Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A facile molecularly imprinted electrochemical sensor based on graphene: application to the selective determination of thiamethoxam in grain†
Tianjiao Xie‡
 a,
Min Zhang‡
a,
Min Zhang‡ a,
Pan Chen‡a,
Haitian Zhao*a,
Xin Yang
a,
Pan Chen‡a,
Haitian Zhao*a,
Xin Yang *a,
Lei Yaoc,
Hua Zhanga,
Aijun Donga,
Jing Wang*b and
Zhenyu Wanga
*a,
Lei Yaoc,
Hua Zhanga,
Aijun Donga,
Jing Wang*b and
Zhenyu Wanga
aDepartment of Food Sciences and Engineering, School of Chemical Engineering and Technology, Harbin Institute of Technology, 150090 Harbin, PR China. E-mail: zhaoht9999@163.com
bKey Laboratory of Agro-Product Quality and Safety, Institute of Quality Standard and Testing Technology for Agro-Product, Chinese Academy of Agricultural Sciences, 100081 Beijing, PR China
cNational Research Center of Soybean Engineering and Technology, Northeast Agricultural University, Harbin 150030, PR China
First published on 8th August 2017
Abstract
In this study, we report a facile method for the preparation of molecularly imprinted polymer based graphene for the electrochemical detection of thiamethoxam residue. We choose p-vinylbenzoic acid as a functional monomer; it can be used to add recognition units and alkenyl units onto the surface of graphene via π–π interactions in just one step, effectively simplifying the reaction process. And an ultra-thin imprinting film (2 nm) is formed by reducing the addition step involving the alkenyl modifier. Due to the irreversible electrochemical reduction characteristics of thiamethoxam, the template can be removed easily using cyclic voltammetry scanning, without the need for organic solvents. The performance of the fabricated sensor was evaluated and the results indicated that the sensor exhibited excellent specific recognition abilities for thiamethoxam detection; the imprinting factor is 2.36. The peak current from thiamethoxam is linearly proportional to its concentration over the range from 0.5 to 20 μM, and the detection limit is 0.04 μM. The practical application of the sensor was also realized in the selective detection of thiamethoxam in real samples.
1. Introduction
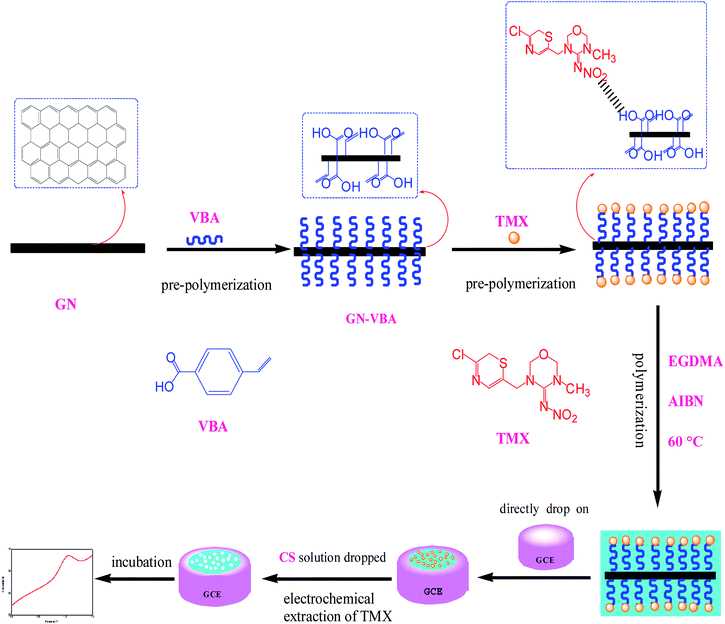
Thiamethoxam (TMX) is a representative compound of new neonicotinoid pesticides, which are the main pesticides used to control rice pests. Thiamethoxam has the characteristics of having high efficiency, low toxicity, a wide insecticide spectrum and long persistence. It is widely used in the world.1–4 However, the impact of residues of thiamethoxam on the environment, and its effect on blindness, human health, and non-target organisms have caused widespread worldwide attention5. At present, many countries have established maximum residue limits (MRLs) for thiamethoxam in food. In Japan, the MRL for thiamethoxam in brown rice is 0.1 mg kg−1. The national standard for food safety published in China stipulates that the MRLs for thiamethoxam in cucumber and brown rice are 0.5 mg kg−1 and 0.1 mg kg−1. The existing analytical techniques for the analysis of thiamethoxam mainly include high-performance liquid chromatography (HPLC),6,7 liquid chromatography and mass spectrometry.8 Despite being sensitive and specific, all these methods require expensive equipment, highly trained personnel, and time-consuming pretreatment. So they can only be carried out in the laboratory and are not convenient for rapid screening on farmland and in the market. Moreover, these methods can involve high levels of material consumption, such as toxic organic solvents, which is again a serious environmental concern. For these reasons, electrochemical sensors that are highly sensitivity, have a fast response, are low cost, are easily portable and have other characteristics might represent an alternative solution.9,10The recognition element on the surface of the electrode is the core of an effective electrochemical sensor.11 Due to the poor electrocatalytic performance of a glassy carbon electrode, we need to modify it. Graphene (GN)10,12–14 with high electrocatalytic activity is preferred, but graphene alone can't meet the need for detecting trace levels and specificity in pesticide analysis. Therefore, it is necessary to synergize with molecular imprinting technology to enhance its use. The molecular imprinting technique involves a kind of cross-linked polymer that exhibits specific binding sites around a template molecule to form a chemically and sterically complementary complex15. Molecularly imprinted electrochemical sensors combine the superiorities of both molecularly imprinted polymers (MIPs) and electrochemical sensors, showing high sensitivity and selectivity, ease of preparation, simplicity, reusability, and the possibility of miniaturization and automation at low cost.16,17 Based on the above, we use graphene as a carrier to prepare a graphene-based surface molecularly imprinted polymer as a recognition element, so as to improve the sensitivity and specificity of pesticide residue analysis.
Up to now, only a few papers have reported surface MIPs based on GN that have been constructed. The inert surface and poor dispersibility make it difficult to merge MIPs and GN18. Zeng et al. developed a strategy for grafting molecularly imprinted films onto the graphene surface using the olefinic bonds of graphene itself. The electrochemical detection limit for 4-nitrophenol was 0.005 μM.19 However, graphene oxide has fewer functional groups, such as alkenyl groups, after reduction, so the molecular imprinting sites for grafting are limited. Luo et al. modified graphene with N-vinylcarbazole to make a surface rich in alkenyl groups, which improved selectivity significantly.20 Compared with Zeng's work, due to the addition of alkenyl modification reagents, the molecular imprinted film was thicker, reaching about 17 nm. The molecular imprinting film is too thick to enhance the electrochemical signal. Luo et al. also developed a strategy to prepare graphene oxide (GO) based molecularly imprinted polymers (MIPs) for a glycoprotein electrochemical sensor, using boronic acid functionalized graphene oxide (GO-APBA) as a supporter by immobilizing them onto the surface of GO-APBA through boronate affinity.21 Chen et al. reported the novel design of magnetic two-dimensional molecularly imprinted polymers for the good recognition and separation of proteins, where dopamine was a reducing agent for graphene oxide.22
In summary, the study of surface molecularly imprinted electrochemical sensors with graphene as the carrier is in the initial stages. At present, graphene should be firstly pretreated, such as vinyl group functionalized graphene, to serve as a support, but more reagents and processes have been introduced.23,24 Luo et al. developed a novel graphene-molecularly imprinted polymer composite for bovine hemoglobin by simply mixing dopamine, graphene oxide and bovine haemoglobin (BHb) in aqueous solution; a thin adherent polydopamine film imprinted with BHb was spontaneously obtained on the surface of the graphene sheets, which was really convenient and easy to scale up.25 So the development of new graphene-modified reagents to study grafted molecular imprinting membranes has great theoretical value.
In this study, we present a facile method for the preparation of graphene based molecularly imprinted polymers (MIPs-GN) for the electrochemical detection of thiamethoxam (TMX) residue. The innovation of this study is in choosing p-vinylbenzoic acid as the functional monomer; the recognition units and alkenyl units can be added onto the surface of graphene via π–π interactions in just one step, effectively simplifying the reaction process. And an ultra-thin imprinting film is formed by reducing the addition step involving the alkenyl modifier. Thanks to the irreversible electrochemical reduction of TMX, the template could easily leach out of the binding sites, and it does not require any organic reagents, only aqueous solution. Besides, the adsorption mechanism and the properties of the MIP layer on the GN surface are carefully discussed. Furthermore, MIP-GN/GCE demonstrated efficiency in the detection of TMX in brown rice.
2. Experimental
2.1. Reagents and apparatus
Thiamethoxam (TMX) standard was obtained from YANGCHENG FLY HIGH CHEMICAL CO., LTD (China). Graphite powder, hydrazine hydrate, and 4-vinylbenzoic acid (VBA, 97%) were purchased from Shanghai Aladdin Chemical Reagent Company (China). Ethylene glycol dimethacrylate (EGDMA, 98%) was obtained from Sigma (China). 2,2-azo-bisisobutyronitrile (AIBN, 98%) and N,N-dimethylformamide (DMF) were purchased from Kemiou Chemical Reagent Co (Tianjing, China). All other chemicals were of analytical grade and used as received without further purification. Doubly distilled water was used throughout the work.Scanning electron microscopy (SEM) images were obtained on a Quantum 200FEG (FEI, U.S.). An Agilent Technology 1100 high performance liquid chromatograph (HPLC) connected to a diode array detector was employed. In addition, an Agilent-C18 column (4.6 × 250 mm, i.e., 5.0 m) was used. All electrochemical measurements such as cyclic voltammetry (CV) and linear sweep voltammetry (LSV) measurements were performed using CHI 660E and GHI 1040C electrochemical work stations (Chenhua Instrument Co, Ltd, Shanghai, China), with three-electrode systems. A conventional three-electrode system was composed of a MIP-GN or NIP-GN modified bare glassy carbon electrode (GCE, Ø 3 mm) as the working electrode, an Ag/AgCl (3 M KCl) electrode as the reference electrode and a platinum wire as the counter electrode.
2.2. Preparation of MIP-GN/GCE and NIP-GN/GCE
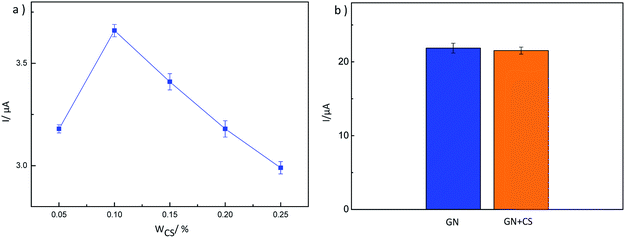
Graphene (GN) was synthesized using a modified method reported by Park et al.26 After determining the preparation scheme for MIP-GN/GCE, the parameters of the molecularly imprinted polymerization system were optimized. We optimized the important factors, including the ratio of functional monomer (VBA) to template molecule (TMX), the ratio of functional monomer to carrier GN, the ratio of functional monomer to crosslinking agent (EGDMA), and the amount of initiator (AIBN) in the molecular imprinting system, determining the best parameters (as shown in Fig. S1–S4†). The experimental parameters and procedures are as follows: firstly, GN (10 mg) was scattered in 10 mL of 0.1 mmol VBA/DMF solution and shake blended for 2 h at room temperature (25 °C) to prepare monomer (VBA) modified GN. Then, 10 mL of 0.1 mmol TMX/DMF was added and mixed fully under shaking for 2 h. Then, the cross-linker (EGDMA, 0.75 mmol) and initiator (AIBN, 0.025 g) were added to a 20 mL dispersion of the functional monomer and template molecule, and this was stirred under nitrogen for 10 min. The polymerization reaction was carried out at 60 °C for 24 h. For comparison, blank non-molecularly imprinted composites (NIP-GN) were prepared under the same conditions only without the addition of a template molecule in the polymerization process.Prior to modification, the glassy carbon electrode (GCE) was polished successively in 0.5 μM alumina slurries, rinsed thoroughly with doubly distilled water and dried in air. Then, the above synthesised MIP-GN (5 μL) was dropped on the well-polished GCE. Then it was dried at room temperature, dropped with 5 μL of 0.1% chitosan (CS, dissolved in HAc) solution and dried again. Here MIP-GN/GCE was successfully prepared.
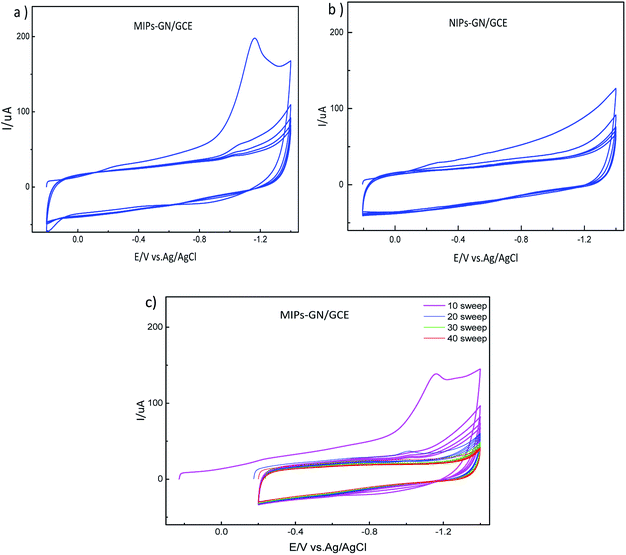
MIP-GN/GCE needs to have the template molecule removed. Firstly, in order to discard redundant reagents, MIP-GN/GCE was washed, under magnetic stirring, carefully with ethanol for 30 s. Then, the template molecules of TMX were removed via CV scanning; MIP-GN/GCE was soaked in 0.1 M phosphate buffered solution (PBS, pH 7.2) under CV over a potential scan range of −0.2 to −1.4 V and with a scan rate of 0.05 V s−1 until there was no signal from TMX, and then it was rinsed thoroughly with DDW. This MIP-GN/GCE was used for electrochemical experiments. For comparison, NIP-GN/GCE was prepared under the same conditions.
2.3. Electrochemical measurements
For electrochemical measurements including cyclic voltammetry (CV) and linear sweep voltammetry (LSV), the three-electrode system was immersed in a 10 mL electrochemical cell containing 0.1 M PBS solution and an appropriate concentration of TMX. After the extraction of the templates, MIP-GN/GCE was incubated in 0.1 M PBS containing different concentrations of TMX for 10 min and examined using LSV in new PBS. The linear sweep voltammograms were recorded from −0.2 V to −1.2 V and at a scan rate of 0.05 V s−1. The frequency range was 0.1 Hz to 105 Hz for electrochemical impedance spectroscopy, which was conducted in 5 mM Fe(CN)63−/4− and 0.1 M KCl solution at a formal potential of 0.25 V, with a signal amplitude of 5 mV.2.4. Sample preparation
The organic brown rice sample was purchased from the local market and crushed, and was detected to contain no TMX. Three levels of standard TMX concentrations (5.0 μM, 10.0 μM, and 15.0 μM) were spiked into 1 g of sample, which was prepared using the efficient method established by our previous group.273. Results
3.1. Preparation of MIP-GN/GCE
The preparation of a glassy carbon electrode modified with molecularly imprinted polymers which are based on graphene (MIP-GN/GCE) is the core of this experiment, and is carried out mainly through three steps. Firstly, a TMX molecularly imprinted polymeric material based on graphene (MIP-GN) was prepared. Subsequently, a facile sensor was obtained by directly dropping the synthetic MIP-GN dispersion on a well-polished electrode. Finally, electrochemical cyclic voltammetry (CV) was used to remove the template molecules.3.2. Characterization of MIP-GN/GCE
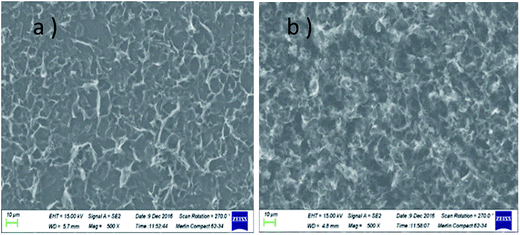
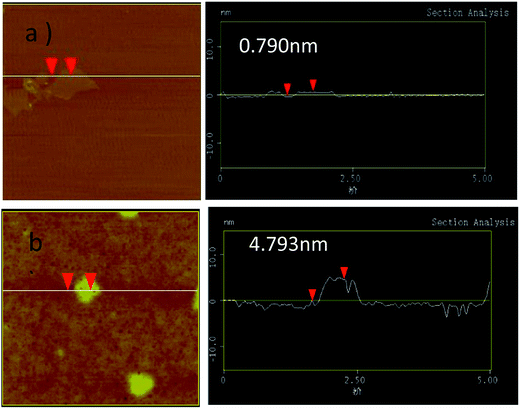
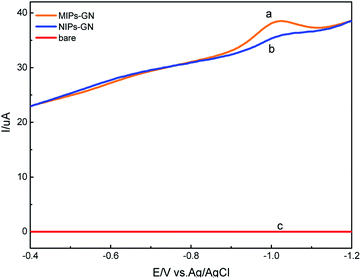
The morphologies of GN/GCE and MIP-GN/GCE were investigated using scanning electron microscopy (Fig. 4). GN sheets showed typically curved, layered structures with fairly smooth surfaces. The typical curved structure can provide a high surface area, providing suitable support for the molecular imprinting process. Compared with GN/GCE, MIP-GN/GCE showed a dense and rather rough surface, indicating that MIP films were uniformly loaded on the GN surface and MIP films were successfully grafted onto the GN surface. The surfaces of the MIP films were further probed using AFM (Fig. 5). The height of the GN as measured from cross section analysis was in the order of 0.790 nm, and thus the image corresponds to single-layer nano-sheets. According to AFM imaging of MIP-GN, the average thickness of the polymer grafted on the GN surface is about 4.793 nm, indicating that an imprinted film was successfully synthesized. Since both sides of the graphene sheet are loaded with molecularly imprinted films, the thickness of the molecularly imprinted film is about 2.0 nm, which is much lower than the reported molecularly imprinted film thickness (17 nm) on a graphene surface in the literature. Because the addition step involving an alkenyl-modifying agent is omitted, an ultra-thin imprinted film is formed. An ultra-thin imprinted film is conducive to the transmission of electrons and improves detection sensitivity. Fig. 6 shows electrochemical impedance spectroscopy (EIS) data for MIP-GN/GCE removable template molecules, MIP-GN/GCE and NIP-GN/GCE in 5.0 μM [Fe(CN)6]3−/4− containing 0.1 M KCl solution. All EIS results include a semicircular region lying on the axis of the plot, followed by a straight line for the three electrodes. The semicircular region observed at higher frequencies corresponds to the electrode transfer-limited process, thus the semicircle diameter equals the surface electrode transfer resistance (Ret). Obviously, the Ret of MIP-GN/GCE becomes lower after the removal of the template molecules. This result was because when the templates were removed from the electrode surface, specific recognition sites appeared. The Ret of MIP-GN/GCE with removable template molecules is also less than NIP-GN/GCE, indicating the high electron transfer rate for MIP-GN films.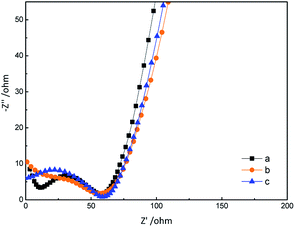 | ||
| Fig. 6 Electrochemical impedance spectroscopy (EIS) data for (a) MIP-GN/GCE with removable template molecules; (b) MIP-GN/GCE; and (c) NIP-GN/GCE. | ||
3.3. Performance of MIP-GN/GCE
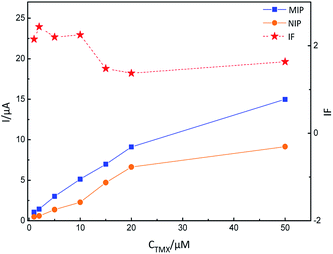
The adsorption mechanism for MIP-GN/GCE and nicotine pesticides was studied via an adsorption isotherm study and an adsorption kinetic study. The selectivity, linear response, stability and reusability of MIP-GN/GCE were also evaluated, along with interference and repeatability studies.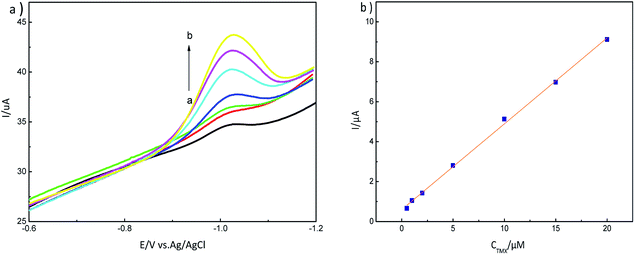 | ||
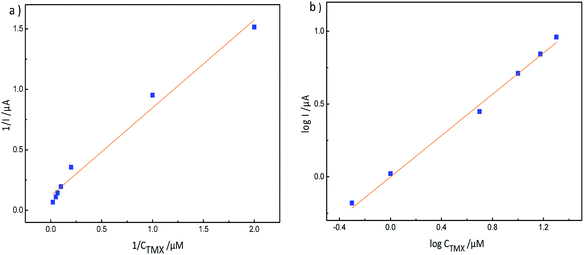
| Fig. 12 (a) LSV response to different concentrations of thiamethoxam, 0.5–20 μM (a → b), on an MIP-GN modified electrode in PBS buffer (pH 7.2); and (b) the calibration curve for TMX on MIP-GN/GCE. | ||
| Electrochemical method | Electrode | Linear range | LOD | Reference |
|---|---|---|---|---|
| Cyclic voltammetry | GCE | 95–1714 μM | 29 μM | Guzsvány et al.28 |
| Differential pulse polarography | Bismuth film electrode (GCE) | 4.3–154.3 μM | 1.3 μM | Guzsvány et al.32 |
| Differential pulse polarography | Mercury film electrode (GCE) | 2.64–154.3 μM | 0.89 μM | Guzsvány et al.32 |
| Differential pulse polarography | Dropping mercury electrode (DME) | 0.11–1.61 μM | 0.032 μM | Guzsvány et al.33 |
| Differential pulse polarography | Nanosilver/SDS/GCE | 0.1–9 μM | 0.1 μM | Kumaravel et al.34 |
| Cyclic voltammetry | Nanosilver/SDS/GCE | 10–90 μM | 4.7 μM | Kumaravel et al.34 |
| Amperometry | Nanosilver/SDS/GCE | 5–25 μM | 0.88 μM | Kumaravel et al.34 |
| Linear sweep voltammetry | MIP-GN/GCE | 0.5–20 μM | 0.04 μM | Present work |
3.4. Application to real sample analysis
To evaluate the practical performance of MIP-GN/GCE for the determination of TMX, rice samples were used. The recoveries obtained using electroanalytical techniques are shown in Table 2. There was no significant difference between the proposed sensor and HPLC analysis methods. A satisfactory result was also achieved with a minimum added concentration of 5 μmol L−1 (equal to 0.001 mg kg−1 of sample weight). The results indicate the practicability of our MIP-GN/GCE sensor, which can be used for the determination of thiamethoxam residue in brown rice samples.| Amount added (μmol L−1) | Amount detected using HPLC (μmol L−1) ± SD | Recovery rate using HPLC (%) ± SD | Amount etected using LSV (μmol L−1) ± SD | Recovery rate using LSV (%) ± SD | |
|---|---|---|---|---|---|
| Brown Rice | 5 | 4.77 ± 0.09 | 95.38 ± 1.75 | 4.49 ± 0.4 | 89.67 ± 8.03 |
| 10 | 9.56 ± 0.15 | 95.62 ± 1.53 | 9.41 ± 0.28 | 93.99 ± 2.74 | |
| 15 | 14.16 ± 0.26 | 94.76 ± 1.19 | 13.31 ± 0.04 | 88.69 ± 0.25 |
4. Conclusions
In conclusion, we present a facile method for the preparation of ultra-thin molecularly imprinted polymers based on graphene for the sensitive and selective determination of TMX residue. We selected 4-vinylbenzoic acid (VBA) as a functional monomer, so that the recognition units and alkenyl units were added onto the surface of GN in just one step. It was essential to simplify the reaction process on the electrochemical sensor using functional monomer modification. The ultra-thin imprinting film (2 nm) was formed by reducing the addition step involving the alkenyl modifier. The ultra-thin imprinted film is conducive to the transmission of electrons and improves detection sensitivity. The experimental results showed that MIP-GN/GCE not only possessed good sensitivity and binding capacity for TMX, but it also exhibited good reproducibility and reusability. In addition, the novel method was further applied to the detection of TMX in brown rice samples with satisfactory results.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 31571798, No. 31401618, and No. 31000831), the Natural Science Foundation of Heilongjiang Province (C2016033), the National Science Foundation for Post-doctoral Scientists of China (2014M551260), the Natural Science Research and Innovation Funds of Harbin Institute of Technology (HIT.NSRIF.2014093), Harbin Young talent reserve project 2014RFYJ179 and the Youth Science Fund Project of Heilongjiang Province (QC2013C026).Notes and references
- P. Jeschke and R. Nauen, Pest Manage. Sci., 2008, 64, 1084–1098 CrossRef CAS PubMed.
- A. Kumaravel and M. Chandrasekaran, Sens. Actuators, B, 2012, 174, 380–388 CrossRef CAS.
- R. Nauen, P. Jeschke and L. Copping, Pest Manage. Sci., 2008, 64, 1081 CrossRef CAS PubMed.
- D. Goulson and D. Kleijn, J. Appl. Ecol., 2013, 50, 977–987 CrossRef.
- J. Kimurakuroda, Y. Komuta, Y. Kuroda, M. Hayashi and H. Kawano, PLoS One, 2012, 7, e32432 CAS.
- P. Wang, X. Yang, J. Wang, J. Cui, A. J. Dong, H. T. Zhao, L. W. Zhang, Z. Y. Wang, R. B. Xu, W. J. Li, Y. C. Zhang, H. Zhang and J. Jing, Food Chem., 2012, 134, 1691–1698 CrossRef CAS PubMed.
- S. Seccia, P. Fidente, D. Montesano and P. Morrica, J. Chromatogr. A, 2008, 1214, 115–120 CrossRef CAS PubMed.
- M. A. Di, P. Fidente, D. A. Barbini, R. Dommarco, S. Seccia and P. Morrica, J. Chromatogr. A, 2006, 1108, 1–6 CrossRef PubMed.
- C. Malitesta, E. Mazzotta, R. A. Picca, A. Poma, I. Chianella and S. A. Piletsky, Anal. Bioanal. Chem., 2011, 402, 1827–1846 CrossRef PubMed.
- J. Wackerlig and P. A. Lieberzeit, Sens. Actuators, B, 2015, 207, 144–157 CrossRef CAS.
- L. Uzun and A. P. Turner, Biosens. Bioelectron., 2016, 76, 131–144 CrossRef CAS PubMed.
- Y. Zeng, Y. Zhou, L. Kong, T. Zhou and G. Shi, Biosens. Bioelectron., 2013, 45, 25–33 CrossRef CAS PubMed.
- Y. Zeng, Y. Zhou, T. Zhou and G. Shi, Electrochim. Acta, 2014, 130, 504–511 CrossRef CAS.
- F. Duan, C. Chen, G. Wang, Y. Yang, X. Liu and Y. Qin, RSC Adv., 2014, 4, 1469–1475 RSC.
- L. Chen, X. Wang, W. Lu, X. Wu and J. Li, Chem. Soc. Rev., 2016, 45, 2137 RSC.
- Y. Yang, Y. Cao, X. Wang, G. Fang and S. Wang, Biosens. Bioelectron., 2015, 64, 247–254 CrossRef CAS PubMed.
- M. L. Yola, T. Eren and N. Atar, Sens. Actuators, B, 2015, 210, 149–157 CrossRef CAS.
- T. Kuila, S. Bose, P. Khanra, A. K. Mishra, N. H. Kim and J. H. Lee, Biosens. Bioelectron., 2011, 26, 4637–4648 CrossRef CAS PubMed.
- Y. Zeng, Y. Zhou, L. Kong, T. Zhou and G. Shi, Biosens. Bioelectron., 2014, 130, 504–511 CAS.
- J. Luo, J. Cong, J. Liu, Y. Gao and X. Liu, Anal. Chim. Acta, 2015, 864, 74–84 CrossRef CAS PubMed.
- J. Luo, J. Huang, J. Cong, W. Wei and X. Liu, ACS Appl. Mater. Interfaces, 2017, 9(8), 7735–7744 CAS.
- F. Chen, W. Zhao, J. Zhang and J. Kong, Phys. Chem. Chem. Phys., 2015, 18, 718–725 RSC.
- Y. Mao, Y. Bao, S. Gan, F. Li and L. Niu, Biosens. Bioelectron., 2011, 28, 291–297 CrossRef CAS PubMed.
- J. Luo, J. Cong, J. Liu, Y. Gao and X. Liu, Anal. Chim. Acta, 2015, 864, 74–84 CrossRef CAS PubMed.
- J. Luo, S. Jiang and X. Liu, Sens. Actuators, B, 2014, 203, 782–789 CrossRef CAS.
- J. A. Sungjin Park, I. Jung, R. D. Piner, S. J. An, X. Li, A. Velamakanni and R. S. Ruoff, Nano Lett., 2009, 9, 1593–1597 CrossRef PubMed.
- M. Zhang, H. T. Zhao, X. Yang, A. J. Dong, H. Zhang, J. Wang, G. Y. Liu and X. C. Zhai, Sens. Actuators, B, 2016, 229, 190–199 CrossRef CAS.
- V. J. Guzsvány, F. F. Gaál, L. J. Bjelica and S. N. Ökrész, J. Serb. Chem. Soc., 2005, 70, 735–743 CrossRef.
- M. Zhang, H. T. Zhao, X. Yang, W. T. Zhang, J. Wang, G. Y. Liu, H. Zhang and A. J. Dong, RSC Adv., 2016, 6, 3714–3722 RSC.
- O. F. Pure and A. Chemistry, Pure Appl. Chem., 1998, 993–1014 Search PubMed.
- L. A. Currie, Pure Appl. Chem., 1995, 1699–1723 CAS.
- V. Guzsvány, M. Kádár, F. Gaál, L. Bjelica and K. Tóth, Electroanalysis, 2010, 18, 1363–1371 CrossRef.
- V. Guzsvány, M. Kádár, F. Gaál, K. Tóth and L. Bjelica, Microchim. Acta, 2006, 154, 321–328 CrossRef.
- A. Kumaravel and M. Chandrasekaran, Sens. Actuators, B, 2012, 174, 380–388 CrossRef CAS.
- J. Guo, Y. Wang, Y. Liu, C. Zhang and Y. Zhou, Talanta, 2015, 144, 411–419 CrossRef CAS PubMed.
- S. Zhao, X. Yang, H. Zhao, A. Dong, J. Wang, M. Zhang and W. Huang, Talanta, 2015, 144, 717–725 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra05167k |
| ‡ These authors contributed equally to this work and should be considered as co-first authors. |
| This journal is © The Royal Society of Chemistry 2017 |