Open Access Article
Open Access ArticleA highly versatile fluorenone-based macrocycle for the sensitive detection of polycyclic aromatic hydrocarbons and fluoride anions†
Ingrid-Suzy Tamgho‡
,
Sauradip Chaudhuri‡,
Molly Verderame,
Dana J. DiScenza and
Mindy Levine *
*
Department of Chemistry, University of Rhode Island, 140 Flagg Road, Kingston, RI 02881, USA. E-mail: mlevine@chm.uri.edu; Fax: +1-401-874-5072; Tel: +1-401-874-4243
First published on 30th May 2017
Abstract
Reported herein is the high yielding synthesis of a new fluorenone-based triazolophane and its sensing capabilities for polycyclic aromatic hydrocarbons (PAHs) and fluoride anions. Fluorescence, UV/Vis and 1H NMR spectroscopy results showed the triazolophane has a high sensitivity for selected PAHs and binds the fluoride anion in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry via C–H hydrogen bonding with the triazole and fluorenone protons.
1 stoichiometry via C–H hydrogen bonding with the triazole and fluorenone protons.
Cyclophanes, or macrocycles that contain aromatic rings linked by aliphatic chains, have been studied in the literature for a range of applications.1 These macrocycles can bind a variety of guests in their interiors, including polycyclic aromatic hydrocarbons (PAHs)2 as well as anions3 and cations,4 through multiple non-covalent interactions. Since the synthesis of the simplest cyclophane, [2.2]paracyclophane, in 1966,5 the number of known cyclophanes has expanded dramatically.
Recent cyclophanes have replaced one or more of the aromatic rings with heteroaromatic moieties,6 including triazole rings for the formation of triazolophane macrocycles.7 Such macrocycles are attractive because of the synthetic accessibility of triazoles8 as well as their ability to bind both cations (via association with the N2 and N3 of the triazole)9 and anions (via hydrogen bonding with the C–H hydrogen bond donor).10
Anions are important targets for binding and detection due to their ubiquitous nature and public health relevance.11 Fluoride, for example, is of interest due to the importance of fluoridated water in promoting dental health;12 excessive amounts of fluoride, by contrast, can lead to fluorosis.13 Other key anions include those with negative health effects including phosphate,14 nitrate,15 thiocyanate16 and cyanide.17 A third class of anions is those that are explosive such as azide.18
Polycyclic aromatic hydrocarbons (PAHs) are another class of important detection targets, with negative health and environmental effects,19 and are formed from the incomplete combustion of petroleum.20 Their environmental stability means that they bioaccumulate and biomagnify,21 which is of concern due to their known and suspected teratogenicity,22 mutagenicity23 and carcinogenicity.24
Work in the Levine group has focused on the detection of toxicants using cyclodextrin-promoted energy transfer25 and cyclodextrin-promoted fluorescence modulation,26 as well as on the use of synthetic macrocycles for the enhanced binding and detection of PAHs.27 One shortcoming is that the previously synthesized macrocycles lacked easily detectable photophysically active components, which in turn meant that an external fluorophore was required to obtain a response signal. Incorporating a UV-active moiety, such as fluorenone, directly into the backbone of the macrocycle would enable the direct use of optical detection methods, and incorporation of a triazole functionality will enable the detection of a broader variety of analytes. Reported herein is the high yielding synthesis of precisely such a macrocycle, compound 1, containing a photophysically active fluorenone unit and two triazole moieties, and its versatility in binding and detecting both PAHs and anions with extremely high sensitivities.
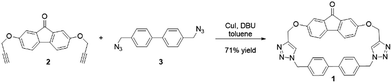
Macrocycle 1 was synthesized from compounds 2 and 3 via a copper catalyzed azide–alkyne cycloaddition (Fig. 1). This reaction proceeded under high dilution conditions28 in toluene to obtain a 71% isolated yield. The low solubility of the macrocycle in toluene caused it to crash out of the reaction mixture, and was crucial in enabling high yields. The formation of the macrocycle was confirmed by NMR spectroscopy and mass spectrometry (see ESI†).
Photophysical characterization of the macrocycle showed a UV-visible absorption spectrum with maxima at 264, 310, and 460 nm, corresponding to the π–π* transition of the biphenyl,29 the electronic transition of the fluorenone,30 and the symmetry forbidden n–π* transition of the carbonyl moiety,31 respectively.
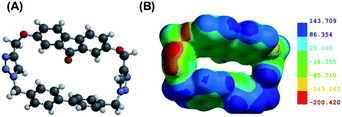
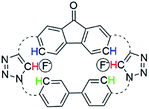
DFT calculations of macrocycle 1 showed a well-defined cavity with dimensions of 10.6 Å × 5.043 Å, with the most stable conformation of the macrocycle having the triazole protons facing opposite sides (i.e. one pointed out of the page and one pointed into the page) (Fig. 2A). Electron density mapping highlighted the strongly electron deficient nature of the macrocycle, making it well-suited for the binding of electron rich aromatic guests (Fig. 2B).
The binding of polycyclic aromatic hydrocarbons 4–7 (Fig. 3) in macrocycle 1 was monitored by UV-visible and fluorescence spectroscopy. In the UV-visible spectra, the absorbance spectrum of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of each analyte and macrocycle 1 was equivalent to the sum of the absorbance spectra of the individual species, indicating no significant complexation-induced absorption changes.
1 mixture of each analyte and macrocycle 1 was equivalent to the sum of the absorbance spectra of the individual species, indicating no significant complexation-induced absorption changes.
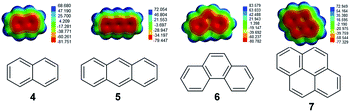 | ||
| Fig. 3 Structures of polycyclic aromatic hydrocarbons 4–7 with electron density mapping of each compound highlighting their electron rich aromatic natures. | ||
In contrast to the limited changes in the absorbance spectra, the fluorescence emission of each of the analytes decreased with the addition of the macrocycle (Table 1), with the decrease in fluorescence quantified according to eqn (1):
| Fluorescence change = (Flm − Fla)/Fla × 100 | (1) |
Of note, these decreases were not accompanied by significant shifts in the emission maxima, in contrast to a report of an analogous system in which such a red shift is observed.32 In that case, the red-shift is probably a result of excited state energy transfer between the anthracene host and guanine guest.
A direct comparison of the fluorescence changes observed in the presence of macrocycle 1 with those observed in the presence of both photophysically active components – 2,7-dihydroxy-9-fluorenone and 4,4′-dimethylbiphenyl (compounds 8 and 9, Fig. 4) indicate that the macrocycle induced fluorescence changes were markedly different from those induced by the components in a mixture (Table 1, Fig. 5), thereby supporting the proposed analyte-macrocycle complexation. For analyte 5, the presence of both 8 and 9 led to noticeable fluorescence quenching as a result of intermolecular co-facial aromatic interactions between the anthracene and fluorenone33 and the anthracene and biphenyl,34 in accordance with literature precedents of analogous quenching phenomena. Once these moieties are geometrically constrained in a macrocycle (Fig. 2), they are no longer completely planar and are not as available for co-facial quenching interactions. Binding of analyte 5 in macrocycle 1, as a result, leads to a much more limited decrease in the observed fluorescence emission.
In the case of analytes 6 and 7, slight fluorescence decreases were observed in the presence of macrocycle 1, while significant fluorescence enhancements were observed in the presence of both 8 and 9. These results indicate different interactions of the macrocycle with analytes 6 and 7 compared to its interactions with 4 and 5. As a result of the larger dimensions of 6 and 7, there is likely weaker binding in the cavity; as a result, limited fluorescence quenching occurred.
In the case of naphthalene (analyte 4), the excitation wavelength of 265 nm is a wavelength at which compounds 1, 8, and 9 have noticeable absorption cross-sections (see ESI†). Although significant wavelength-dependent fluorescence decreases were observed, these observed changes are indicative of an inner filter mechanism, where the macrocycle absorbs energy and filters some of that energy from reaching the analyte.35
The limits of detection of analytes 4–7 using this method were calculated following literature-reported procedures (Table 2).36 For analyte 4, the calculated detection limit is a result of the inner filter effect-induced fluorescence changes.35 The nanomolar detection limits obtained for the analytes are close to or below the literature-reported levels of concern for three out of the four analytes (compounds 4, 5, and 7),37 which highlights the high sensitivity of this fluorescence method for PAH binding and concomitant detection. The limits of detection for the analytes in the absence of the macrocycle were higher, which highlights the role of the macrocycle in enhancing fluorescence sensitivities.
| Analyte | Limit of detection with compound 1a (nM) | Limit of detection without compound 1a (nM) | Literature-reported values (nM) |
|---|---|---|---|
| a Details for the limit of detection calculations can be found in the ESI. All results represent an average of at least 3 trials. | |||
| 4 | 28.7 ± 0.1 | 166.5 ± 1.4 | 78.0 (ref. 38) |
| 5 | 2.2 ± 0.8 | 30.1 ± 0.9 | 0.8 (ref. 39) |
| 6 | 37.2 ± 0.1 | 59.5 ± 0.7 | 0.6 (ref. 38) |
| 7 | 4.2 ± 0.0 | 204.8 ± 1.1 | 0.8 (ref. 39) |
In addition to binding PAHs in the cavity interior, macrocycle 1 (10 μM in DMSO) was also investigated for its ability to bind anions. Among all anions studied (fluoride, cyanide, azide, and thiocyanate), only fluoride exhibited a noticeable spectroscopic change (Fig. 6) with increases in the molar absorptivity of the macrocycle's λmax bands at 264 and 305 nm. The response for fluoride is likely due to its ability to act as a hydrogen bond acceptor, as a result of its small size, high electronegativity, and high charge density.40
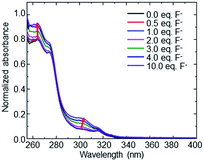 | ||
| Fig. 6 Illustration of changes in the UV-visible absorption spectrum of macrocycle 1 with the addition of up to 10 equivalents of fluoride anion. | ||
The fluoride binding was confirmed by nonlinear curve fitting of the NMR titration data to host-guest binding models (Fig. 7 and Table 3). An excellent non-linear fit was obtained for a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 binding stoichiometry between macrocycle 1 and two fluoride anions, and this stoichiometry was confirmed with a Job plot analysis that showed a maximum at a molar ratio of 0.66 (see ESI† for details). The calculated binding constants indicate anti-cooperativity, with the binding of the first fluoride (K1 = 522 M−1) preferred compared to binding of the second fluoride anion (K2 = 333.25 M−1). This phenomenon could be attributed to the fact that the fluorenone flexibility is constrained by the first binding, reducing the conformational flexibility for the second fluoride binding. Each fluoride anion interacts with the triazole proton (He, red), and is additionally assisted by the fluorenone and biphenyl protons (Hb, blue and Hg, green) (Fig. 8), as shown through the chemical shift changes of these protons with the addition of up to 10 equivalents of fluoride anion (Table 3).
2 binding stoichiometry between macrocycle 1 and two fluoride anions, and this stoichiometry was confirmed with a Job plot analysis that showed a maximum at a molar ratio of 0.66 (see ESI† for details). The calculated binding constants indicate anti-cooperativity, with the binding of the first fluoride (K1 = 522 M−1) preferred compared to binding of the second fluoride anion (K2 = 333.25 M−1). This phenomenon could be attributed to the fact that the fluorenone flexibility is constrained by the first binding, reducing the conformational flexibility for the second fluoride binding. Each fluoride anion interacts with the triazole proton (He, red), and is additionally assisted by the fluorenone and biphenyl protons (Hb, blue and Hg, green) (Fig. 8), as shown through the chemical shift changes of these protons with the addition of up to 10 equivalents of fluoride anion (Table 3).
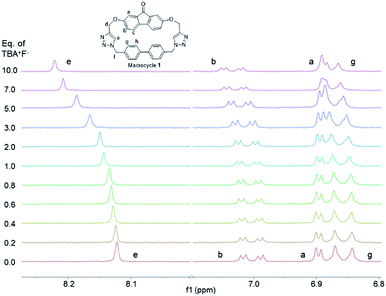 | ||
| Fig. 7 Illustration of changes in the 1H NMR chemical shifts of macrocycle 1 with the titration of fluoride anions. | ||
| Equivalents of fluoride | Change in δ (ppm) | |||
|---|---|---|---|---|
| He | Hb | Ha | Hg | |
| a Detailed methods for the 1H NMR titration are shown in the ESI. | ||||
| 1 | 0.014 | 0.004 | −0.000 | 0.002 |
| 3 | 0.044 | 0.016 | −0.003 | 0.009 |
| 5 | 0.066 | 0.022 | −0.005 | 0.014 |
| 10 | 0.100 | 0.031 | −0.010 | 0.022 |
The small size of fluoride makes it compatible with the binding pockets in each arm of the macrocycle. This compatibility results in selective binding of fluoride, with significantly higher chemical shift changes compared to the other anions (Table 4).
| Anion | Δδ (ppm) |
|---|---|
| a Detailed methods for the 1H NMR titration are shown in the ESI. | |
| F− | 0.1002 |
| CN− | 0.0021 |
| SCN− | 0.0025 |
| N3− | 0.0024 |
Moreover, the solvent used in these NMR titration experiments has a significant effect on the magnitude of the shifts observed. Chemical shift changes of higher magnitude have been reported in the literature with [HF2]− and triazolophane hosts in deuterated dichloromethane.41 Because of solubility constraints, binding analyses were carried out in d6-DMSO. Even though hydrogen fluoride and HF2− anions are present to a minor extent, their relatively small amounts (see ESI†) means that they are unlikely to have a significant effect on fluoride binding. Moreover, chemical shift changes of the tetrabutylammonium indicated no significant association between the counterion and the macrocycle–fluoride complex.
In conclusion, we have successfully synthesized a new macrocycle composed of biphenyl and fluorenone moieties linked by two triazoles. We demonstrated that macrocycle 1 is sensitive towards small amounts of PAHs with limits of detections in the nanomolar range. Additionally, compound 1 is able to bind selectively to fluoride in 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry through the use of triazole, fluorenone and biphenyl-facilitated C–H binding. This macrocycle can be used as a scaffold for additional detection applications as well as a crucial tool in our efforts to understand fundamental intermolecular interactions. Results of these and other investigations are currently underway in our laboratory, and results will be reported in due course.
1 stoichiometry through the use of triazole, fluorenone and biphenyl-facilitated C–H binding. This macrocycle can be used as a scaffold for additional detection applications as well as a crucial tool in our efforts to understand fundamental intermolecular interactions. Results of these and other investigations are currently underway in our laboratory, and results will be reported in due course.
Acknowledgements
The authors acknowledge Dr Li Li (MIT) and the Smith-Oxley research group (University of Rhode Island) for their help with mass spectrometry. This research was supported by the University of Rhode Island Department of Chemistry.Notes and references
- E. J. Dale, N. A. Vermeulen, M. Juricek, J. C. Barnes, R. M. Young, M. R. Wasielewski and J. F. Stoddart, Acc. Chem. Res., 2016, 49, 262 CrossRef CAS PubMed.
- J. C. Barnes, M. Juricek, N. L. Strutt, M. Frasconi, S. Sampath, M. A. Giesener, P. L. McGrier, C. J. Bruns, C. L. Stern, A. A. Sarjeant and J. F. Stoddart, J. Am. Chem. Soc., 2013, 135, 183 CrossRef CAS PubMed.
- A. Kim, R. Ali, S. H. Park, Y.-H. Kim and J. S. Park, Chem. Commun., 2016, 52, 11139 RSC.
- E. Makrlik, S. Bohm, D. Sykora, B. Klepetarova, V. Petr and M. Polasek, Chem. Phys. Lett., 2015, 642, 39 CrossRef CAS.
- D. J. Cram, C. S. Montgomery and G. R. Knox, J. Am. Chem. Soc., 1966, 88, 515 CrossRef CAS.
- S.-i. Kato, N. Yamazaki, T. Tajima and Y. Nakamura, Chem. Lett., 2013, 42, 401 CrossRef CAS.
- M. Busch, M. Cayir, M. Nieger, W. R. Thiel and S. Brase, Eur. J. Org. Chem., 2013, 2013, 6108 CrossRef CAS.
- F. Wei, W. Wang, Y. Ma, C.-H. Tung and Z. Xu, Chem. Commun., 2016, 52, 14188 RSC.
- Y. H. Lau, P. J. Rutledge, M. Watkinson and M. H. Todd, Chem. Soc. Rev., 2011, 40, 2848 RSC.
- J. Cai and J. L. Sessler, Chem. Soc. Rev., 2014, 43, 6198 RSC.
- L. S. Thakur and P. Semil, Int. J. ChemTech Res., 2013, 5, 1299 CAS.
- C. A. Palmer and J. A. Gilbert, J. Acad. Nutr. Diet., 2012, 112, 1443 CrossRef CAS PubMed.
- M. Borysewicz-Lewicka and J. Opydo-Szymaczek, Pol. J. Environ. Stud., 2016, 25, 9 CrossRef CAS.
- H. Komaba and M. Fukagawa, Kidney Int., 2016, 90, 753 CrossRef CAS PubMed.
- A. H. Gorenjak and A. Cencic, Acta Aliment., 2013, 42, 158 CrossRef CAS.
- T. J. Barrett and C. L. Hawkins, Chem. Res. Toxicol., 2012, 25, 263 CrossRef CAS PubMed.
- R. Jackson and B. A. Logue, Anal. Chim. Acta, 2017, 960, 18 CrossRef CAS PubMed.
- D. M. Badgujar, M. B. Talawar, S. N. Asthana and P. P. Mahulikar, J. Hazard. Mater., 2008, 151, 289 CrossRef CAS PubMed.
- S. Marzooghi and D. M. Di Toro, Environ. Toxicol. Chem., 2017, 36, 1138–1148 CrossRef CAS PubMed.
- L. D. Claxton, Mutat. Res., Rev. Mutat. Res., 2014, 762, 108 CrossRef CAS PubMed.
- A. J. Mearns, D. J. Reish, P. S. Oshida, T. Ginn, M. A. Rempel-Hester, C. Arthur, N. Rutherford and R. Pryor, Water Environ. Res., 2015, 87, 1718 CrossRef CAS PubMed.
- N. Verma, M. Pink, A. W. Rettenmeier and S. Schmitz-Spanke, Proteomics, 2012, 12, 1731 CrossRef CAS PubMed.
- E. B. Balcioglu, Toxin Rev., 2016, 35, 98 CrossRef CAS.
- C. Ceccaroli, A. Pulliero, M. Geretto and A. Izzotti, J. Environ. Sci. Health, Part C: Environ. Carcinog. Ecotoxicol. Rev., 2015, 33, 188 CrossRef CAS PubMed.
- N. Serio, D. F. Moyano, V. M. Rotello and M. Levine, Chem. Commun., 2015, 51, 11615 RSC.
- D. J. DiScenza and M. Levine, Supramol. Chem., 2016, 28, 881 CrossRef CAS; D. J. DiScenza and M. Levine, New J. Chem., 2016, 40, 789 RSC.
- B. Radaram and M. Levine, Eur. J. Org. Chem., 2015, 2015, 6194 CrossRef CAS; B. Radaram, J. Potvin and M. Levine, Chem. Commun., 2013, 49, 8259 RSC.
- P. Knops, N. Sendhoff, H. B. Mekelburger and F. Voegtle, Top. Curr. Chem., 1992, 161, 1 CrossRef CAS.
- T. Ohana, M. Kaise, S. Nimura, O. Kikuchi and A. Yabe, Chem. Lett., 1993, 765 CrossRef CAS.
- W.-L. Yu, J. Pei, W. Huang and A. J. Heeger, Adv. Mater., 2000, 12, 828 CrossRef CAS.
- F. Uckert, S. Setayesh and K. Mullen, Macromolecules, 1999, 32, 4519 CrossRef CAS.
- J. Y. Kwon, N. J. Singh, H. N. Kim, S. K. Kim, K. S. Kim and J. Yoon, J. Am. Chem. Soc., 2004, 126, 8892 CrossRef CAS PubMed.
- H.-D. Becker, C. Burgdorff and H.-G. Loehmannsroeben, J. Photochem. Photobiol., A, 1995, 86, 133 CrossRef CAS.
- Y.-H. Chen, K.-C. Tang, Y.-T. Chen, J.-Y. Shen, Y.-S. Wu, S.-H. Liu, C.-S. Lee, C.-H. Chen, T.-Y. Lai, S.-H. Tung, R.-J. Jeng, W.-Y. Hung, M. Jiao, C.-C. Wu and P.-T. Chou, Chem. Sci., 2016, 7, 3556 RSC.
- F. Akhgari, N. Samadi and K. Farhadi, J. Fluoresc., 2017, 27, 921–927 CrossRef CAS PubMed; P. Marks, B. Radaram, M. Levine and I. A. Levitsky, Chem. Commun., 2015, 51, 7061 RSC.
- B. Saute and R. Narayanan, J. Raman Spectrosc., 2013, 44, 1518 CrossRef CAS.
- K. Blasch, J. Kolivosky and B. Hill, Inhalation Toxicol., 2016, 28, 216 CrossRef CAS PubMed.
- Center for Disease Control and Prevention, The National Institute for Occupational Safety and Health (NIOSH): Naphthalene, https://www.cdc.gov/niosh/npg/npgd0439.html, accessed, Mar 27, 2017 Search PubMed.
- Center for Disease Control and Prevention, The National Institute for Occupational Safety and Health (NIOSH): Coal tar pitch volatiles, https://www.cdc.gov/niosh/npg/npgd0145.html, accessed, Mar 27, 2017 Search PubMed.
- M. Cametti and K. Rissanen, Chem. Commun., 2009, 2809 RSC.
- R. O. Ramabhadran, Y. Liu, Y. Hua, M. Ciardi, A. H. Flood and K. Raghavachari, J. Am. Chem. Soc., 2014, 136, 5078 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthetic procedures, spectral characterization of compounds 1–3, experimental methods for all photophysical experiments, copies of all spectra, LOD procedures and data. See DOI: 10.1039/c7ra05404a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2017 |