Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Antimicrobials tethering on suture surface through a hydrogel: a novel strategy to combat postoperative wound infections†
Himadri Kalita‡
*a,
Ankita Hazarika‡*a,
Sanjeeb Kalita§
b,
Raghuram Kandimalla§b and
Rajlakshmi Devi *a
*a
aLife Sciences Division, Institute of Advanced Study in Science and Technology (IASST), Paschim Boragaon, Garchuk, Guwahati-781035, Assam, India. E-mail: biochemistry.iasst@gmail.com; kalitahimadri123@gmail.com; ankitahazarikabiotech@gmail.com; Tel: +91-9706033567 Tel: +91-9706107073 Tel: +91-9706053605
bDrug Discovery Laboratory, IASST, Paschim Boragaon, Garchuk, Guwahati-781035, Assam, India
First published on 27th June 2017
Abstract
The present study aimed to develop a novel biocompatible suture biomaterial from Eri silk waste to avoid surgical site infections. To achieve this, silk waste fibers were reeled through a five-loop technique into a suture and embedded with an antimicrobial agents-growth factor cocktail in a hydrogel base comprising of Aloe-Vera and gum acacia (PRWSc). Characterization techniques like scanning electron microscopy (SEM), attenuated total reflection Fourier infrared spectroscopy (ATR-FTIR), thermo gravimetric analysis (TGA), and tensile properties analysis revealed the surface morphology, functionalization analysis, thermal and mechanical stability of PRWSc. Drug release study confirms the sustained release of the drugs from the suture. PRWSc was found to be biocompatible towards mammalian cells. In vitro antimicrobial study revealed that the PRWSc could inhibit the growth of Gram +ve, Gram −ve bacteria and opportunistic fungus. Further, confocal microscopy analysis confirmed the successful inhibition of biofilm on PRWSc surface. Clinical examination of the wound sutured with PRWSc revealed the successful wound healing which was confirmed by reduced microbial burden (CFU load) and inflammatory markers at the surgical site. This result was further confirmed by histopathology data where all skin adnexal structures were observed. This advanced suture material (PRWSc) can reduce the unwanted side effects of antibiotic overdose and can avoid serious problems related to postoperative complications.
1. Introduction
Wound healing is a complex process that restores external and internal physical integrity of the body structure and involves several other complex interactions between the cells and other external environmental factors. The healing process starts as soon as the tissue injury occurs and it is categorized into four sequential and overlapping phases: homeostasis, inflammation, proliferation and remodelling.1 These phases require perfectly coordinated cellular and molecular events involving several biological processes such as proliferation, differentiation, cell migration and increased biochemical activities. These biochemical events involve the components such as the extracellular matrix, cytokines, blood cells and growth factors. Growth factors are responsible for cell proliferation by the process of angiogenesis, myelogenesis, and gene transcription for accelerating the healing process.1–3With the extensive use of sutures and other medical devices, the associated surgical site infections have been receiving greater attention. With an estimated 27 million surgical procedures each year, approximately 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–500
000–500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 surgical site infections can be predicted to occur annually.4 Infections associated with medical devices and sutures account for 44% of all of the nosocomial infections.5 The consequence of surgical site infections includes longer hospital stays, extra financial costs, increased physiological and psychological burdens on patients and their families, and sometimes requiring an additional surgery to remove the infected implant.6 One of the leading causes of infection at the wound site is the formation of biofilm on the surface of the surgical suture.7 Incorporation of antimicrobial agents and growth factors on the suture biomaterial increases the rate of wound healing and prevents biofilm formation.5 At the surgical site, infection due to bacteria like Staphylococcus aureus, Staphylococcus epidermidis, and opportunistic fungal pathogen like Candida albicans are more common.8 So, using broad spectrum antimicrobial agents like amoxicillin, vancomycin, and amphotericin b can safely work against these microbial infections.
000 surgical site infections can be predicted to occur annually.4 Infections associated with medical devices and sutures account for 44% of all of the nosocomial infections.5 The consequence of surgical site infections includes longer hospital stays, extra financial costs, increased physiological and psychological burdens on patients and their families, and sometimes requiring an additional surgery to remove the infected implant.6 One of the leading causes of infection at the wound site is the formation of biofilm on the surface of the surgical suture.7 Incorporation of antimicrobial agents and growth factors on the suture biomaterial increases the rate of wound healing and prevents biofilm formation.5 At the surgical site, infection due to bacteria like Staphylococcus aureus, Staphylococcus epidermidis, and opportunistic fungal pathogen like Candida albicans are more common.8 So, using broad spectrum antimicrobial agents like amoxicillin, vancomycin, and amphotericin b can safely work against these microbial infections.
Natural non-absorbable biopolymer like silk fibroin is considered as promising biomaterial because of its desirable characteristics. Silk fibroins are classified into two main types namely mulberry (Bombyx mori) and non-mulberry varieties including muga (Antheraea assama), eri (Philosamia ricini), tasar (Antheraea mylitta).9 Bombyx mori silk fibroin (BMSF) has been characterised and commercially available biomaterial worldwide for centuries.10 Among the non-mulberry silk P. ricini and A. assama has been widely studied in the field of biomaterial. The potential applicability of P. ricini waste fiber (PRWF) as suture biomaterial is yet to be fully explored. Although the utility of this waste material has been reported in the field of textile as conventional clothing material,11 there is no scientific evaluation of the PRWF in the field of biomaterial till now. So in this present study, we have selected PRWF for the development of suture material. In this context, the present study was designed to develop a novel suture biomaterial to overcome all the drawbacks related to surgical site infections by impregnating the suture made from PRWF (PRWS) with broad spectrum antimicrobial agents (amoxicillin & amphotericin-b) and growth factors. A plant-based hydrogel system composed of gum acacia (GA) and Aloe-Vera (AV) has been used for efficient impregnation of drugs on the suture surface. Plant based gels and gum are reported to possess superior wound healing ability which accelerates the healing process. Hydrogel obtained from AV has an inherent ability to increase the collagen cross linking at wound site and accelerate the healing process. This is achieved by enhancing the wound contraction and breaking strength of the repaired tissue through escalation of the collagen type-III synthesis.12,13 Natural gum, GA is composed of arabinogalactan, oligosaccharide, polysaccharides and is often used as a binding agent. This binding ability of GA can contribute to the efficient impregnation of antimicrobial agents on to the suture surface. GA also has antimicrobial activity, which can further contribute to faster tissue regeneration at the surgical site.14 In view of this, it has been hypothesized in this study that the presence of this AV–GA hydrogel system can potentially serve the dual purpose of efficient drug loading on the suture surface and accelerate the wound healing cascade. The outcome of the study can fabricate a potential antimicrobial releasing suture biomaterial for prevention of postoperative wound infections.
2. Materials and methods
2.1. Materials
P. ricini (Eri) waste fiber was collected from Central silk Board, Guwahati, Assam, India. Processed fiber was sterilized by moist heat sterilization method in the autoclave (121 °C at 100 kPa pressure) for 20 min for further use. All the drugs and chemicals used in this study were procured from Sigma Aldrich, USA and Merck, Germany. Interleukin kites were procured from R&D systems, USA. BacLight bacterial viability kit was purchased from Life technologies, USA.2.2. Methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and nerve growth factor (250 ng mL−1) and epithelial growth factor (1 ng mL−1) in a mixture of 0.1% hydrogel consist of AV and GA. Further PRWS was dipped in the coating solution and dried at 37 °C for 24 h (PRWSc).
1) and nerve growth factor (250 ng mL−1) and epithelial growth factor (1 ng mL−1) in a mixture of 0.1% hydrogel consist of AV and GA. Further PRWS was dipped in the coating solution and dried at 37 °C for 24 h (PRWSc).2.3. Biocompatibility evaluation
Before conducting in vivo analysis biocompatibility evaluation of blood contacting biomaterial is very much essential. In this study both the sutures PRWS and PRWSc were screened for biocompatibility towards mammalian cells by following experiments-| Viability (%) = abs test/abs control × 100 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 ratio). After one hour tubes were centrifuged at 1500 rpm for 10 minutes. The absorbance of the supernatant was measured at 545 nm in a UV-visible spectrophotometer (Shimadzu, UV 1800, Japan).16 The percentage of hemolysis was calculated by following formula-
9 ratio). After one hour tubes were centrifuged at 1500 rpm for 10 minutes. The absorbance of the supernatant was measured at 545 nm in a UV-visible spectrophotometer (Shimadzu, UV 1800, Japan).16 The percentage of hemolysis was calculated by following formula-2.4. Evaluation of anti-thrombogenic property
The antithrombogenic properties of PRWS, PRWSc, and BMSF were evaluated using an in vitro kinetic method.10 Citrate dextrose stabilized blood (2 mL) was incubated in micro centrifuge tubes for 1 h, each containing PRWS, PRWSc, and BMSF separately at 37 °C. After incubation, blood (0.2 mL) was taken from each tube and placed onto a clean sterilized glass plate. The glass plates were sterilized by autoclaving at high pressure saturated steam at 121 °C for around 15–20 min prior to perform the experiment. After the addition of 0.02 mL 0.1 M calcium chloride solution to the citrate dextrose treated blood, the clotting test was performed by the weight measurement of the amount of thrombus (clot) formed. The storage time of the blood was maintained at 30, 45 and 60 min. The blood clot was fixed in 5 mL formaldehyde solution (36% v/v) for 5 min after each particular time interval and further washed with distilled water, blotted with tissue paper and finally weighed the clot. The citrate dextrose treated blood without suture material was taken as control. The experiment is carried out in triplicate and repeated three times, and the result is reported as mean ± S.D.2.5. Evaluation of in vitro antimicrobial activity
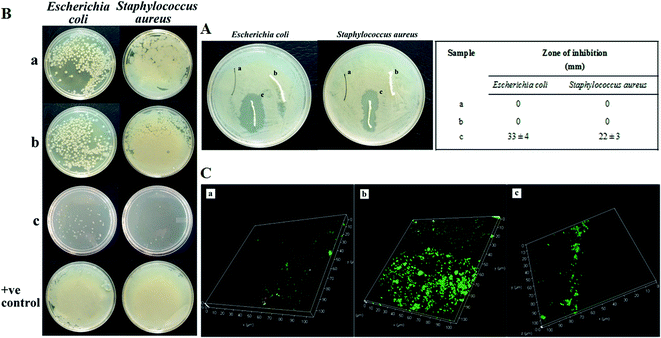
Antimicrobial property of the suture materials (PRWS & PRWSc) were examined by standard agar diffusion test and direct contact method. Further anti-biofilm property of the sutures was determined by confocal imaging of the suture through LIVE/DEAD Baclight bacterial viability assay kit.2.6. Drug release kinetics of PRWSc
The drug release profile of antimicrobial drugs and growth factors from the PRWSc suture was measured in PBS to study the drug release during usage. PRWSc (10 cm each) were placed in 1.5 mL centrifuge tube containing releasing medium (1.33 mL) of different pH (6.3, 6.6, and 7.7) which correspond to the different skin pH during healing and incubated at 37 °C with continuous shaking at 60 rpm. At different time points (24, 48, 72, 96, 120, and 144 h) 150 μL of each sample was withdrawn to measure the drug concentration and replaced with fresh media. The absorbance of the recovered aliquots were measured by using Multimode Reader, VARIOSKAN FLASH at different wavelengths 290 nm (pH 6.3), 289 nm (pH 6.6), and 287 nm (pH 7.7).202.7. In vitro degradation study
Enzymatic degradation study of the sutures was performed using a previously described method with slight modification.21 Briefly, an enzyme solution was prepared by dissolving α-chymotrypsin Type I-S (Sigma, C7762) in Tris–HCL (pH-7.8). BMSF, PRWS, and PRWSc sutures were incubated in the enzyme solution at 37 °C for 14 days. After incubation period sutures were removed from the enzyme solution and dried under vacuum at 50 °C. The weight of the dried sutures was measured and tensile strength evaluated. All the experiments were performed in triplicate and results were expressed in mean ± S.D.2.8. Evaluation of healing ability of sutures on infected wounds
Male rats of Wister strain weighing 150–200 g was selected for the study and divided into three groups (n = 6). All the animals were housed at 25 ± 1 °C with a relative humidity of 45–55% and 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 h dark/light cycle. Animals were free to access water and standard pellet diet (Provimi, India) throughout the experimental period.22 This study was performed in strict accordance with Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) guidelines (registration number: 1706/GO/c/13/CPCSEA) and the protocol was approved by the Institutional Animal Ethical Committee of Institute of Advanced Study in Science and Technology, Guwahati-35, Assam, India (IASST/IAEC/2015-16/751).
12 h dark/light cycle. Animals were free to access water and standard pellet diet (Provimi, India) throughout the experimental period.22 This study was performed in strict accordance with Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) guidelines (registration number: 1706/GO/c/13/CPCSEA) and the protocol was approved by the Institutional Animal Ethical Committee of Institute of Advanced Study in Science and Technology, Guwahati-35, Assam, India (IASST/IAEC/2015-16/751).
BMSF. Animals sutured with BMSF and infected with S. aureus (2 × 108 cells per mL) after 24 h of surgery.
PRWS. Animals were sutured with PRWS and infected with S. aureus (2 × 108 cells per mL) after 24 h of surgery.
PRWSc. Animals were sutured with PRWSc and infected with S. aureus (2 × 108 cells per mL) after 24 h of surgery.
2.9. Determination of bacterial load/CFU count
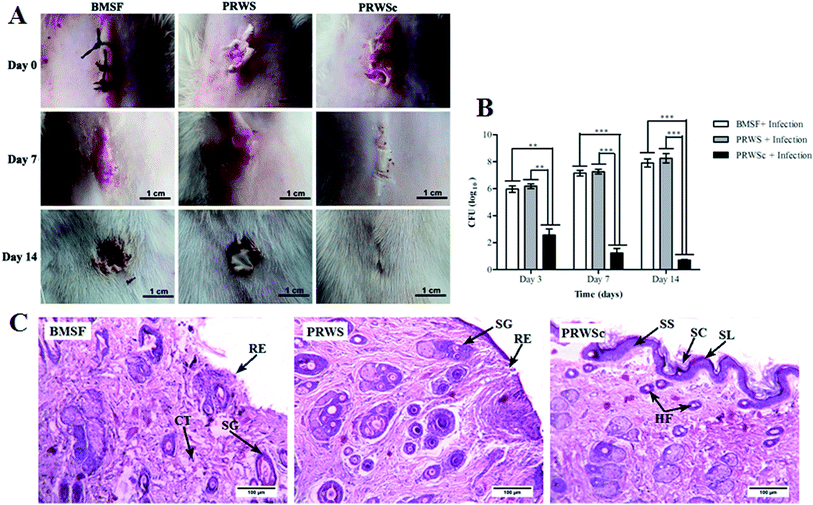
At 3rd, 7th, and 14th post operative day tissue samples from the wounded area were excised, pulverized, and homogenized in sterile PBS. The resulting suspension was diluted and plated on nutrient agar and further incubated at 37 °C for 24 h. Results were normalized based on the tissue collected for determination of bacterial load.82.10. Measurement of inflammatory markers
Serum was separated by centrifuging the blood at 1500 rpm for 10 min and stored at −80 °C for further use. Inflammatory cytokines like (IL-1β & TNF-α) were estimated by using ELISA kits from R&D systems as per the instructions given by the manufacturer.2.11. Histopathological analysis
Skin tissue from the surgical site was collected from all the groups and fixed in 10% buffered formaldehyde. Fixed samples were subjected to ethanol dehydration process (70, 90, and 100%) and embedded in the paraffin block. Sections of 5 μm thickness were made by using rotary microtome and stained with hematoxylin–eosin stain. The slides were examined under light microscope (LEICA EC3, Germany) for determination of pathological changes.23–253. Results
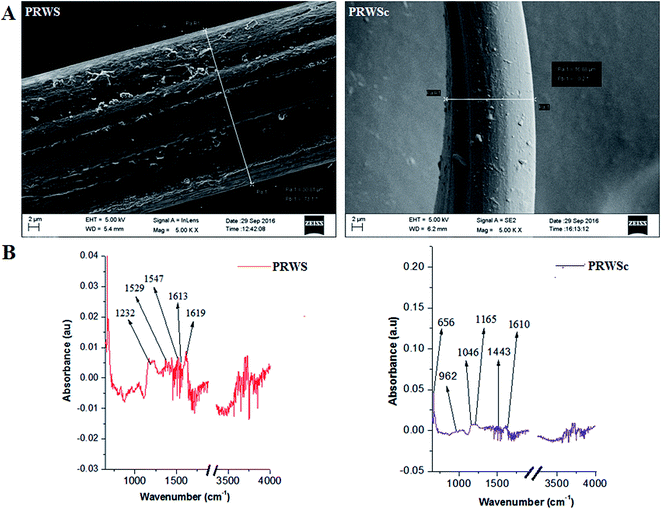
3.1. Characterization of suture material
| Sample | Maximum load (g) | Strain at maximum (%) | Young's modulus (g per den) | Tenacity at break (g per den) | Tenacity at maximum (g per den) | Toughness (g per den) |
|---|---|---|---|---|---|---|
| a All the results were expressed in mean ± S.D (n = 3). | ||||||
| BMSF | 2000 ± 170.05 | 43.97 ± 5.9 | 1453 ± 54.9 | 200 ± 12 | 290 ± 12 | 117 ± 7 |
| PRWS | 1998.49 ± 96 | 40.21 ± 5.28 | 1324 ± 115 | 178.75 ± 73 | 216.41 ± 16.04 | 110.48 ± 5.12 |
| PRWSc | 1990 ± 100 | 40.23 ± 3 | 1350 ± 50 | 180 ± 40 | 225 ± 20 | 115 ± 10 |
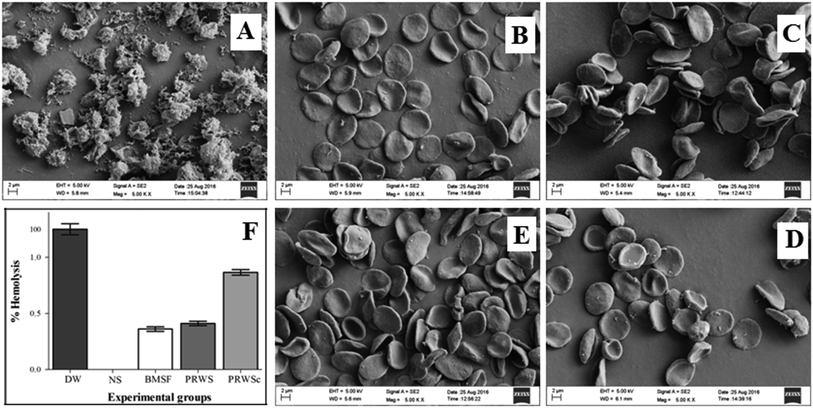
3.2. Biocompatibility evaluation
3.3. In vitro antithrombogenic property
For evaluating the in vitro antithrombogenic property in vitro blood clotting test was performed (ESI Fig. S5†). Thrombus formation was observed before and after the clotting time of 30, 45, and 60 min for control (A), BMSF (B), PRWS (C), and PRWSc (D). Non coated and coated sutures did not show any significant difference in the blood clotting process at different time interval.3.4. Antimicrobial activity
3.5. Release kinetics of the drug from PRWSc
PRWSc demonstrated continuous drug release up to 5–6 days (Fig. 4). The initial burst of drug release was started at 24 h and remained stable after 96 h. The natural base material AV and GA facilitate continuous release of the antibacterial drug from the PRWSc at 72, 78, and 85% in various pH 6.3, 6.6, and 7.7 conditions. The results suggested that maximum drug release was observed in alkaline pH and lowest in acidic pH.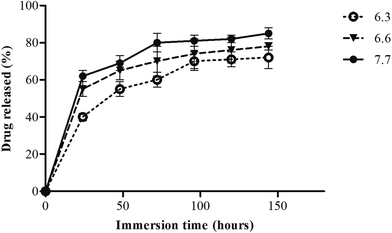 | ||
| Fig. 4 In vitro amoxicillin release percentage from coated P. ricini waste suture (PRWSc) at three different pH 6.3, 6.6, and 7.7 up to 144 h. | ||
3.6. Degradation study
After 14 days of incubation period, changes were observed in terms of percentage loss in dry weight and tensile properties of the sutures. The non-coated suture (PRWS) showed a dry weight loss of 9 ± 1.32% and tensile strength loss of 7.01 ± 1%. However, the hydrogel coated suture (PRWSc) demonstrated 10.99 ± 2% and 7.32 ± 1.2% loss of dry weight and tensile strength respectively. All the comparisons were made with commercial suture (BMSF) which exhibited 8.2 ± 1% loss in dry weight and 6.5 ± 1.1% loss in tensile strength (ESI Table S1†).3.7. Wound healing assessment
Wound healing is well supported by CFU count with a significant reduction in viable S. aureus in the wound area of the animal sutured with PRWSc. Significant increase in bacterial colonization (CFU) was observed in animals sutured with PRWS and BMSF at 3rd, 7th, and 14th day of post operation. On the contrary, CFU count decreased surprisingly in PRWSc sutured animal, which indicates that S. aureus count was reduced significantly for the PRWSc sutured wound as compared to PRWS and BMSF (Fig. 5B).
4. Discussion
Recent understanding of tissue remodeling and wound healing processes enable the researchers to contribute towards the development of cost effective wound management products.26 Surgical suture mediated infections poses major concern during post-operative period that further lead to delayed wound healing and prolonged hospital stay. Antimicrobial drug releasing sutures avert biofilm formation and subsequent deeper tissue colonization of bacteria which can potentially reduce the complications of surgical site infection.27Till date, to the best of our knowledge, no such suture material is available in the market which possesses the characteristics of an ideal suture like tissue compatibility, thermal stability, and superior tensile strength. In this context, the present proposition was made for the development of an antimicrobial drugs releasing suture having the ideal suture characteristics. Biomaterials from B. mori, A. assama, and P. ricini are already reported, however the silk from these mulberry and non-mulberry silkworms are mainly used in the textile industry.28 Among these silk fibers, P. ricini is the cheapest and easy to rear without any environmental hazards.29 During the reeling of the silk from the cocoon, tons of fibrous waste materials are generated every year.30 The utility of this fibrous waste is yet to be realized for value-added purposes like fabrication of cost effective suture. In this study, fibrous waste of P. ricini was exploited for the development of a novel antimicrobial suture biomaterial. Tensile strength is the most crucial and primary requisite in the development of a suture material to reduce the possibility of breakage during the time of surgery.31 Previous study reported that the greater percentage of C–H, C–C, and C–N unit at the surface of the fiber contribute to its tensile strength.32 Hence the increased percentage of C and H on PRWSc might have resulted in its higher tensile strength.
Also, antimicrobial drug amoxicillin revealed the presence of characteristic peak for O13–H, C–H, N–H, C16–H, O25–H, CH2 bending and C16–NH2, C–C ring 2 stretching.33 The detection of these functional groups on the PRWSc suture depicts the successful impregnation of amoxicillin on the suture surface. This have further lead to the prominent inhibition of infection in the surgical site sutured with PRWSc. Study reveals that aliphatic amine (C–N bending) and O–H bending are the markers of AV and GA.34,35 Detection of these groups confirmed the presence of AV–GA based hydrogel system on the PRWSc suture surface. Inorganic mineral, potassium (K) is a component of AV hydrogel, which is essential for different enzymatic reactions modulating the wound healing process.36 Thus, the identified K on PRWSc surface upon elemental analysis have come from the AV gel. Further TGA analysis of developed suture depicts that the suture biomaterial stable up to 600 °C. Interestingly the observed delay in thermal degradation of PRWS and PRWSc as compared to BMSF confirms that the fabricated suture is more thermally stable than BMSF.
The presence of broad spectrum antibiotic amoxicillin inhibits the growth of S. aureus.37 This could be the reason for the prominent zone of inhibition exhibited by PRWSc against pathogenic S. aureus. The inhibited growth of S. aureus might have led to the prevention of biofilm formation on the suture surface as observed upon confocal analysis of PRWSc (Fig. 3C). The antifungal agent, amphotericin b is reported to inhibit the growth of opportunistic fungal pathogen C. albicans.38 The observed zone of inhibition against C. albicans in this could be attributed to the efficient coating of amphotericin b on the suture surface. This might have further contributed to the prevention of SSI and faster wound healing as observed in vivo analysis of PRWSc. A sustained drug release profile is necessary for prolonged antimicrobial activity and faster healing process.17 Biomaterials prepared by AV gel blended with cellulose, chitosan, and gelatine is reported to exhibit anti-inflammatory and antibacterial properties.39 This is due to the presence of polymers like galactan, glucomannan, and polysaccharide in AV.39 The presence of this polymeric matrix contributes to the release of the drug from the AV-drug amalgam by leaching process further leading to its diffusion into the wound area, following a biphasic release mechanism.40 GA acts as a natural binding agent, which possesses antimicrobial activity and finds wide use in therapeutic applications like wound dressing materials.41 Thus, the biphasic drug release profile of PRWSc observed in this study is due to the presence of AV–GA hydrogel base. This might have also contributed to the faster wound healing of the surgical wound sutured with PRWSc by inhibiting microbial colonization on the sutures surface. It is important to analyze the in vitro biocompatibility and cytocompatibility of the suture biomaterials prior to in vivo application.16 Studies have reported that the permissible limit of hemolysis of blood exposed to biomaterials is 5%.16 In this study percentage hemolysis of PRWSc is below this permissible limit, which confirms its biocompatibility as a suture material. Hence it can be predicted that PRWSc would not show any adverse effects on human erythrocytes and this supports the possible coexistence of the fabricated suture in the human body without toxicity.
Growth factors in the wound site leads to tissue remodelling by enhancing the collagen synthesis which further induces the wound healing process.42 Collagen plays an important role in the synthesis of connective tissue, which provides a framework in the regeneration of tissue in the healing area.43,44 The prominent improvements in the ultra-structure of skin tissue stitched with PRWSc might have resulted due to the presence of growth factors in the coating material. Studies also reported that AV hydrogel contributes to the synthesis of collagen in wound site. This is rendered by the presence of muco-polysaccharide and acemannan in the hydrogel.40 In this view, it can be predicted that the presence of AV and growth factors led to the formation of dense connective tissue in the PRWSc sutured wound site which resulted in its faster healing. AV has anti-inflammatory activity due to the presence of brady kinase enzyme, which helps to reduce excessive inflammation upon topical application on skin. This helps in stimulating immune defence system at the wound site and fasted the healing process.45 The observed reduction in the level of inflammatory markers, TNF-α and IL-1β in the PRWSc sutured rats could be due to the anti-inflammatory activity of AV hydrogel. Reduction of these inflammatory marker levels in PRWSc sutured animal can be correlated with the accelerated wound healing process and thus support the present findings.
5. Conclusion
In this study we have prepared novel antimicrobial and growth factor impregnated suture, PRWSc from P. ricini (Eri) silk waste fiber using AV–GA hydrogel system. The prepared suture showed antimicrobial efficacy against S. aureus, E. coli, and C. albicans and could effectively prevent bacterial adherence on it. Slow and sustained release of drug was realized by diffusion of drug in aqueous medium, which was facilitated by the hydrogel. The hydrogel system also possesses inherent wound healing, anti-scar, antimicrobial properties which are helpful in fastening the healing process. Further, PRWSc prevents the surgical site infections and accelerates the wound healing process. This invention can outrage the applications of P. ricini silk waste fiber and introduces a novel strategy to combat surgical site infections for better healing of surgical wounds.Author contributions
All authors made a significant contribution to the study and are in agreement with the content of the manuscript. Himadri Kalita and Ankita Hazarika conceived and designed the experiment, performed the experiments and analyzed the data and jointly wrote the paper as well and thus made an equal contribution to this study. Sanjeeb Kalita and Raghuram Kandimalla helped in conceiving the experiment and also in critical revision of the manuscript for important intellectual content. The final approval of the manuscript was done by Rajlakshmi Devi.Conflict of interest
A portion of this work was used to file an Indian patent application (201631039604) dated 22nd November 2016.Acknowledgements
The authors acknowledge Department of Science and Technology, Government of India, New Delhi, for financial support. We also acknowledge Institute of Advanced Study in Science and Technology (IASST), Guwahati, Assam, for providing necessary facilities. The authors acknowledge Central Silk Board, Assam, India for providing the Eri silk waste material. The authors thank Subrata Goswami, technical assistant, IASST for evaluating mechanical properties; Bikash Sarma and Achyut Konwar, PhD scholar, IASST for sample analysis using FESEM and TGA respectively. All the authors are grateful to Dr Anupam Banerjee, Manager Application Support, Leica Microsystem and Dr Bula Choudhury, Senior Scientist, Guwahati Biotech Park for their immense help with confocal microscopy.References
- M. Sengupta, J. Sengupta, P. Banerjee, M. Ghosh and S. Paul, Muller J. Med. Sci. Res., 2015, 6, 27–30 CrossRef
.
- S. Werner and R. Grose, Physiol. Rev., 2003, 83, 835–870 CAS
.
- D. Roth, M. Piekarek, M. Paulsson, H. Christ, W. Bloch, T. Krieg, J. M. Davidson and S. A. Eming, Am. J. Pathol., 2006, 168, 670–684 CrossRef CAS PubMed
.
- C. Mingmalairak, Antimicrobial Sutures: New Strategy in Surgical Site Infections, A. Mendez-Vilas, Formatex Research Center, Spain, 2011, pp. 313–323 Search PubMed
.
- S. Guo and L. A. Dipietro, J. Dent. Res., 2010, 89, 219–229 CrossRef CAS PubMed
.
- X. Liu, T. Lin, J. Fang, G. Yao, H. Zhao, M. Dodson and X. Wang, J. Biomed. Mater. Res., Part A, 2010, 94, 499–508 Search PubMed
.
- J. Meyle, Periodontal Pract. Today, 2006, 3, 253–268 Search PubMed
.
- S. Kalita, R. Kandimalla, B. Devi, B. Kalita, K. Kalita, M. Deka, A. Chandra Kataki, A. Sharma and J. Kotoky, RSC Adv., 2017, 7, 1749–1758 RSC
.
- D. Gogoi, A. J. Choudhury, J. Chutia, A. R. Pal, M. Khan, M. Choudhury, P. Pathak, G. Das and D. S. Patil, Biopolymers, 2014, 101, 355–365 CrossRef CAS PubMed
.
- A. Jyoti, D. Gogoi, R. Kandimalla, S. Kalita, Y. B. Chaudhari, M. R. Khan, J. Kotoky and J. Chutia, Mater. Sci. Eng., C, 2016, 60, 475–484 CrossRef PubMed
.
- D. Gogoi, A. J. Choudhury, J. Chutia, A. R. Pal, N. N. Dass, D. Devi and D. S. Patil, Appl. Surf. Sci., 2011, 258, 126–135 CrossRef CAS
.
- A. Surjushe, R. Vasani and D. G. Saple, Indian J. Dermatol., 2008, 53, 163–166 CrossRef PubMed
.
- S. K. Verma and S. M. M, Inter. J Biol. Med. Res., 2011, 2, 466–471 Search PubMed
.
- P. M. Sivakumar, V. Prabhawathi, R. Neelakandan and M. Doble, Biomater. Sci., 2014, 2, 990–995 RSC
.
- R. Kandimalla, S. Dash, S. Kalita, B. Choudhury, S. Malampati, R. Devi, M. Ramanathan, N. C. Talukdar and J. Kotoky, Front. Cell. Neurosci., 2017 DOI:10.3389/fncel.2017.00073
.
- R. Kandimalla, S. Kalita, B. Choudhury, D. Devi, D. Kalita, K. Kalita, S. Dash and J. Kotoky, Mater. Sci. Eng., C, 2016, 62, 816–822 CrossRef CAS PubMed
.
- A. Konwar, S. Kalita, J. Kotoky and D. Chowdhury, ACS Appl. Mater. Interfaces, 2016, 8, 20625–20634 CAS
.
- J. Li, G. Wang, H. Zhu, M. Zhang, X. Zheng, Z. Di, X. Liu and X. Wang, Sci. Rep., 2014 DOI:10.1038/srep04359
.
- G. Limbert, R. Bryan, R. Cotton, P. Young, L. Hall-stoodley, S. Kathju and P. Stoodley, Acta Biomater., 2013, 9, 6641–6652.
- X. Chen, D. Hou, L. Wang, Q. Zhang, J. Zou and G. Sun, ACS Appl. Mater. Interfaces, 2015, 7, 22394–22403 CAS
.
- T. Arai, G. Freddi, R. Innocenti and M. Tsukada, J. Appl. Polym. Sci., 2004, 91, 2383–2390 CrossRef CAS
.
- A. Hazarika, H. Kalita, D. C. Boruah, M. C. Kalita and R. Devi, Nutrition, 2016, 32, 1081–1091 CrossRef CAS PubMed
.
- A. Hazarika, H. Kalita, M. Chandra, Kalita and R. Devi, Nutrition, 2017, 38, 95–101 CrossRef CAS PubMed
.
- H. Kalita, D. C. Boruah, M. Deori, A. Hazarika, R. Sarma, S. Kumari, R. Kandimalla, J. Kotoky and R. Devi, Front. Pharmacol., 2016 DOI:10.3389/fphar.2016.00102
.
- N. Bhardwaj, Y. P. Singh, D. Devi, R. Kandimalla, J. Kotoky and B. B. Mandal, J. Mater. Chem. B, 2016, 4, 3670–3684 RSC
.
- C. Vepari and D. L. Kaplan, Prog. Polym. Sci., 2007, 32, 991–1007 CrossRef CAS PubMed
.
- J. Pedro, C. Serrano, L. García-fern, M. Barbeck, S. Ghanaati, R. Unger, J. Kirkpatrick, E. Arzt, L. Funk and P. Tur, Biomaterials, 2015, 52, 291–300 CrossRef PubMed
.
- B. Talukdar, M. Saikia, J. Handique and D. Devi, Int. J. Pure Appl. Sci. Technol., 2011, 7, 81–86 CAS
.
- S. Dutta, B. Talukdar, R. Bharali, R. Rajkhowa and D. Devi, Biopolymers, 2013, 99, 326–333 CrossRef CAS PubMed
.
- U. C. Javali, N. V. Padaki, B. Das and K. B. Malali, Developments in the use of silk by-product and silk waste, 2015, DOI:10.1016/B978-1-78242-311-9.00013-6
.
- D. Wu, H. Cui, J. Zhu, X. Qin and T. Xie, J. Mater. Chem. B, 2016, 4, 2606–2613 RSC
.
- K. Mai-ngam, K. Boonkitpattarakul and J. Jaipaew, J. Biomater. Sci., Polym. Ed., 2011, 22, 2001–2022 CrossRef CAS PubMed
.
- A. Bebu, L. Szabó, N. Leopold, C. Berindean and L. David, J. Mol. Struct., 2011, 993, 52–56 CrossRef CAS
.
- F. Bouaziz, M. Koubaa, F. Barba, S. Roohinejad and S. Chaabouni, Antioxidants, 2016 DOI:10.3390/antiox5030026
.
- L. S. Kassama, International Journal of Contemporary Medical Research, 2015, 5, 30–39 Search PubMed
.
- A. Femenia, E. S. Sánchez, S. Simal and C. Rosselló, Carbohydr. Polym., 1999, 39, 109–117 CrossRef CAS
.
- K. D. Mlynek, M. T. Callahan, A. V. Shimkevitch, J. T. Farmer, J. L. Endres, M. Marchand, K. W. Bayles, A. R. Horswill and J. B. Kaplan, Antimicrob. Agents Chemother., 2016, 60, 2639–2651 CrossRef CAS PubMed
.
- H. Nailis, D. Vandenbosch, D. Deforce, H. J. Nelis and T. Coenye, Res. Microbiol., 2010, 161, 284–292 CrossRef CAS PubMed
.
- S. S. Silva, M. B. Oliveira, J. F. Mano and R. L. Reis, Carbohydr. Polym., 2014, 112, 264–270 CrossRef CAS PubMed
.
- M. Tummalapalli, M. Berthet, B. Verrier, B. L. Deopura, M. S. Alam and B. Gupta, Int. J. Biol. Macromol., 2016, 82, 104–113 CrossRef CAS PubMed
.
- B. A. Aderibigbe, K. Varaprasad, E. R. Sadiku, S. S. Ray, X. Y. Mbianda, M. C. Fotsing, S. J. Owonubi and S. C. Agwuncha, Int. J. Biol. Macromol., 2015, 73, 115–123 CrossRef CAS PubMed
.
- A. Paige, V. Giuseppe and A. Travaglia, J. Inorg. Biochem., 2016, 161, 1–8 CrossRef PubMed
.
- E. Carolina, D. João, D. Masi, A. Carlos, L. Campos, F. David, J. De Masi, M. Aurelio, S. Ratti, I. Shin, R. David and J. De Mais, Braz. J. Otorhinolaryngol., 2016, 82, 512–521 CrossRef PubMed
.
- D. Grande, T. Lee, D. Ph and O. Limpisvasti, Arthrosc. J. Arthrosc. Relat. Surg., 2010 DOI:10.1016/j.arthro.2010.02.025
.
- S. Choi and M. H. Chung, Semin. Integr. Med., 2003, 1, 53–62 CrossRef
.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra04888b |
| ‡ Himadri Kalita and Ankita Hazarika are co-first authors. |
| § Sanjeeb Kalita and Raghuram Kandimalla are co-second authors. |
| This journal is © The Royal Society of Chemistry 2017 |