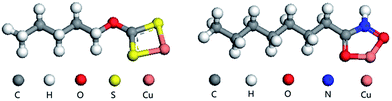Open Access Article
Open Access ArticleActivation of chrysocolla flotation by organic chelating agents
D. Jiang a,
J. Lanb,
W. Zhaoc,
Z. Zhangd and
Y. Lan*e
a,
J. Lanb,
W. Zhaoc,
Z. Zhangd and
Y. Lan*e
aSchool of Materials Science and Engineering, Shanghai University, Shanghai 200072, China
bShanghai Film Academy, Shanghai University, Shanghai 200072, China
cKunming Professional College of Arts, Kunming 650073, China
dYuxi Normal University, 653100, China
eYunnan University, Kunming 650091, China. E-mail: 1504635629@qq.com
First published on 19th July 2017
Abstract
Activation of chrysocolla by organic Cu-chelating agents was studied using flotation tests, instrumental analytical techniques, and computational methods, revealing that the performance of these agents was related to their chemical activity and chrysocolla dissolution properties. The above activation was mainly ascribed to the high chemical activity of organic chelators, which significantly increased collector adsorption capacity.
Introduction
Although surface hydrophobicity is a necessary condition for mineral flotation, most natural insoluble oxide ores possess strongly hydrophilic surfaces. Consequently, poor surface metal ion diffusion complicates the leaching of these ions into the aqueous phase, hindering the chemical adsorption of conventional collectors on the surface of these minerals and thus leading to unsatisfactory flotation effects.The development of, and research on, organic chelating collectors has attracted great interest,1 because they can form metal chelates on the oxide surface that are more stable than ordinary ionic and covalent metal salts. In addition, organic chelating agents can alter the chemical adsorption capacity of mineral surfaces.2 For a long time, chelate collectors have been treated as better selective collectors with seemingly better flotation conventional collectors.3
Chrysocolla is a typical insoluble and surface-porous metal oxide mineral containing large amounts of physically and chemically adsorbed water and OH–. So far, the effective flotation-based recovery of chrysocolla is still one of the three major problems of copper oxide flotation. To mitigate this problem, the mineral's surface can be activated by small-molecule organic chelators that form hydrophobic metal chelates, which enable effective adsorption and strengthening the interaction with the above surface. Herein, we use this representative mineral to study activation by copper-chelating agents and explore the parameters and mechanism of organic chelator-activated insoluble mineral flotation, thereby contributing to the design of novel methods for the same purpose.
Experimental
Chrysocolla (CuSiO3·2H2O) ore sourced from the Dongchuan mine and hand-separated from malachite exhibited flotation and test sample purities above 80 and 90%, respectively, with the main impurities being CaO and MgO. Samples used for flotation tests and adsorption capacity determination exhibited −100 mesh particle sizes, with the Brunauer–Emmett–Teller surface area of the latter samples equaling 132 m2 g−1. Quartz and calcite showed purities above 98%.Pentyl xanthate exhibited a purity above 96%, with other pharmaceuticals being pure or pure for chemical analysis. Distilled water was used in all tests, with pH adjustments performed using H2SO4 and NaOH.
Single mineral tests were carried out for 1 g samples in an improved Hallimond single-bubble tube with a flotation volume of 45 mL and an inflation rate of 60 mL min−1. Mixed ore samples (1 g of chrysocolla mixed with 4 g of calcite or quartz) were separated in a 30 mL-trough flotation machine utilizing a flotation time of 5 min. Adsorption capacities were determined by the residual concentration method at a solid-to-liquid ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 using an spw-200 UV-Vis spectrophotometer, and ξ potentials were determined by microscopic electrophoresis. X-ray photoelectron spectroscopy (XPS) analyses were performed at the Yunnan University test center with a PHI5500 (PHI, USA) using Mg Kα ray excitation of 1253.6 eV and X-ray source power of 200 W. The background vacuum was greater than 10−7 Pa, while the energy scale was corrected with the binding energy of contaminating carbon C (1s) (284.8 eV).
45 using an spw-200 UV-Vis spectrophotometer, and ξ potentials were determined by microscopic electrophoresis. X-ray photoelectron spectroscopy (XPS) analyses were performed at the Yunnan University test center with a PHI5500 (PHI, USA) using Mg Kα ray excitation of 1253.6 eV and X-ray source power of 200 W. The background vacuum was greater than 10−7 Pa, while the energy scale was corrected with the binding energy of contaminating carbon C (1s) (284.8 eV).
All calculations were carried out using the Cambridge Serial Total Energy Package (CASTEP) of Material Studio (Accelrys, USA), employing a first-principle pseudopotential method based on the density functional theory (DFT).4 The exchange correlation functional used was the generalized gradient approximation (GGA) developed by Perdew, Burke and Ernzerhof (PBE).5 DFT calculations employing plane wave (PW) basis sets and ultrasoft pseudopotentials were performed.6,7 The interactions between valence electrons and ionic cores were represented by ultrasoft pseudopotentials. The kinetic energy cut-off (310 eV) of the PW basis was used throughout the study, and the Brillouin zone was sampled with Monkhorst and Pack special k-points of a 4 × 4 × 4 grid for all structure calculations.8 For self-consistent electronic minimization, the Pulay Density Mixing method was employed with a convergence tolerance of 2.0 × 10−6 eV per atom. The energy tolerance was 2.0 × 10−5 eV per atom, the force tolerance 0.05 eV Å−1, and the displacement tolerance 0.002 Å.
The crystal structure data of chrysocolla obtained from the Crystallography Open Database(http://www.crystallography.net/).9
Results and discussion
Chelator/pentyl xanthate synergistic effect on chrysocolla activation
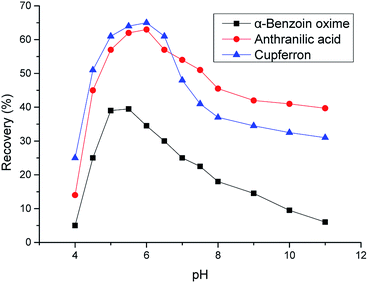
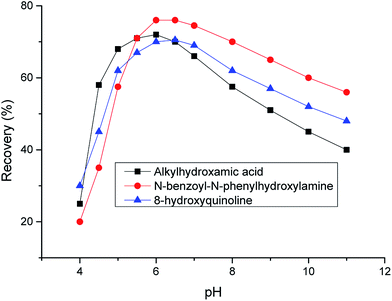
Fig. 1 and 2 show the effects of copper-chelating agents on the flotation recovery of chrysocolla and malachite, respectively. The addition of α-benzoin oxime, anthranilic acid, and cupferron reagents resulted in maximum flotation recoveries of chrysocolla of 40, 63, and 65%, respectively. For malachite, the addition of alkylhydroxamic acid, N-benzoyl-N-phenylhydroxylamine, and 8-hydroxyquinoline resulted in maximum flotation recoveries of 72, 80, and 70%, respectively. In addition, these figures reveal that the optimum pH value corresponded to weakly acidic conditions (pH 5–6), which differs from the typically pH associated with copper ion precipitation and malachite flotation. Thus, the optimum pH for copper ion precipitation or malachite activation was mainly controlled by chelator chemical activity.Chrysocolla is a poorly soluble mineral, exhibiting surface properties similar to those of silica or copper oxide in alkaline or acidic media, respectively, e.g., the copper ions on its surface can dissolve at an appropriate pH. At pH < 5, dissolved copper(II) ions mainly diffuse into the liquid phase and are not easily adsorbed on the mineral surface; they easily adsorb onto the chrysocolla surface in the form of CuOH+ at pH 5–6, which corresponds to the maximum copper ion concentration on the surface. The above results show that chrysocolla flotation is controlled by both mineral dissolution characteristics and the chemical activity of chelating agents.
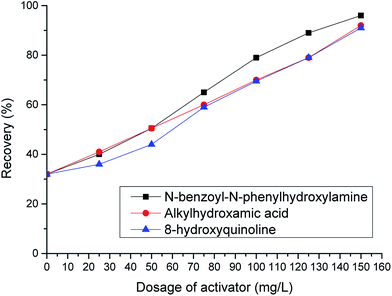
Fig. 3 shows that the use of activators such as N-benzoyl-N-phenylhydroxylamine, 8-hydroxyquinoline, or octylhydroxamic acid results in chrysocolla flotation recoveries of 90% or more, as compared to values of <40% when using the silicon sulfide/xanthate flotation method.
Fig. 1 to 3 also reveal that chrysocolla activation was enhanced by increasing the activator amount, which can be attributed to the poor solubility and large surface area of this mineral. Thus, a relatively large amount of hydrophobic agent needs to be adsorbed on the chrysocolla surface to increase its hydrophobicity. Pentyl xanthate, on the other hand, exhibits a relatively poor adsorption capacity, requiring a sufficiently high activator concentration to facilitate its adsorption by enhancing the role of surface copper(II) ions and improving the rate of the reaction between the activator and the mineral surface.
Mechanism of the activation effect
Although the chrysocolla activation effect depends on the nature of the chelating agent, the activation mechanisms corresponding to the agents should be similar. Herein, octylhydroxamic acid was chosen as a representative chelator to investigate its synergetic effects with pentyl xanthate adsorption on chrysocolla activation.| Sample | Binding energy | Valence | |||
|---|---|---|---|---|---|
| Cu 2p3/2 | Cu 2p1/2 | Si 2p | Cu | Si | |
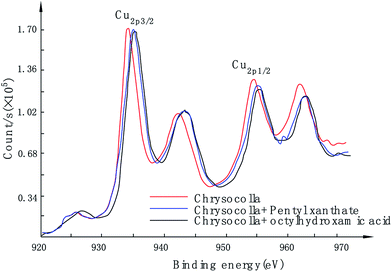
| Pristine chrysocolla | 934.68 | 955.02 | 102.33 | +2 | +4 |
| Chrysocolla/pentyl xanthate | 935.57 | 955.64 | 102.54 | +2 | +4 |
| Chrysocolla/octylhydroxamic acid | 935.79 | 955.69 | 102.53 | +2 | +4 |
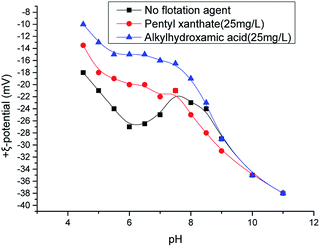
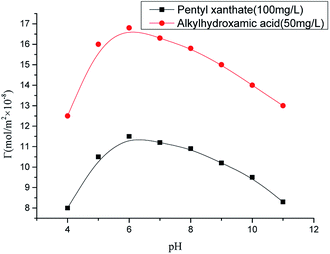
Fig. 6 shows that octylhydroxamic acid and pentyl xanthate co-adsorbed onto the chrysocolla surface, with maximum adsorption capacity achieved at pH 6. This value also corresponds to the optimum pH of activated flotation, revealing that the adsorption capacity of these agents directly affected the flotation effect. As mentioned above, the concentration of copper ions (as CuOH+) adsorbed onto the chrysocolla surface is the highest at pH 5–6, and these ions also diffuse very easily to the surface. Under these conditions, the rate and capacity of octylhydroxamic acid adsorption onto the chrysocolla surface are maximized, inducing maximal initial surface hydrophobicity. The thus achieved activation maximizes the pentyl xanthate adsorption capacity, further demonstrating that mineral surface dissolution characteristics and activator chemical activity control the flotation effect.
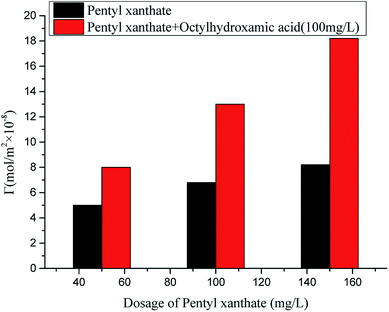
Fig. 7 shows that activation by octylhydroxamic acid effectively improved the adsorption capacity of pentyl xanthate and mineral surface hydrophobicity, more than doubling the former parameter at elevated pentyl xanthate levels.
Table 2 shows that both collectors strongly interact with Cu(II). Considering population values, the bond between S8 and Cu(II) is of similar strength to that between O10 and Cu(II), while bonding between S9 and Cu(II) was stronger than that between O11 and Cu(II). However, analysis of the bond length parameters shows that the bond between octylhydroxamic acid and Cu is shorter (i.e., stronger) than the bond between pentyl xanthate and Cu. The Cu chelation energy of octylhydroxamic acid (−30.59 eV) was calculated to be greater than that of pentyl xanthate (−27.33 eV), implying that the O, O-5 ring chelate formed by octylhydroxamic acid and Cu is more stable than the corresponding pentyl xanthate chelate, since the ring strain of the former (five-membered ring) is smaller than that of the latter (four-membered ring). Therefore, we can conclude that octylhydroxamic acid is a highly efficient copper collector that is superior to pentyl xanthate (Fig. 9 and 10).12,13
| Pentyl xanthate–Cu | Octylhydroxamic acid–Cu | ||||
|---|---|---|---|---|---|
| Bond type | Population value | Bond length (Å) | Bond type | Population value | Bond length (Å) |
| S8–Cu | 0.32 | 2.223 | O10–Cu | 0.31 | 1.911 |
| S9–Cu | 0.27 | 2.248 | O11–Cu | 0.19 | 2.001 |
| C7–Cu | −0.38 | 2.469 | N9–Cu | −0.27 | 2.517 |
| C8–Cu | −0.28 | 2.571 | |||
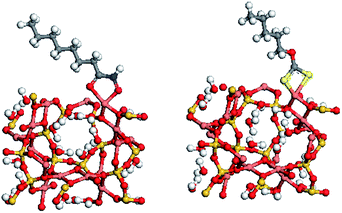 | ||
| Fig. 10 Interactions of flotation agents with chrysocolla: (left) octylhydroxamic acid and (right) pentyl xanthate. | ||
The energy of octylhydroxamic acid adsorption on the chrysocolla surface (−56.4 kcal mol−1) was determined to be significantly higher than that of pentyl xanthate (−33.4 kcal mol−1), in agreement with the above results.
Conclusions
Herein, we demonstrated the ability of N-benzoyl-N-phenylhydroxylamine, alkylhydroxamic acids, 8-hydroxyquinoline, and other organic chelating agents to effectively activate chrysocolla, achieving flotation recovery rates above 90% and thus indicating that certain insoluble metal oxides can be activated by choosing organic chelators with appropriate structures and activities.Flotation tests showed that the above activation was controlled by both the chrysocolla dissolution characteristics and chemical activity of the chelating agent.
X-ray photoelectron spectroscopy, ξ-potential tests, and quantum chemical calculations further showed that both octylhydroxamic acid and pentyl xanthate could be adsorbed onto the chrysocolla surface, with the strong chemical adsorption of the former effectively increasing the adsorption capacity of the latter, thereby improving the surface hydrophobicity to achieve chrysocolla activation.
Adsorption capacity tests showed that activation by octylhydroxamic acid effectively improved the adsorption capacity of pentyl xanthate and mineral surface hydrophobicity, almost doubling the former parameter at elevated xanthate levels.
Acknowledgements
This work was supported by NSFC-YN (UI037603) and the Yunnan Provincial Science and Technology Department (2009cc011).Notes and references
- J. S. Lee, D. R. Nagaraj and J. E. Coe, Miner. Eng., 1998, 11, 929 CrossRef CAS.
- M. Minakshi, M. I. Barmi and R. T. Jones, Dalton Trans., 2017, 46, 3588 RSC.
- S. N. Tan, A. Jiang and J. J. Liau, et al., Miner. Process., 2009, 93, 194 CrossRef CAS.
- M. C. Payne, M. P. Teter, D. C. Allan, T. A. Arias and J. D. Joannopoulos, Rev. Mod. Phys., 1992, 64, 1045 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865 CrossRef CAS PubMed.
- J. P. Perdew and Y. Wang, Phys. Rev. B: Condens. Matter Mater. Phys., 1992, 45, 13244 CrossRef.
- D. Vanderbilt, Phys. Rev., 1990, 41, 7892 Search PubMed.
- H. J. Monkhorst and J. D. Pack, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 13, 5188 CrossRef.
- P. H. Ribbe, G. V. Gibbs and M. A. Hamil, Am. Mineral., 1977, 62, 807 CAS.
- L. Bangrul, The Chelating Flotation Agent, Metallurgical Industry Press, Beijing, China, 1992 Search PubMed.
- Z. Guoxi, Physical Chemistry of Surface Active Agent, Peking University Press, Beijing, China, 1984 Search PubMed.
- T. Watcharatharapong, M. M. Sundaram, G. M. Shafiullah, R. D. Aughterson and R. Ahuja, ACS Appl. Mater. Interfaces, 2017, 9, 17977 CAS.
- M. Minakshi, D. Mitchell, R. Jones, F. Alenazey, T. Watcharatharapong, S. Chakraborty and R. Ahuja, Nanoscale, 2016, 8, 111291 RSC.
| This journal is © The Royal Society of Chemistry 2017 |