Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Acid-ionic polymer as recyclable catalyst for one-pot three-component Mannich reaction†
W. Senapak,
R. Saeeng and
U. Sirion *
*
Department of Chemistry and Center for Innovation in Chemistry, Faculty of Science, Burapha University, Sangsook, Chonburi 20131, Thailand. E-mail: uthaiwan@buu.ac.th; Fax: +66-3-839-3494; Tel: +66-98-026-2181
First published on 12th June 2017
Abstract
A recyclable solid-catalyst, acid-ionic polymer bearing imidazolium trifluoromethanesulfonate, [PS-Im][OTf] was presented as an efficient catalyst in a one-pot, three-component Mannich reaction of aldehydes, amines and ketones. The protocol was conducted successfully under mild, convenient and metal-free conditions to afford the corresponding products in moderate to excellent yields. In addition, the catalyst was reused four times with no significant loss in activity.
Introduction
The Mannich reaction is one of the most powerful tools for carbon–carbon bond-forming.1 It produces β-amino carbonyl compounds, which are crucial building blocks for the synthesis of a wide variety of nitrogen-containing drugs and natural products.2 Some of these compounds exhibit biological significance,3 for examples anticancer,4 antimicrobial5 anti-HIV6 and antimalarial7 properties (Fig. 1). As a consequence, new synthetic methods to prepare these compounds have been continually developed, especially direct-Mannich reaction in a one-pot three-component process of aldehydes, amines and various nucleophiles.Generally, one-pot, three-component Mannich reactions have been reported with various catalyst systems. Most catalysts are Lewis acids,8 Bronsted acids,9 organocatalyst10 and recently nanoparticles.11,16 However, most of these catalysts have serious disadvantages such as their high cost, toxicity and hazardous nature, in addition to being difficult to separate from the product. Some are unstable, moisture sensitive, environmental contaminants and not reusable. Therefore, high performance non-toxic, inexpensive, reusable and efficient catalysts are desirable. Additionally, practical and metal-free methods with an environmentally benign synthetic process under green context merits more recent attention for one-pot Mannich reactions.
Solid-supported catalysts are currently of great interest as green and reusable catalysts, providing high yields of desirable products for many useful organic reactions.12 The advantages of these catalysts are moisture resistance, air tolerance, low-toxicity, easily separated post reaction by simple filtration and recyclable. In particular, there are practical advantages in medicinal and pharmacological production because of their non-toxicity and high-throughput synthesis. Mannich reactions that promoted by solid-supported Lewis and Bronsted acid-catalysts have been rare.13–16 Kobayashi's group in 2003 used a hydrophobic polymer-supported sulfonic acid as a catalyst for three-component Mannich reaction.14
Subsequently, in 2007, Wu's group employed SiO2–OSO3H as the catalyst for the direct-Mannich reaction.15 Recently, in 2014, Zareyee' group described the use of a hydrophobic sulfonic acid based nanosilica as the catalyst for one-pot three-component Mannich reaction.16
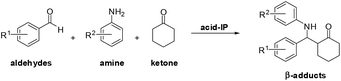
Bronsted acidic ionic liquids (BAILs) have received attention in past decades as efficient acidic catalysts under a wide range of reaction conditions.17,18 Recently, BAILs has proven to be effective catalysts in the Mannich reactions.18 Solid-supported acidic ionic liquid catalysts19–26 have been reported for acetalization reaction,20 condensation,21 Hantzsch reaction,22 multicomponent reaction,23 esterification,24 hydrolysis of cellulose25 and biodiesel synthesis.26 Herein, we would like to present the utilization of polystyrene bearing acidic ionic liquid (acid-IP) as a reusable heterogeneous catalyst for direct-Mannich reaction of aldehydes, amines and ketones in one-pot process under mild, convenient and metal-free conditions at ambient temperature (Scheme 1).
Results and discussion
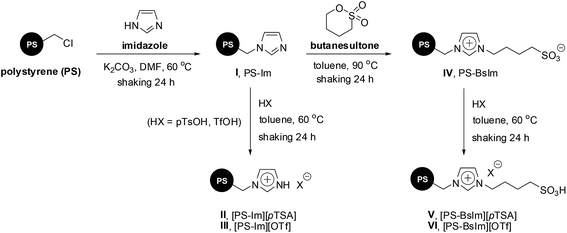
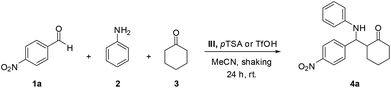
A series of acid-ionic polymers (acid-IP) bearing imidazolium cation with two different acid-anions (pTSA and OTf) was investigated as a recyclable heterogeneous catalyst for one-pot, three-component Mannich reaction of aldehydes, amines, and ketones. Preparation of these acid-ionic polymers used commercially available polystyrene beads (PS) with 1.80 mmol g−1 of chloride loading (Scheme 2, see ESI†).The formation of β-amino carbonyl compound 4a through one-pot three-component Mannich reaction catalyzed by acid-ionic polymer-based imidazolium salts was investigated using 4-nitrobenzaldehyde 1a, aniline 2 and cyclohexanone 3 as model substrates (Table 1). Initial experiments, 10 mol% of catalysts were tested using 1.0 mmol of each substrate in acetonitrile (MeCN) at room temperature for 24 h (entries 1–4). The highest yield of the desired product 4a was obtained with catalyst III in 82% (entry 2). Subsequently, with the best catalyst III, various solvents were screened in the same reaction condition. Results demonstrated the high yield of desired product 4a was achieved with polar solvents (MeCN and DMF). This was probably due to the generous swelling of acid-ionic polymer in polar solvents that lead to reaction rate enhancement. However, MeCN was selected as the best solvent for this protocol due to more efficient recyclability.
| Entry | Catalyst | Solvent | Yield 4ab (%) |
|---|---|---|---|
| a All reactions were conducted with 1.0 mmol of 1, 2 and 3 using 10 mol% of catalyst in 3.0 mL of solvent, shaking 800 rpm at room temperature for 24 h.b Isolated yields.c Second run.d Third run.e Fourth run. | |||
| 1 | II, [PS-Im][pTSA] | MeCN | 72 |
| 2 | III, [PS-Im][OTf] | MeCN | 82(83)c(81)d(80)e |
| 3 | V, [PS-BsIm][pTSA] | MeCN | 41 |
| 4 | VI, [PS-BsIm][OTf] | MeCN | 36 |
| 5 | III, [PS-Im][OTf] | DMF | 81(74)c(54)d(48)e |
| 6 | III, [PS-Im][OTf] | DCM | 38 |
| 7 | III, [PS-Im][OTf] | DCE | 48 |
| 8 | III, [PS-Im][OTf] | Toluene | 27 |
| 9 | III, [PS-Im][OTf] | THF | 60 |
| 10 | III, [PS-Im][OTf] | 1,4-Dioxane | 38 |
| 11 | III, [PS-Im][OTf] | H2O | 49 |
| 12 | III, [PS-Im][OTf] | MeOH | 56 |
To improve yield of desired product 4a, ratio of substrates was adjusted (Table 2). Slight increase of cyclohexanone 3 to 1.2 equivalents, desired product 4a was produced in higher yield, 89% for 24 h and 93% for 48 h (entry 1). However, reaction time was investigated also from 3–48 h and the result indicated that the reaction was almost completed in 48 h.
| Entry | SM (1a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) (mmol) 3) (mmol) |
Catalyst | Yield 4ab (%) |
|---|---|---|---|
| a All reactions were conducted with 1.0 mmol of 1, 1–5 mmol of 2 and 1–5 mmol of 3 using 10 mol% of catalyst in 3.0 mL of MeCN, shaking at 800 rpm at room temperature for 24 h.b Isolated yields.c 48 h.d 1.0 mL MeCN.e 20 mol% of III.f 5 mol% of III. | |||
| 1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
III, [PS-Im][OTf] | 89(93)c |
| 2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.0 2.0 |
III, [PS-Im][OTf] | 86 |
| 3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.0 5.0 |
III, [PS-Im][OTf] | 86 |
| 4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
III, [PS-Im][OTf] | 75 |
| 5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.0 2.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
III, [PS-Im][OTf] | 71 |
| 6 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.0 5.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
III, [PS-Im][OTf] | 50 |
| 7d | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
III, [PS-Im][OTf] | 81 |
| 8e | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
III, [PS-Im][OTf] | 77 |
| 9f | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
III, [PS-Im][OTf] | 80 |
| 10 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
pTSA | 73 |
| 11 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
TfOH | 77 |
| 12 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
— | 0 |
For comparison, the reaction was investigated with 24 h and showed yield was not improved when substrate 3 was increased further to 2.0–5.0 equivalents (entries 2 and 3). Unfortunately, when amount of amine substrate 2 was increased yield of the desired product 4a declined (entries 4–6). This was attributed to excess amine base quenching the acidity of catalytic system. Highly concentrated reaction volume produced the slight decrease in yield (entry 7). Amount of catalyst III with 20 and 5 mol% were examined, and resulted in lower yields of desired product 4a in 77 and 80% respectively (entries 8 and 9). Due to greater acidity with 20 mol% of catalyst amine substrate was reduced by reacting with acid catalyst, while reaction was incomplete with 5 mol% of catalyst. In addition, confirmation of catalytic activity of catalyst III, the control reactions were tested with general acids such as pTSA and TfOH and found to give lower yields of the desired product 4a (entries 10 and 11) and no product in the absence of catalyst (entry 12).
Recycle performance of catalyst III was also examined with the optimum reaction over 48 h (Table 2, entry 1). After the first run, the resin catalyst III was recovered by simple filtration and washed with ethyl acetate and dried under vacuum pump. Recovered resin catalyst III was reused, giving similar yields even after four cycles with 93, 91, 92 and 90% of the desired product 4a, respectively.
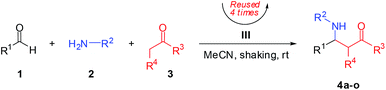
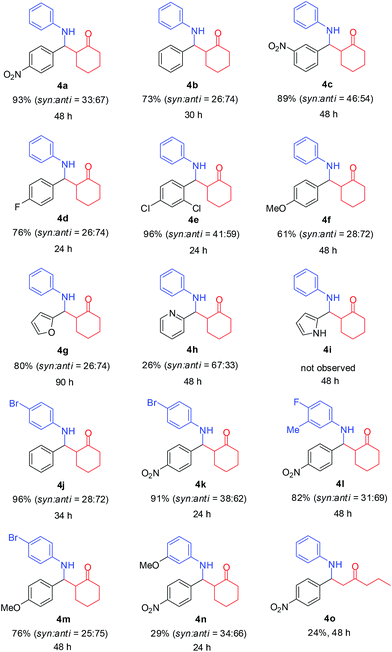
Under optimum reaction condition (Table 2, entry 1), a variety of both aldehydes and anilines were investigated for their performance (Table 3). Corresponding product yields depended on steric hindrance and electronic effect of substrates. Reaction of aromatic aldehyde substrates with electron-withdrawing group (–NO2, –F, –Cl, including –H) produced good to excellent yields (73–96% for products 4a–e and 4j–l). Notably, electron-withdrawing group at para-position gave a superior yield than at meta-position (4a compared to 4c; –NO2). While aromatic aldehyde substrates with an electron-donating group (–OMe) provided products in moderate and good yields (61 and 76% of 4f and 4m, respectively). Moreover, heteoaromatic aldehydes gave good yields with furan-2-carbaldehyde (80%; 4g), low yield with 2-pyridinecarboxaldehyde (26%; 4h), and no desirable product (4i) with pyrrole-2-carbaldehyde. This result is likely due to basicity of nitrogen atom on substrates that could easily attach to an acid-catalyst and reduce catalytic activity. Aniline substrates with an electron-withdrawing group (–Br, –F) could improve product yields (4j–m), by providing a higher electrophilicily of an iminium ion intermediate. Unfortunately, yields were lower in the case of an electron-donating group (–OMe) on aniline substrate (29%; 4n) because the instability of an iminium ion intermediate and product led to generating undesirable products. Moreover, 2-pentanone was investigated as a nucleophile, resulting in low yields of desired products with 24% (4o).
a All reactions were conducted with aldehyde (1, 1.0 mmol), amine (2, 1.0 mmol) and ketone (3, 1.2 mmol) using 10 mol% of catalyst III in MeCN (3.0 mL), shaking 800 rpm at room temperature; % yields were reported as isolated yields; the ratio of syn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) anti was determined by NMR. anti was determined by NMR. |
|---|
 |
Conclusions
In summary, we have demonstrated a metal-free and convenient method for the preparation of β-amino carbonyl compounds via one-pot three-component Mannich reaction of aldehydes, amines and cyclohexanone at ambient temperature catalyzed by recyclable heterogeneous acid-ionic polymer. The acid-ionic polymer-based imidazolium cation with OTf anion, [PS-Im][OTf] (III) exhibited the most efficient catalyst to produce desirable products in moderate to excellent yields with a wide range of aldehyde and amine substrates. In addition, the catalysts are easily separated from the reaction mixture by simple filtration and reusability of catalyst was possible for subsequent reactions.Acknowledgements
This work was financially supported by Thailand Research Fund (Grant no. TRG5780298), the Center of Excellence for Innovation in Chemistry (PERCH-CIC) and the Research Grant of Burapha University through National Research Council of Thailand (Grant no. 28/2555 and 16/2556). Special thanks to Prof. Dr Frederick W. H. Beamish, Faculty of Science, Burapha University, for his comments and English correction and Dr Byoung Se Lee (FutureChem Co. Ltd., Korea) for helpful discussion.Notes and references
- (a) M. Arend, B. Westermann and N. Risch, Angew. Chem., Int. Ed., 1998, 37, 1044–1070 CrossRef; (b) S. Kobayashi and H. Ishitani, Chem. Rev., 1999, 99, 1069–1094 CrossRef CAS PubMed; (c) C. Mannich and W. Krösche, Arch. Pharm., 1912, 250, 674–667 Search PubMed.
- (a) F. A. Davis, Y. Zhang and G. Anilkumar, J. Org. Chem., 2003, 68, 8061–8064 CrossRef CAS PubMed; (b) G. B. Evans, R. H. Furneaux, P. C. Tyler and V. L. Schramm, Org. Lett., 2003, 5, 3639–3640 CrossRef CAS PubMed.
- (a) S. Bala, N. Sharma, A. Kajal, S. Kamboj and V. Saini, J. Med. Chem., 2014, 191072, 15 Search PubMed; (b) S. G. Subramaniapillai, J. Chem. Sci., 2013, 125, 467–482 CrossRef CAS.
- A. Y. Shaw, C.-Y. Chang, M. Y. Hsu, P.-J. Lu, C. N. Yang, H. L. Chen, C.-W. Lo, C.-W. Shiau and M.-K. Chern, Eur. J. Med. Chem., 2010, 45, 2860–2867 CrossRef CAS PubMed.
- (a) A. Idhayadhulla, R. S. Kumar, A. J. A. Nasser and A. Manilal, J. Chem. Pharm. Res., 2011, 3, 904–911 CAS; (b) M. Ashok, B. S. Holla and B. Poojary, Eur. J. Med. Chem., 2007, 42, 1095–1101 CrossRef CAS PubMed; (c) K. K. Sivakumar, A. Rajasekaran and P. Senthilkumar, Bioorg. Med. Chem. Lett., 2014, 24, 2940–2944 CrossRef CAS PubMed.
- S. N. Pandeya, D. Sriram, G. Nath and E. D. Clercq, Eur. J. Med. Chem., 2000, 35, 249–255 CrossRef CAS PubMed.
- F. Lopes, R. Capela, J. O. Goncaves, P. N. Horton, M. B. Hursthouse, J. Iley, C. M. Casimiro, J. Bom and R. Moreira, Tetrahedron Lett., 2004, 45, 7663–7666 CrossRef CAS.
- (a) P. Phukan, D. Kataki and P. Chakraborty, Tetrahedron Lett., 2006, 47, 5523–5525 CrossRef CAS; (b) W.-B. Yi and C. Cai, J. Fluorine Chem., 2006, 127, 1515–1521 CrossRef CAS; (c) M. Wu, H. Jing and T. Chang, Catal. Commun., 2007, 8, 2217–2221 CrossRef CAS; (d) C. Zhang, J. Dong, T. Cheng and R. Li, Tetrahedron Lett., 2001, 42, 461–463 CrossRef CAS; (e) W.-G. Shou, Y.-Y. Yang and Y.-G. Wang, Tetrahedron Lett., 2006, 47, 1845–1847 CrossRef CAS; (f) S. Kogayashi, M. Araki and M. Yasuda, Tetrahedron Lett., 1995, 36, 5773–5776 CrossRef; (g) T. Ollevier, E. Nadeau and A.-A. Guay-Bégin, Tetrahedron Lett., 2006, 47, 8351–8354 CrossRef CAS; (h) T. Ollevier and E. Nadeau, J. Org. Chem., 2004, 69, 9292–9295 CrossRef CAS PubMed; (i) H. Li, H.-y. Zeng and H.-w. Shao, Tetrahedron Lett., 2009, 50, 6858–6860 CrossRef CAS; (j) Y. Dai, B. D. Li, H. D. Quan and C. X. Lü, Chin. Chem. Lett., 2010, 21, 31–34 CrossRef CAS; (k) R. I. Kureshy, S. Agrawal, S. Saravanan, N. H. Khan, A. K. Shah, S. H. R. Abdi, H. C. Bajaj and E. Suresh, Tetrahedron Lett., 2010, 51, 489–494 CrossRef CAS; (l) X. Zhang, S. Yin, R. Qiu, J. Xia, W. Dai, Z. Yu, C.-T. Au and W.-W. Wong, J. Organomet. Chem., 2009, 694, 3559–3564 CrossRef CAS; (m) T.-P. Loh and S.-L. Chen, Org. Lett., 2002, 4, 3647–3650 CrossRef CAS PubMed; (n) T. P. Loh and L.-L. Wei, Tetrahedron Lett., 1998, 39, 323–326 CrossRef CAS; (o) T.-P. Loh, S. B. K. W. Liung, K.-L. Tan and L.-L. Wei, Tetrahedron, 2000, 56, 3227–3237 CrossRef CAS; (p) G. Pandey, R. P. Singh, A. Garg and V. K. Singh, Tetrahedron Lett., 2005, 46, 2137–2140 CrossRef CAS; (q) R. Wang, B. g. Li, T.-k. Huang, L. Shi and X.-x. Lu, Tetrahedron Lett., 2007, 48, 2071–2073 CrossRef CAS; (r) L. Wang, J. Han, J. Sheng, H. Tian and Z. Fan, Catal. Commun., 2005, 6, 201–204 CrossRef CAS; (s) Y.-Y. Yang, W.-G. Shou and Y.-G. Wang, Tetrahedron, 2006, 62, 10079–10086 CrossRef CAS; (t) W.-G. Shou, Y.-Y. Yang and Y.-G. Wang, Tetrahedron Lett., 2006, 47, 1845–1847 CrossRef CAS; (u) X. M. Chen, X. S. Li and A. S. C. Chan, Chin. Chem. Lett., 2009, 20, 407–410 CrossRef CAS; (v) D. Hong, Y.-Y. Yang, Y.-G. Wang and X.-F. Lin, Synlett, 2009, 1107–1110 CAS.
- (a) C. Mukhopadhyay, A. Datta and R. J. Butcher, Tetrahedron Lett., 2009, 50, 4246–4250 CrossRef CAS; (b) X. C. Wang, L. J. Zhang, Z. Zhang and Z. J. Quan, Chin. Chem. Lett., 2012, 23, 423–426 CrossRef CAS; (c) H.-T. Luo, Y.-R. Kang, H.-Y. Nie and L.-M. Yang, J. Chin. Chem. Soc., 2009, 56, 186–195 CrossRef CAS; (d) H. Zeng, H. Li and H. Shao, Ultrason. Sonochem., 2009, 16, 758–762 CrossRef CAS PubMed; (e) M. Xia and Y.-D. Lu, J. Fluorine Chem., 2006, 127, 1119–1124 CrossRef CAS; (f) C. Chen, X. Zhu, Y. Wu, H. Sun, G. Zhang, W. Zhang and Z. Gao, J. Mol. Catal. A: Chem., 2014, 395, 124–127 CrossRef CAS; (g) Y.-S. Wu, J. Cai, Z.-Y. Hu and G.-X. Lin, Tetrahedron Lett., 2004, 45, 8949–8952 CrossRef CAS; (h) K. Manabe, Y. Mori and S. Kobayashi, Tetrahedron, 2001, 57, 2537–2544 CrossRef CAS; (i) G. Dagousset, F. Drouet, G. Masson and J. Zhu, Org. Lett., 2009, 11, 5546–5549 CrossRef CAS PubMed.
- (a) M. Rachwalski, T. Leenders, S. Kaczmarczyk, P. Kiełbasiński, S. Leśniak and F. P. J. T. Rutjes, Org. Biomol. Chem., 2013, 11, 4207–4213 RSC; (b) L. Cheng, X. Wu and Y. Lu, Org. Biomol. Chem., 2007, 5, 1018–1020 RSC; (c) J. M. M. Verkade, L. J. C. van Hemert, P. J. L. M. Quaedflieg and F. P. J. T. Rutjes, Chem. Soc. Rev., 2008, 37, 29–41 RSC; (d) P. Goswami and B. Das, Tetrahedron Lett., 2009, 50, 2384–2388 CAS; (e) W. Notz, K. Sakthivel, T. Bui, G. Zhong and C. F. Barbas, Tetrahedron Lett., 2001, 42, 199–201 CrossRef CAS; (f) M. L. Kantam, C. V. Rajasekhar, G. Gopikrishna, K. R. Reddy and B. M. Choudary, Tetrahedron Lett., 2006, 47, 5965–5967 CrossRef CAS; (g) H. Wu, X.-m. Chen, Y. Wan, L. Ye, H.-q. Xin, H.-h. Xu, C.-h. Yue, L.-l. Pang, R. Ma and D.-q. Shi, Tetrahedron Lett., 2009, 50, 1062–1065 CrossRef CAS; (h) E. Alza, C. Rodríguez-Escrich, S. Sayalero, A. Bastero and M. A. Pericàs, Chem.–Eur. J., 2009, 15, 10167–10172 CrossRef CAS PubMed; (i) Y. Hayashi, W. Tsuboi, I. Ashimine, T. Urushima, M. Shji and K. Sakai, Angew. Chem., Int. Ed., 2003, 42, 3677–3680 CrossRef CAS PubMed.
- M. Kidwai, N. K. Mishra, V. Bansal, A. Kumar and S. Mozumdar, Tetrahedron Lett., 2009, 50, 1355–1358 CrossRef CAS.
- (a) D. H. Drewry, D. M. Coe and S. Poon, Med. Res. Rev., 1999, 19, 97–148 CrossRef CAS PubMed; (b) Y. R. de Miguel, J. Chem. Soc., Perkin Trans. 1, 2000, 4213–4221 RSC; (c) M. M. Dell'Anna, G. Romanazzi and P. Mastrorilli, Curr. Org. Chem., 2013, 17, 1236–1273 CrossRef.
- (a) N. Azizi, L. Torkiyan and M. R. Saidi, Org. Lett., 2006, 8, 2079–2082 CrossRef CAS PubMed; (b) E. Rafiee, S. Eavani, F. K. Nejad and M. Joshaghani, Tetrahedron, 2010, 66, 6858–6863 CrossRef CAS; (c) M. A. Bigdeli, F. Nemati and G. H. Mahdavinia, Tetrahedron Lett., 2007, 48, 6801–6804 CrossRef CAS; (d) Z. Li, X. Ma, J. Liu, X. Feng, G. Tian and A. Zhu, J. Mol. Catal. A: Chem., 2007, 272, 132–135 CrossRef CAS; (e) W.-Y. Li, Y.-X. Zong, J.-K. Wang and Y.-Y. Niu, Chin. Chem. Lett., 2014, 25, 575–578 CrossRef CAS; (f) R. K. Sharma, D. Rawat and G. Gaba, Catal. Commun., 2012, 19, 31–36 CrossRef CAS.
- S. Iimura, D. Nobutou, K. Manabe and S. Kobayashi, Chem. Commun., 2003, 1644–1645 RSC.
- H. Wu, Y. Shen, L.-y. Fan, Y. Wan, P. Zhang, C.-f. Chen and W.-x. Wang, Tetrahedron, 2007, 63, 2404–2408 CrossRef CAS.
- D. Zareyee and H. Alizadeh, RSC Adv., 2014, 4, 37941–37946 RSC.
- (a) A. C. Cole, J. L. Jensen, L. Ntai, K. L. T. Tran, K. J. Weaver, D. C. Forbes and J. H. Davis, J. Am. Chem. Soc., 2002, 124, 5962–5963 CrossRef CAS PubMed; (b) H. Xing, T. Wang, Z. Zhou and Y. Dai, Ind. Eng. Chem. Res., 2005, 44, 4147–4150 CrossRef CAS; (c) H.-P. Zhu, F. Yang, J. Tang and M.-Y. He, Green Chem., 2003, 5, 38–39 RSC; (d) D. C. Forbes and K. J. Weaver, J. Mol. Catal. A: Chem., 2004, 124, 129–132 CrossRef; (e) T. Joseph, S. Sahoo and S. B. Halligudi, J. Mol. Catal. A: Chem., 2005, 234, 107–110 CrossRef CAS; (f) A. K. Bagdi and A. Hajra, RSC Adv., 2014, 4, 23287–23291 RSC; (g) A. Taheri, C. Liu, B. Lai, C. Cheng, X. Pan and Y. Gu, Green Chem., 2014, 16, 3715–3719 RSC; (h) A. R. Hajipour, Y. Ghayeb, N. Sheikhan and A. E. Ruoho, Tetrahedron Lett., 2009, 50, 5649–5651 CrossRef CAS; (i) A. S. Amarasekara and M. A. Hasan, Tetrahedron Lett., 2014, 55, 3319–3321 CrossRef CAS; (j) A. S. Amarasekara, Chem. Rev., 2016, 116, 6133–6183 CrossRef CAS PubMed; (k) A. K. Bagdi and A. Hajra, RSC Adv., 2014, 4, 23287–23291 RSC; (l) A. Monopoli, P. Cotugno, F. Iannone, F. Ciminale, M. M. Dell'Anna, P. Mastrorilli and A. Nacci, Eur. J. Org. Chem., 2014, 27, 5925–5931 CrossRef.
- (a) C. B. Yue, T. F. Yi, C. B. Zhu and G. Liu, J. Ind. Eng. Chem., 2009, 15, 653–656 CrossRef CAS; (b) G. Zhao, T. Jiang, H. Gao, B. Han, J. Huang and D. Sun, Green Chem., 2004, 6, 75–77 RSC; (c) S. Sahoo, T. Joseph and S. B. Halligudi, J. Mol. Catal. A: Chem., 2006, 244, 179–182 CrossRef CAS; (d) T. Chang, L. He, L. Bian, H. Han, M. Yuan and X. Gao, RSC Adv., 2014, 4, 727–731 RSC; (e) L. He, S. Qin, T. Chang, Y. Sun and J. Zhao, Int. J. Mol. Sci., 2014, 15, 8656–8666 CrossRef PubMed; (f) J. Li, Y. Peng and G. Song, Catal. Lett., 2005, 102, 159–162 CrossRef CAS; (g) F. Dong, F. Zhenghao and L. Zuliang, Catal. Commun., 2009, 10, 1267–1270 CrossRef CAS; (h) F. Dong, J. Luo, X.-L. Zhou and Z.-L. Liu, Catal. Lett., 2007, 116, 76–80 CrossRef.
- R. Skoda-Földes, Molecules, 2014, 19, 8840–8884 CrossRef PubMed.
- R. Sugimura, K. Qiao, D. Tomida and C. Yokoyama, Catal. Commun., 2007, 8, 770–772 CrossRef CAS.
- J. Wang, Y. Zong, R. Fu, Y. Niu, G. Yue, Z. Quan, X. Wang and Y. Pan, Ultrason. Sonochem., 2014, 21, 29–34 CrossRef CAS PubMed.
- B. Jahanbin, A. Davoodnia, H. Behmadi and N. Tavakoli-Hoseini, Bull. Korean Chem. Soc., 2012, 33, 2140–2144 CrossRef CAS.
- D. A. Kotadia and S. S. Soni, J. Mol. Catal. A: Chem., 2012, 354, 44–49 CrossRef.
- Z. Xu, H. Wan, J. Miao, M. Han, C. Yang and G. Guan, J. Mol. Catal. A: Chem., 2010, 332, 152–157 CrossRef CAS.
- A. S. Amarasekara and O. S. Owereh, Catal. Commun., 2010, 11, 1072–1075 CrossRef CAS.
- Y. Cao, H. Zhou and J. Li, Renewable Sustainable Energy Rev., 2016, 58, 871–875 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra04834c |
| This journal is © The Royal Society of Chemistry 2017 |