + | − | − | − |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
+ | + | + | − | − | − |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
+ | + | + | − | − | − |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Poly(TPSDPA-co-TBDPA) | ||||||
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
± | ± | ± | − | − | − |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
± | ± | ± | − | − | − |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
± | ± | ± | − | − | − |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
± | ± | ± | − | − | − |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
± | ± | ± | − | − | − |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| DSpoly(TMSDPA-co-TBDPA) | ||||||
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
− | − | − | − | − | − |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
− | − | − | − | − | − |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
− | − | − | − | − | − |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
− | ± | ± | − | − | − |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
± | ± | ± | − | − | − |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| DSpoly(TESDPA-co-TBDPA) | ||||||
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
− | − | − | − | − | − |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
− | − | − | − | − | − |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
− | + | + | − | − | − |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
+ | + | + | − | − | − |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
+ | + | + | − | − | − |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| DSpoly(DMOSDPA-co-TBDPA) | ||||||
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
− | − | − | − | − | − |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
− | − | − | − | − | − |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
− | + | ± | − | − | − |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
+ | + | + | − | − | − |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
+ | + | + | − | − | − |
Tough free-standing membranes could be fabricated by casting polymers from their toluene solutions for poly(TMSDPA-co-TBDPA)s, poly(TESDPA-co-TBDPA)s, and poly(DMOSDPA-co-TBDPA)s. However, it was difficult to prepare a membrane of poly(TPSDPA-co-TBDPA) by solution-casting because of too poor solubility. The desilylation was carried out in a mixture of hexane/trifluoroacetic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio) at room temperature for 24 h to afford the desilylated membranes. It is known that the aryl–Si bond is easily cleaved by proton acids.18,19 Fig. 1 shows the IR spectra of membranes of poly(DMOSDPA-co-TBDPA) and desilylated analogue [DSpoly(DMOSDPA-co-TBDPA)] in the feed ratio of 1
1 volume ratio) at room temperature for 24 h to afford the desilylated membranes. It is known that the aryl–Si bond is easily cleaved by proton acids.18,19 Fig. 1 shows the IR spectra of membranes of poly(DMOSDPA-co-TBDPA) and desilylated analogue [DSpoly(DMOSDPA-co-TBDPA)] in the feed ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4. The absorptions at 1250 cm−1 derived from the stretching of SiC–H bonds and at 1120 cm−1 derived from the vibration of Si–C completely disappeared in the spectrum after desilylation reaction. This indicates that the silyl groups were completely eliminated from the polymer membrane even the silyl groups were bulky dimethyl-n-octylsilyl groups. For poly(TMSDPA-co-TBDPA)s and poly(TESDPA-co-TBDPA)s, the completion of desilylation was confirmed by IR spectra of the polymer membranes. The color of polymer membranes changed from yellow to orange through desilylation, which would suggest that the conjugated main chains were maintained during the reaction. The results of desilylation are the same as the previous studies.10,12
4. The absorptions at 1250 cm−1 derived from the stretching of SiC–H bonds and at 1120 cm−1 derived from the vibration of Si–C completely disappeared in the spectrum after desilylation reaction. This indicates that the silyl groups were completely eliminated from the polymer membrane even the silyl groups were bulky dimethyl-n-octylsilyl groups. For poly(TMSDPA-co-TBDPA)s and poly(TESDPA-co-TBDPA)s, the completion of desilylation was confirmed by IR spectra of the polymer membranes. The color of polymer membranes changed from yellow to orange through desilylation, which would suggest that the conjugated main chains were maintained during the reaction. The results of desilylation are the same as the previous studies.10,12
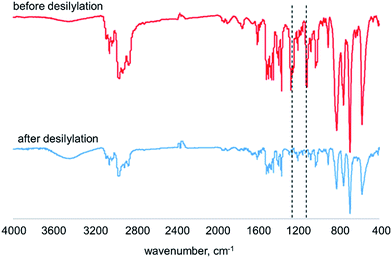 | ||
Fig. 1 IR spectra of membranes of poly(DMOSDPA-co-TBDPA) and DSpoly(DMOSDPA-co-TBDPA) in the feed ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4. 4. | ||
The solubility of the desilylated copolymers, DSpoly(TMSDPA-co-TBDPA)s, DSpoly(TESDPA-co-TBDPA)s, and DSpoly(DMOSDPA-co-TBDPA)s was examined (Table 2). The desilylated copolymers showed less solubility than silyl group-containing copolymers. DSpoly(TMSDPA-co-TBDPA)s, DSpoly(TESDPA-co-TBDPA)s, and DSpoly(DMOSDPA-co-TBDPA)s in the feed ratios of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were practically insoluble copolymers in any solvents, indicating that the incorporation of the particular content of tert-butyl groups is essential for the solubility of poly(diphenylacetylene). The desilylated copolymers in the feed ratios of 1
1 were practically insoluble copolymers in any solvents, indicating that the incorporation of the particular content of tert-butyl groups is essential for the solubility of poly(diphenylacetylene). The desilylated copolymers in the feed ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 indeed showed better solubility in toluene, CHCl3, and THF in general because they had tert-butyl groups in relatively high contents.
4 indeed showed better solubility in toluene, CHCl3, and THF in general because they had tert-butyl groups in relatively high contents.
Oxygen permeability of the copolymers
Copolymer membranes were immersed in methanol for 24 h and dried to constant weight at room temperature, and then their permeability was measured at 25 °C. The oxygen and nitrogen permeability coefficients of membranes of poly(TMSDPA-co-TBDPA)s, poly(TESDPA-co-TBDPA)s, poly(DMOSDPA-co-TBDPA)s, and the desilylated analogues were summarized in Tables 3–5.| Membrane | Ratio | PO2 | PN2 | PO2/PN2 |
|---|---|---|---|---|
| a In the units of 1 × 10−10 cm3 (STP) cm cm−2 s−1 cmHg−1 (=1 barrer). | ||||
| Poly(TMSDPA-co-TBDPA) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
1500 | 680 | 2.2 |
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2200 | 1200 | 1.8 | |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1900 | 1000 | 1.9 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2300 | 1300 | 1.8 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
2100 | 1100 | 1.9 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
2700 | 1.700 | 1.6 | |
0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1100 | 690 | 1.6 | |
| DSpoly(TMSDPA-co-TBDPA) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
900 | 410 | 2.2 |
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1600 | 1000 | 1.6 | |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1900 | 1000 | 1.9 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2700 | 1600 | 1.7 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
2800 | 1800 | 1.6 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
3200 | 2100 | 1.5 | |
0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | — | — | |
| Membrane | Ratio | PO2 | PN2 | PO2/PN2 |
|---|---|---|---|---|
| a In the units of 1 × 10−10 cm3 (STP) cm cm−2 s−1 cmHg−1 (=1 barrer). | ||||
| Poly(TESDPA-co-TBDPA) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
190 | 80 | 2.4 |
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
290 | 120 | 2.4 | |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
480 | 200 | 2.4 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
880 | 410 | 2.1 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
1500 | 780 | 1.9 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
1400 | 690 | 2.0 | |
0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1100 | 690 | 1.6 | |
| DSpoly(TESDPA-co-TBDPA) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
760 | 440 | 1.7 |
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1100 | 590 | 1.9 | |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1900 | 1100 | 1.7 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1900 | 1100 | 1.7 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
2700 | 1.500 | 1.8 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
2100 | 1100 | 1.8 | |
0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | — | — | |
| Membrane | Ratio | PO2 | PN2 | PO2/PN2 |
|---|---|---|---|---|
| a In the units of 1 × 10−10 cm3 (STP) cm cm−2 s−1 cmHg−1 (=1 barrer). | ||||
| Poly(DMOSDPA-co-TBDPA) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
27 | 8.2 | 3.3 |
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
28 | 8.5 | 3.3 | |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
31 | 9.1 | 3.4 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
52 | 24 | 2.2 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
230 | 150 | 1.5 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
300 | 120 | 2.5 | |
0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1100 | 690 | 1.6 | |
| DSpoly(DMOSDPA-co-TBDPA) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
650 | 300 | 2.2 |
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
670 | 310 | 2.2 | |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1300 | 670 | 1.9 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1800 | 1100 | 1.6 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
2000 | 1200 | 1.7 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
3300 | 2100 | 1.6 | |
0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | — | — | |
The PO2 value of poly(TMSDPA-co-TBDPA) (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) obtained by the polymerization in feed ratio of 4
1) obtained by the polymerization in feed ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was 2200 barrer. The other poly(TMSDPA-co-TBDPA)s also exhibited high gas permeability and their PO2 values were more than 1900 barrer. Poly(TMSDPA-co-TBDPA) (1
1 was 2200 barrer. The other poly(TMSDPA-co-TBDPA)s also exhibited high gas permeability and their PO2 values were more than 1900 barrer. Poly(TMSDPA-co-TBDPA) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) exhibited the highest permeability and its PO2 value was as large as 2700 barrer. Interestingly, all the poly(TMSDPA-co-TBDPA)s exhibited high oxygen permeability compared to homopolymers. The PO2 values of homopolymers of TMSDPA and TBDPA were 1500 and 1100 barrer, respectively. The higher gas permeability of copolymers may be due to the heterogeneous structure; i.e., the copolymers has both trimethylsilyl and tert-butyl groups as substituents. It is thought that the heterogeneity of substituents generates micro-scale voids when the membrane is formed. Desilylation of homopolymer of TMSDPA decreased the PO2 value from 1500 to 900 barrer. Similarly, the desilylated poly(TMSDPA-co-TBDPA) in the low contents of TBDPA, DSpoly(TMSDPA-co-TBDPA) (4
4) exhibited the highest permeability and its PO2 value was as large as 2700 barrer. Interestingly, all the poly(TMSDPA-co-TBDPA)s exhibited high oxygen permeability compared to homopolymers. The PO2 values of homopolymers of TMSDPA and TBDPA were 1500 and 1100 barrer, respectively. The higher gas permeability of copolymers may be due to the heterogeneous structure; i.e., the copolymers has both trimethylsilyl and tert-butyl groups as substituents. It is thought that the heterogeneity of substituents generates micro-scale voids when the membrane is formed. Desilylation of homopolymer of TMSDPA decreased the PO2 value from 1500 to 900 barrer. Similarly, the desilylated poly(TMSDPA-co-TBDPA) in the low contents of TBDPA, DSpoly(TMSDPA-co-TBDPA) (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), showed the lower oxygen permeability than poly(TMSDPA-co-TBDPA) (4
1), showed the lower oxygen permeability than poly(TMSDPA-co-TBDPA) (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). This is because the membrane became dense by the elimination of bulky spherical trimethylsilyl groups. However, the desilylation of poly(TMSDPA-co-TBDPA)s with high contents of TBDPA increased the gas permeability. DSpoly(TMSDPA-co-TBDPA) (1
1). This is because the membrane became dense by the elimination of bulky spherical trimethylsilyl groups. However, the desilylation of poly(TMSDPA-co-TBDPA)s with high contents of TBDPA increased the gas permeability. DSpoly(TMSDPA-co-TBDPA) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) showed the highest oxygen permeability and the PO2 value was as large as 3200 barrer. It is probably because that the spaces occupied by trimethylsilyl groups were maintained as micro-scale voids due to the steric repulsion of tert-butyl groups. These findings indicate that tert-butyl groups play an important role to generate micro-scale voids through desilylation.
4) showed the highest oxygen permeability and the PO2 value was as large as 3200 barrer. It is probably because that the spaces occupied by trimethylsilyl groups were maintained as micro-scale voids due to the steric repulsion of tert-butyl groups. These findings indicate that tert-butyl groups play an important role to generate micro-scale voids through desilylation.
The PO2 values of poly(TESDPA-co-TBDPA)s were 290–1500 barrer, which are larger than that of homopolymer, poly(TESDPA). Poly(TESDPA) has flexible ethyl chains, which would occupy the free volume in the membrane.10 Therefore, poly(TESDPA) exhibits lower gas permeability than poly(TMSDPA) and poly(TBDPA). The PO2 values of poly(TESDPA-co-TBDPA)s in low TBDPA contents were smaller than that of poly(TBDPA), but the PO2 values of poly(TESDPA-co-TBDPA)s in feed ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 were larger than that of poly(TBDPA) irrespective the copolymers contained relatively low gas-permeable TESDPA component. This result also suggests that the heterogeneity of substituent enhances the gas permeability. In the previous study, the desilylation of triethylsilyl groups increased the gas permeability because the free volume in the membrane were increased due to the elimination of bulkier silyl groups.10 All the DSpoly(TESDPA-co-TBDPA)s showed higher gas permeability than poly(TESDPA-co-TBDPA)s.
4 were larger than that of poly(TBDPA) irrespective the copolymers contained relatively low gas-permeable TESDPA component. This result also suggests that the heterogeneity of substituent enhances the gas permeability. In the previous study, the desilylation of triethylsilyl groups increased the gas permeability because the free volume in the membrane were increased due to the elimination of bulkier silyl groups.10 All the DSpoly(TESDPA-co-TBDPA)s showed higher gas permeability than poly(TESDPA-co-TBDPA)s.
Poly(DMOSDPA) has more flexible long alkyl chains and shows much lower gas permeability.10 Therefore, the PO2 values of poly(DMOSDPA-co-TBDPA)s were smaller than that of poly(TBDPA). However, the desilylation drastically enhanced the gas permeability and DSpoly(DMOSDPA-co-TBDPA) in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 feed ratio showed the highest permeability. In this way, the presence of tert-butyl groups is important to achieve high gas permeability when the bulkier silyl groups were eliminated.
4 feed ratio showed the highest permeability. In this way, the presence of tert-butyl groups is important to achieve high gas permeability when the bulkier silyl groups were eliminated.
The oxygen/nitrogen separation factors (PO2/PN2) of all the copolymer membranes before and after desilylation were in the range 1.5–3.4, and the values tended to decrease as the PO2 increased. These results agree with the general tendency of gas permeation through polymer membranes.20
Diffusivity and solubility of gases in the copolymer membranes
To inspect the gas permeability of the present polymers in detail, gas diffusion coefficients (D) were measured by time lag method and gas solubility coefficients (S) were calculated from P and D values. Tables 6–8 listed the diffusion and solubility coefficients of poly(TMSDPA-co-TBDPA)s, poly(TESDPA-co-TBDPA)s, poly(DMOSDPA-co-TBDPA)s, and their desilylated analogues.| Membrane | Ratio | DO2 × 107 | DN2 × 107 | SO2 × 103 | SN2 × 103 |
|---|---|---|---|---|---|
| a Determined by the “time lag” method at 25 °C. In the units of cm2 s−1.b Calculated by using equation, S = P/D. In the units of cm3 (STP) cm−3 cmHg−1. | |||||
| Poly(TMSDPA-co-TBDPA) | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
55 | 46 | 40 | 26 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
75 | 62 | 25 | 16 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
94 | 80 | 24 | 16 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
69 | 65 | 30 | 17 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
87 | 81 | 31 | 21 | |
| DSpoly(TMSDPA-co-TBDPA) | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
34 | 32 | 47 | 31 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
78 | 62 | 24 | 16 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
110 | 95 | 25 | 17 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
110 | 93 | 25 | 19 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
120 | 100 | 27 | 21 | |
| Membrane | Ratio | DO2 × 107 | DN2 × 107 | SO2 × 103 | SN2 × 103 |
|---|---|---|---|---|---|
| a Determined by the “time lag” method at 25 °C. In the units of cm2 s−1.b Calculated by using equation, S = P/D. In the units of cm3 (STP) cm−3 cmHg−1. | |||||
| Poly(TESDPA-co-TBDPA) | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
41 | 35 | 7.1 | 3.4 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
62 | 53 | 7.7 | 3.8 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
73 | 55 | 12 | 7.5 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
100 | 65 | 15 | 12 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
98 | 51 | 14 | 14 | |
| DSpoly(TESDPA-co-TBDPA) | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
83 | 70 | 13 | 8.4 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
100 | 88 | 19 | 13 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
110 | 95 | 17 | 12 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
140 | 120 | 19 | 13 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
130 | 94 | 16 | 12 | |
| Membrane | Ratio | DO2 × 107 | DN2 × 107 | SO2 × 103 | SN2 × 103 |
|---|---|---|---|---|---|
| a Determined by the “time lag” method at 25 °C. In the units of cm2 s−1.b Calculated by using equation, S = P/D. In the units of cm3 (STP) cm−3 cmHg−1. | |||||
| Poly(DMOSDPA-co-TBDPA) | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
12 | 3.8 | 2.3 | 2.2 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
14 | 7.0 | 2.2 | 1.3 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
27 | 12 | 1.9 | 2.0 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
30 | 25 | 7.7 | 6.0 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
31 | 15 | 9.7 | 8.0 | |
| DSpoly(DMOSDPA-co-TBDPA) | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
48 | 36 | 14 | 8.6 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
55 | 50 | 24 | 13 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99 | 80 | 18 | 14 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
110 | 85 | 18 | 14 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
180 | 150 | 18 | 14 | |
The D values of DSpoly(TMSDPA-co-TBDPA) with low contents of TBDPA was lower than Si-containing membranes. However, the desilylation of poly(TMSDPA-co-TBDPA) with high contents of TBDPA increased the gas diffusivity. The increases of diffusivity would be due to the increment of free volume of membrane. In addition, DSpoly(TMSDPA-co-TBDPA)s had nearly the same gas solubility as poly(TMSDPA-co-TBDPA)s. The elimination of silyl groups affected the gas diffusivity more effectively than the gas solubility. On the other hand, the desilylation of poly(TESDPA-co-TBDPA)s increased both the D and S values. Especially in high TESDPA ratios, the large enhancement of solubility was observed through desilylation. Since both SO2 and SN2 increased in a similar fashion, the increment in solubility may be due to the increase of the free volume.6 For poly(DMOSDPA-co-TBDPA)s, the enhancement of D and S through desilylation was also quite noticeable, and the D and S values considerably increased after desilylation.
Comparison of three types of copolymer membranes
The PO2 values of three types of copolymer membranes before and after desilylation were shown in Fig. 2. The copolymer membrane having longer alkyl chains exhibited lower oxygen permeability. This is because the flexible alkyl chains of ethyl and octyl groups would occupy the free volume in the polymer matrix. For poly(TESDPA-co-TBDPA) and poly(DMOSDPA-co-TBDPA), the permeability increased as the feed ratio of TBDPA increased. However, poly(DMOSDPA-co-TBDPA) showed still low gas permeability in 80% feed ratio of TBDPA, indicating that even a small content of long alkyl chain caused a significant decrease in gas permeability.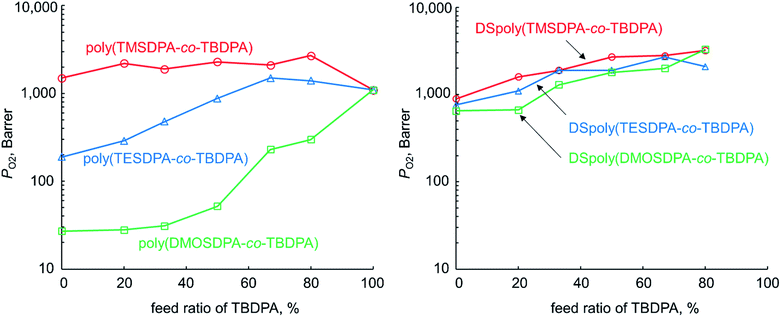 | ||
| Fig. 2 Relationship between PO2 and TBDPA ratio in copolymers for poly(TMSDPA-co-TBDPA)s, poly(TESDPA-co-TBDPA)s, poly(DMOSDPA-co-TBDPA)s, and the desilylated analogues. | ||
The three type's desilylated copolymer showed similar oxygen permeability. This means that as the alkyl group becomes longer, an extent of increment in PO2 through desilylation became larger. In this reason, DSpoly(DMOSDPA-co-TBDPA) exhibited the highest PO2 enhancement. Similarly to the case of copolymers before desilylation, the permeability of the desilylated copolymers increased as increasing TBDPA ratio. It suggests that tert-butyl groups are important to generate micro-scale voids after desilylation as well as before desilylation.
Conclusions
Four types of copolymers having silyl and tert-butyl groups were synthesized by the copolymerization of the corresponding monomers with various feed ratios. Poly(TMSDPA-co-TBDPA)s exhibited higher gas permeability than homopolymers, poly(TMSDPA) and poly(TBDPA), because of heterogeneity of substituents. Poly(TESDPA-co-TBDPA)s and poly(DMOSDPA-co-TBDPA)s showed lower gas permeability than poly(TMSDPA-co-TBDPA)s because the flexible alkyl chains occupy the free volume. The desilylation of poly(TMSDPA) decreased the gas permeability, but the PO2 values of poly(TMSDPA-co-TBDPA)s increased through desilylation in high feed ratios of tert-butyl group. This indicates that tert-butyl groups play an important role to maintain micro-scale voids through desilylation. When bulkier silyl groups were removed, the PO2 increased to larger extents. The gas diffusivity and gas solubility were also increased upon desilylation due to the increase of free volume that eliminated of silyl groups.Notes and references
- J. R. Weidman and R. Guo, Ind. Eng. Chem. Res., 2017, 56, 4220–4236 CrossRef CAS.
- R. Catro-Munoz, V. Fila and C. T. Dung, Chem. Eng. Commun., 2017, 204, 295–309 CrossRef.
- R. W. Baker and B. T. Low, Macromolecules, 2014, 47, 6999–7013 CrossRef CAS.
- Y. Huang, T. C. Merkel and R. W. Baker, J. Membr. Sci., 2014, 463, 33–40 CrossRef CAS.
- T. C. Merkel, H. Lin, X. Wei and R. W. Baker, J. Membr. Sci., 2010, 359, 126–139 CrossRef CAS.
- Y. Yampolskii, Macromolecules, 2012, 45, 3298–3311 CrossRef CAS.
- T. Aoki, T. Kaneko and M. Teraguchi, Polymer, 2006, 47, 4867–4892 CrossRef CAS.
- K. Nagai, T. Masuda, T. Nakagawa, B. D. Freeman and I. Pinnau, Prog. Polym. Sci., 2001, 26, 721–798 CrossRef CAS.
- K. Tsuchihara, T. Masuda and T. Higashimura, Macromolecules, 1992, 25, 5816–5820 CrossRef CAS.
- T. Sakaguchi, K. Yumoto, M. Shiotsuki, F. Sanda, M. Yoshikawa and T. Masuda, Macromolecules, 2005, 38, 2704–2709 CrossRef CAS.
- H. Kouzai, T. Masuda and T. Higashimura, J. Polym. Sci., Part A: Polym. Chem., 1994, 32, 2523–2530 CrossRef CAS.
- M. Teraguchi and T. Masuda, Macromolecules, 2002, 35, 1149–1151 CrossRef CAS.
- K. Tsuchihara, T. Masuda and T. Higashimura, J. Polym. Sci., Part A: Polym. Chem., 1993, 31, 547–552 CrossRef CAS.
- M. Teraguchi and T. Masuda, J. Polym. Sci., Part A: Polym. Chem., 1998, 36, 2721–2725 CrossRef CAS.
- F. Sanda, M. Shiotsuki and T. Masuda, Alkyne Polymerization, in Polymer Science: A comprehensive, ed. K. Matyjaszewski and M. Möller, Elsevier BV, Amsterdam, 2012, vol. 3, pp. 875–954 Search PubMed.
- T. Masuda, F. Sanda and M. Shiotsuki, in Comprehensive organometallic chemistry III, ed. R. Crabtree and M. Mingos, Elsevier, Oxford, 2007, vol. 11, ch. 16, pp. 557–593 Search PubMed.
- T. Masuda and F. Sanda, in Handbook of metathesis, ed. R. H. Grubbs, Wiley-VCH, Weinheim, 2003, vol. 3, pp. 375–406 Search PubMed.
- D. Habich and F. Effenberger, Synthesis, 1979, 841–876 CrossRef.
- R. A. Benkeser, R. A. Hickner, D. I. Hoke and O. H. Thomas, J. Am. Chem. Soc., 1958, 80, 5289–5294 CrossRef CAS.
- L. M. Robeson, J. Membr. Sci., 2008, 320, 390–400 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |