 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Current advances in the utility of functionalized SBA mesoporous silica for developing encapsulated nanocatalysts: state of the art
S. Sadjadi
*a and
M. M. Heravi
 *b
*b
aGas Conversion Department, Faculty of Petrochemicals, Iran Polymer and Petrochemical Institute, PO Box 14975-112, Tehran, Iran. E-mail: samahesadjadi@yahoo.com
bDepartment of Chemistry, School of Science, Alzahra University, PO Box 1993891176, Vanak, Tehran, Iran. Fax: +98 21 88041344; Tel: +98 21 88044051
First published on 14th June 2017
Abstract
The cavities of SBA mesoporous silica materials can be used as nanoreactors for embedding catalytic species such as nanoparticles, complexes and heteropolyacids etc. The encapsulated catalysts are renowned as superior catalytic systems regarding their catalytic activity, selectivity and reusability as well as suppressed leaching. An efficient method to improve the encapsulation of these catalysts, is in creating anchors for capturing catalytic species. It has been performed by functionalization of the surface of SBA mesoporous silica materials with various groups including sulfur, phosphorus and amine-based functional groups. The aim of this article is to review the recent advances (2014–2017) in the field of the utility of functionalized SBA for developing encapsulated catalysts. In most cases the reusability of the catalyst and the leaching of the catalytic active species were discussed to disclose the role of functionalized SBA in heterogenizing and suppressing the leaching. Moreover, to show the superiority of these developed encapsulated catalysts, with a couple of examples we compared their catalytic activities with those of the conventional ones.
1. Introduction
The 21st century has witnessed considerable progress in the research area of novel catalysts. In this regard, developing biomimetic catalysts through confinement of a catalytic species within the cavity of a porous system, mostly referred to as nanoreactors, has proved to be a very promising procedure.1 To date, various nanoreactors including dendrimers,2 metal organic frameworks (MOFs),3,4 mesoporous silica, carbon nanostructures, polymers,5–10 clays,11 zeolites12 etc. have been applied for embedding catalytic species.13 It is noteworthy that the encapsulated catalysts are distinguished from the conventional supported catalysts in which the catalytic species are immobilized on the surface of a heterogeneous support.1 The most outstanding features of the encapsulated catalysts are their superior selectivity, activity and reusability as well as the low leaching of the catalytic species.Mesoporous silica materials14–20 have not only proved their utilities as heterogeneous catalysts21–29 for promoting various organic transformations,30–37 but also find interesting applications38,39 in other research fields such as food chemistry,40 production of bio-mass derived chemicals,41–45 petrochemisry,46–48 sensors, waste treatment,49,50 optical materials and carriers. One of the most important advances in this field is developing hybrid systems through functionalization of mesoporous silica materials.51–56 In this context using sulfur,57–62 phosphorous and amine-based functional groups63,64 has attracted growing attention and resulted in introducing novel hybrid systems65–68 with outstanding utilities for catalysis.69–75 Not only the functionalized mesoporous silica can be employed as catalysts, they can be exploited as nanoreactors for incorporation of another catalytic species such as nanoparticles,76–79 complexes80,81 for developing state of the art encapsulated catalysts.82–86
Recently, Rostamnia et al. reviewed the hybrid functionalized silica mesostructures containing sulphonic acid and amine based moieties and their utilities as green organocatalysts for promoting various organic transformations.87 They also covered a book chapter on the utility of functionalized mesoporous silica for Pd encapsulation.1 Karimi et al. also provide a comprehensive review on the applications of functionalized ordered/periodic mesoporous silicas and organosilicas as catalyst support in C–C coupling reactions.88 Moreover, Mohammadi Ziarani et al. review the utility of sulfonic acid-functionalized SBA in organic synthesis.89 All these review articles highlight the importance of mesoporous silica materials in catalysis. Although all of those review articles were well organized and informative, the first two review articles covered various mesoporous silica including SBA and MCM and the review article covered by Mohammadi Ziarani only considered one functionality (sulfonic acid). Moreover, the review article authored by Karimi et al. targeted only one class of organic transformation or catalyst. We recently published a review article regarding Pd(0) nanoparticles in which we disclosed the utility of mesoporous silica for the encapsulation of Pd nanoparticles.13 In attempt to expand the concept of encapsulated catalysts9,11,90,91 and in continuation of our recent review article on encapsulated catalysts,13 herein we consider mesoporous silica materials from a new point of view and cover the recent advances (2014–2017) in utility of the functionalized SBA mesoporous silica for embedding various catalytic species or second functionality for developing novel hybrid catalysts. Noteworthy, we make effort to highlight the advantageous of these state of the art catalysts.
2. Functionalized SBA for developing hybrid catalysts through incorporation of complexes
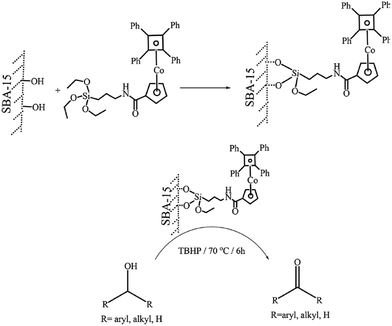
One of the challenges in the field of metal containing catalysts such as complexes is leaching of the metallic species. This issue not only reduce the catalytic activity, it can also make the purification of the products difficult. To suppress the metal leaching, the complex can be encapsulated within the pores of SBA. The inorganic SBA not only physically protect the encapsulated complex, it can also affect the catalytic performance through synergistic effects between the SBA and complex. In this regard, surface functionalization of SBA can improve the encapsulation of the catalyst. Moreover, according to the complex size, the size of the SBA pores can be controlled. In the following some recent advances in this field have been discussed.The hydrolysis and co-condensation of tetraethylorthosilicate (TEOS) and 3-chloropropyltrimethoxysilane (CPTES) were exploited for preparation of Cl-functionalized mesoporous silica, Cl–SiO2. Subsequently, reaction with p-salicylidine aminobenzoic acid followed by incorporation of MoO2(acac)2 was employed to afford an inorganic–organic hybrid catalyst, Mo–SB–Cl–SiO2, (Fig. 1) for liquid-phase epoxidation of various olefins in the presence of t-BuOOH as the oxygen source under mild reaction condition in 1,2-dichloroethane. The observed catalytic activity (conversion: 45.3–87.1%, selectivity: 56.4 to >99%, TON: 15.9–368.1 h−1) was comparable with a homogeneous system and depended on the steric and electronic features of the substrates. Moreover, the novel catalyst exhibited superior catalytic activity compared with the catalyst obtained through a post-grafting assisted method. This observation was attributed to higher density of MoVI active sites in the Mo–SB–Cl–SiO2.92 The catalyst could be used for four times with preserving its catalytic activity.
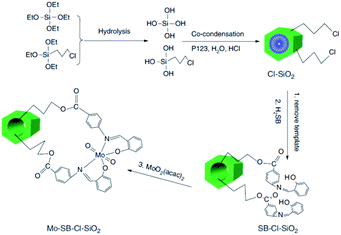 | ||
| Fig. 1 Preparation of ordered Mo–SB–Cl–SiO2. Adapted from Zhang et al. (2016), ref. 92, with permission from CSIRO Publishing. | ||
Singh et al. developed a novel catalyst, 3-[N,N′-bis-3-(salicylidenamino)ethyltriamine] Mo(VI)O2@SBA-15, through incorporation of 3-[N,N-bis-3-(salicylidenamino)ethyltriamine] ligand (L or Salpr) in inner space of SBA-15 functionalized with 3-chloropropyl trimethoxy silane and dimethoxydimethyl silane followed by complexation with Mo(VI)O2(acac)2 (Fig. 2). The novel hybrid system was used as a heterogeneous catalyst for epoxidation of cycloalkenes in CHCl3 with anhydrous tert-butyl hydroperoxide as oxidant and sulfoxidation of various sulphides in acetonitrile with hydrogen peroxide.93 In the case of epoxidation, the substrate with more electron rich C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C led to better results. Regarding sulfoxidation, unsubstituted substrates exhibited better results. The catalytic activity of Mo(VI)O2@SBA-15 was also compared with free (L)Mo(VI)O2 complex. The results established the superior catalytic performance of the former. Moreover, the catalyst was reusable and could be used for consecutive reaction runs without negligible loss of activity.
C led to better results. Regarding sulfoxidation, unsubstituted substrates exhibited better results. The catalytic activity of Mo(VI)O2@SBA-15 was also compared with free (L)Mo(VI)O2 complex. The results established the superior catalytic performance of the former. Moreover, the catalyst was reusable and could be used for consecutive reaction runs without negligible loss of activity.
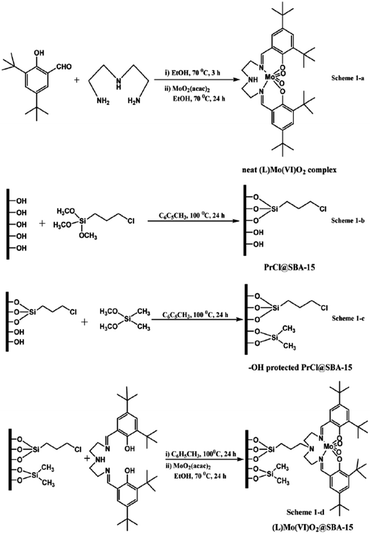 | ||
| Fig. 2 Organofunctionalization and immobilization of the neat (L)Mo(VI)O2 complex over SBA-15, (1-a) synthesis of homogeneous complex [(L)Mo(VI)O2 complex], (1-b) chloro functionalization of SBA-15 [PrCl@SBA-15], (1-c) capping of PrCl@SBA-15 [–OH protected PrCl@SBA-15], (1-d) heterogenization of homogeneous complex [(L)Mo(VI)O2@SBA-15]. Reprinted from ref. 93 with permission of Royal Society of Chemistry. | ||
Recently, Zare et al. reported grafting oxoperoxo molybdenum(VI) complex on the Cl-functionalized SBA-15 and the utility of the obtained catalyst, MoO3(sal-phz)/SBA-15 (where sal-phz is salicylidene 2-picoloyl hydrazine), for the olefins epoxidation with tert-BuOOH in decane as oxidant.94 It was found that the solvent and reaction temperature could affect the process. The best results were obtained at 95 °C and decane as the solvent. Catalytic activity of the heterogeneous catalyst was compared with the heterogeneous counterpart, MoO3(sal-phz). The catalytic activities of both catalysts were comparable. However, the heterogeneous catalyst was chemically stable and could be recovered and reused for six reaction runs with preserving its catalytic activity.
Mn(salen)Cl complexes were immobilized on amine-functionalized SBA-15 to afford a series of catalyst with different Mn loading (0.293–0.765 mmol Mn per g) (Fig. 3). The catalysts were successfully used for liquid phase oxidation of benzyl alcohol in the presence of tert-butylhydroperoxide as the oxidant. The authors studied the effects of reaction variables such as reaction time, temperature and solvent. It was found that polar protic solvents such as CH3CN at 90 °C resulted in the best yields. Studying the effect of oxidant established that the conversion had a linear relationship with the amount of the oxidant. Notably, selectivity showed a reversible relationship with the ratio of benzaldehyde to the oxidant which was due to the consecutive reaction of the transformation of benzaldehyde to benzoic acid. High efficiency, low complex leaching which emerged from interactions of amine moieties and complex, reusability of the catalyst and low amount of the required catalyst were the merits of this protocol.95
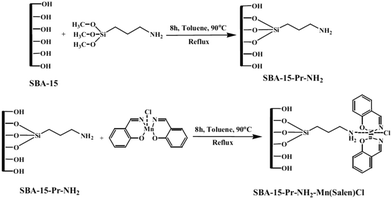 | ||
| Fig. 3 Preparation of SBA-15–Pr-NH2-supported Mn-salen complexes. Reprinted from ref. 95 with permission of Springer. | ||
The amine functionalized SBA-15 was used for coupling with 2-thiophenecarboxaldehyde followed by grafting to manganese (Fig. 4). The catalytic activity of the system was investigated for Knoevenagel condensation of various aldehydes and malononitrile.96 High yields (92–97%), sort reaction time (15–100 min) broad substrate scope and reusability of the catalyst for five reaction runs were the merits of this protocol. The comparison of the catalytic activity of this catalyst with some reported ones such as SO4/ZrO2, TiO2, nitrided MCM-48, zeolite imidazolate and ZrO2 established its good comparability with them in terms of product yield and reaction time.
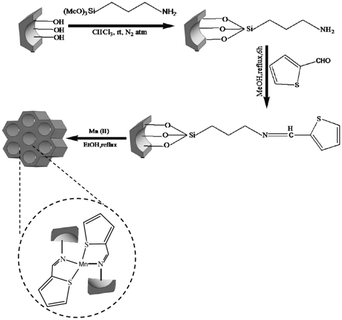 | ||
| Fig. 4 Synthetic pathway for the formation of Mn–SBA-15. Reprinted from ref. 96 with permission of Springer. | ||
Chen et al. reported a novel catalyst based on supporting vanadyl(IV) acetylacetonate (VO(acac)2) on amine-functionalized SBA-15 (Fig. 5) for the direct hydroxylation of benzene to phenol in the presence of oxygen as the oxidant.97 The authors believed that the reaction condition could dramatically affect the yield of the desired product. Optimizing the reaction condition, 13.3% phenol was obtained. The comparison of the catalytic activity of this catalyst with those of previously reported ones such as V/Al2O3, TBA–PW11V, V–AlPO5, VOx/SBA-15, VxOy@C and CsPMoV2 proved the superior activity of this novel catalyst. The catalyst exhibited high stability and reusability, which emerged from the strong interaction of vanadium atoms and amine group.
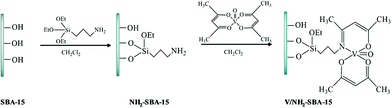 | ||
| Fig. 5 Schematic illustration for the preparation of V/NH2–SBA-15. Reprinted from ref. 97 with permission of Springer. | ||
Post-synthesis method was employed for SBA-15 functionalization with 3-(2-aminoethylamino) propyldimethoxymethylsilane to afford amine functionalized SBA-15, MPAOS, which subsequently coupled with 2-thiophene carboxaldehyde as Schiff-base to furnish MPIOS. The hybrid system was successfully used for anchoring Cu by reacting with CuCl2·H2O (Fig. 6). The obtained catalyst, Cu-MPIOS was proved to be an efficient heterogeneous catalyst for one-pot three-component C–S cross coupling reaction of thiourea benzyl bromides and bromoanisole under microwave irradiation in aqueous medium.98 Noteworthy, the catalytic performance of Cu-MPIOS was superior to CuCl2·H2O indicating the role of surface area and mesoporosity of the Cu-MPIOS in catalysis. To study the copper leaching, hot filtration and AAS analysis was performed.
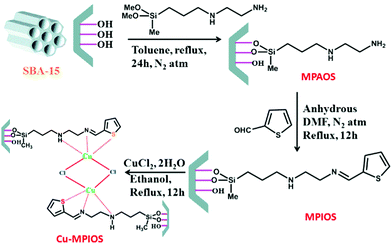 | ||
| Fig. 6 Schematic representation for the formation of Cu-MPIOS material. Reprinted from ref. 98 with permission of Elsevier. | ||
The results ruled out the leaching of the copper and prove the heterogeneous nature of the catalyst. Investigating the reusability of the catalyst for five reaction runs demonstrated only 8% decrease of the yield of the desired product indicating the excellent reusability of the catalyst.
Bhaumik and Ali et al. introduced a novel catalyst for solvent-free oxidation of olefins. The catalyst was prepared by amine functionalization of SBA-15 with 3-aminopropyl-triethoxysilane (APTES) and subsequent reaction with Schiff base diacetylmonooxime followed by incorporation of Ni through reaction with Ni(ClO4)2 (Fig. 7). The obtained catalyst, SBA-15–NH2–DAMO–Ni could promote one-pot oxidation of a broad range of olefins in the presence of tert-butylhydroperoxide as oxidant. The catalyst was also reusable and could be recovered and reused with slight loss of the activity. Investigation of the effect of Ni loading was performed by comparing the performances of three catalysts with three different Ni loading (1.29, 0.63 and 1.95 wt%). The results established that the higher the Ni loading, the higher conversion was obtained. However, the TON value changed in order of 1.95 < 0.63 < 1.29 wt%. This can be due to the decrease of surface area and ordering of 2D hexagonal mesophase due to higher Ni loading.99
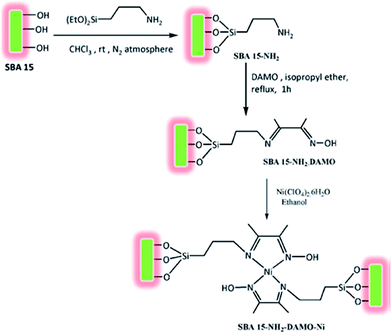 | ||
| Fig. 7 Route to synthesize functionalized SBA-15 supported nickel(II)-oxime–imine catalysts. Reprinted from ref. 99 with permission of Elsevier. | ||
Sakthivel et al. disclosed a novel catalytic system based on conjugating SBA-15 with cobalticinium complex containing siloxane moiety on ligand centre (Fig. 8).100 The hybrid system was used as an efficient heterogeneous catalyst (conversion: 57–91%, TON: 356–568) for oxidation of various alcohols in the presence of TBHP in decane as oxidant with good selectivity of ketone. Noteworthy, the catalyst was reusable and preserved its catalytic activity after recycling for two runs of reaction. FTIR analysis of the spend catalyst and comparing it with fresh one established that both two catalysts exhibited similar vibrational bands indicating their identical features. However, due to the adsorption of organic compounds on the spend catalyst, higher intensities of the bands for spend catalyst was observed. Moreover, leaching of cobalt species was not detected.
 | ||
| Fig. 8 Schematic representation of complex (6) heterogenization on SBA-15 and its application on alcohol oxidation. Reprinted from ref. 100 with permission of Springer. | ||
In attempt to develop an efficient visible-light-photocatalyst for oxidation of H2O, aminopropyl-functionalized SBA-15 was reacted with 2-acetylpyridine and cobalt nitrate (Fig. 9).101 The catalytic activity (turnover frequency: 59.64 mol of O2 per h per mol per Co) of the obtained catalyst, cobalt salophen functionalized SBA-15, (MC) was remarkably higher than that of corresponding Schiff-base. The high catalytic activity was ascribed to the large surface area and tuneable porous structures. Furthermore, the catalyst exhibited high reusability. This economical heterogeneous catalyst was reusable and could be reused for four reaction runs. The analysis of the reused catalyst proved that upon reusing, the Schiff-base remained immobilized on MC and the salophen did not degrade. However, it was found that the coordination environment of Co was altered in reused catalyst.
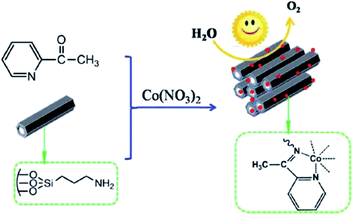 | ||
| Fig. 9 Preparation of MC catalyst. Reprinted from ref. 101 with permission of Royal Society of Chemistry. | ||
Molybdenum(VI) Schiff base complexes were incorporated in SBA-15 via initial functionalization of SBA-15 with APTES and coupling with salicylaldehyde followed by reaction with MoO2(acac)2 (Fig. 10). The hybrid system was then used as an efficient and reusable catalyst for epoxidation of cyclohexene (conversion = 97.78% and selectivity = 93.99%). It was found that silylation of the residual Si–OH groups could increase the catalytic activity of the catalyst. This was attributed to higher content of Mo active sites.102 Moreover, the catalyst was reusable and only a slight loss of the catalytic activity was observed upon reusing for four times. The results obtained from hot filtration established negligible leaching of Mo(VI) and predominance of the heterogeneous catalytic process.
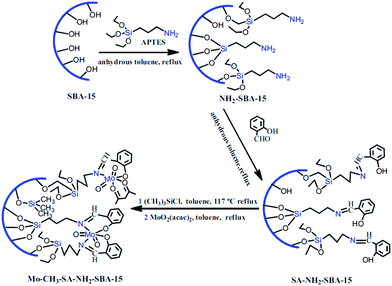 | ||
| Fig. 10 Procedures of the synthesis of Mo–CH3–SA–NH2–SBA-15. Reprinted from ref. 102 with permission of Springer. | ||
Manganese(III) complex of meso-tetrakis(4-carboxyphenyl)porphine was immobilized on two types of SBA-15 functionalized with short-chain 3-aminopropyltriethoxysilane and long chain N-(3-trimethoxysilylpropyl)diethylenetriamine to furnish two catalysts, [SBA-15-short-chain-NH2@Mn(TCPP)OAc] and [SBA-15-long-chain-NH2@Mn(TCPP)OAc] with utilities for promoting olefins oxidation in the presence of acetic anhydride as an activator, urea hydrogen peroxide (UHP) as oxidant and imidazole as co-catalyst.103
Both catalysts were reusable. However, the long-chain catalyst, [SBA-15-long-chain-NH2@Mn(TCPP)OAc], proved to be more active than the short-chain counterpart and exhibited comparable catalytic activity with homogeneous (Mn(TCPP)OAc).
Bardajee et al. designed a novel reusable and non-leaching organometallic catalyst, Cu(II)–DiAmSar/SBA-15, based on coordination of Cu(II) ions with diaminosarcophagine ligand followed by conjugating with SBA-15 (Fig. 11). The novel catalyst was successfully used for solvent-free synthesis of a series of heterocycles including benzimidazole, benzothiazole and benzoxazole derivatives.104 The protocol showed broad substrate scope and desired heterocycles could be achieved in high yields under mild reaction condition. Moreover, the catalyst was reusable and could be recovered and reused for 6 reaction runs with negligible loss of the activity (only 1%). The presence of ordered mesoporous channels in the catalyst was proved by various analyses. This characteristic of the catalyst facilitated the ingress of reagents into the pores of the catalyst and resulted in high dispersion of copper species.
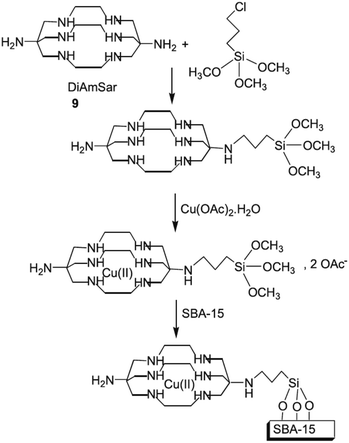 | ||
| Fig. 11 Preparation of Cu(II)–DiAmSar/SBA-15. Reprinted from ref. 104 with permission of Wiley. | ||
Vinu et al. developed a highly acidic SBA-15 through functionalization with triflic acid. Using FTIR spectroscopy the binding of triflic acid on the SBA was proved. Changing the amount of triflic acid, pore diameters was controlled. The authors investigated the interaction of the different amounts of triflic acid with the SBA-15. The acidity of the catalyst was estimated by using TPD technique. It was found that upon using higher amount of triflic acid, the acidity of the catalyst was improved. The authors reported that SBA with the largest pore diameter could not be served as an efficient support as it exhibited low acidity and surface area as well as poor degree of structural order. The obtained catalyst was employed for the synthesis of coumarin. The results established the superior catalytic activity of this catalyst compared to some conventional catalysts such as zeolites and bare SBA-15.105
Click reaction was exploited for the synthesis of a novel functionalized SBA-15, D-2PAPd(II)@SBA-15. The synthetic procedure (Fig. 12) included the synthesis of azido-functionalized mesoporous SBA-15 followed by its cycloaddition reaction with N,N-dimethyl-2-propynylamine (D-2PA) and subsequently the complexation with PdCl2. The catalytic activity of the novel catalytic system was investigated for Suzuki coupling reaction of phenylboronic acid with aryl halide derivatives. The authors investigated the effects of reaction variables such as temperature, solvent and base. Inorganic base such as K2CO3 and DMF were found as the best base and solvent respectively. Additionally, performing the reaction at 120 °C led to the highest yields. Broad substrate scope, high TON and yields and reusability of the catalyst were the merits of this protocol.106
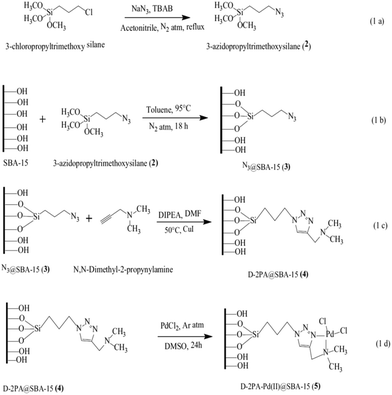 | ||
| Fig. 12 (a) Synthesis of 3-azidopropyltrimethoxysilane 2. (b) Azide functionalization over SBA-15 [N3@SBA-15 3]. (c) Cycloaddition of N,N-dimethyl-2-propynylamine and N3@SBA-15 3 via the click reaction [D-2PA@SBA-15 4]. (d) Metallation of D-2PA@SBA-15 4 by PdCl2 [D-2PA-Pd(II)@SBA-15 5]. Reprinted from ref. 106 with permission of Springer. | ||
In an innovative design, Singh et al. employed amine-functionalized SBA-15 for grafting Pd–EDTA complex and developing a novel catalyst, SBA-15–EDTA–Pd(II) (Fig. 13). The authors fully characterized the catalysts by using various analyses such as TEM, SEM, TGA-DTA, XRD, FTIR and 13C, 29S NMR. The oxidation state of the Pd was found to be Pd2+ by XPS technique. The catalytic utility of the catalyst was investigated for Suzuki and Sonogashira coupling reactions. The results established high catalytic performance, TON and reusability. Broad substrate scope, promoting the reaction in the absence of any ligand or copper co-catalysts and reusability of the catalyst were other merits of this protocol.107
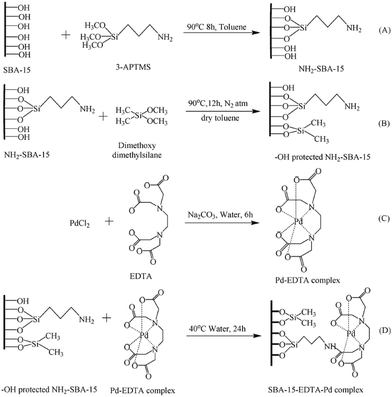 | ||
| Fig. 13 Schematic diagram of SBA-15 functionalization and heterogenization of Pd–EDTA–SBA-15. 1(A) Amino (–NH2) functionalization, 1(B) capping of SBA-15, 1(C) Pd–EDTA complex formation, 1(D) anchoring of Pd–EDTA complex over modified surface of SBA-15. Reprinted from ref. 107 with permission of Royal Society of Chemistry. | ||
A novel organic–inorganic hybrid catalyst, SBA-15–TAT–Pd(II), was designed and synthesized trough reaction of thio-functionalized SBA-15 with 2,4,6-triallyloxy-1,3,5-triazine and azobisisobutyronitrile (AIBN) (initiator) followed by anchoring of palladium (Fig. 14).108 The novel hybrid system was successfully used for promoting C–C coupling reactions including Heck, “copper-free” Sonogashira, Suzuki and Hiyama cross coupling reactions in DMF at 120 °C. In comparison to the previously reported palladium containing SBA-15 catalysts, the novel system exhibited higher performance in terms of reaction time, yield and Pd loading (0.62 mmol%). Moreover, the catalyst was reusable and could be recovered and reused for five reaction runs with comparable catalytic performance. Additionally, the XPS analyses of fresh and reused catalysts established the identical oxidation state of Pd in both catalysts. The hot filtration tests also ruled out the leaching of Pd and confirmed the heterogeneous nature of the catalysis.
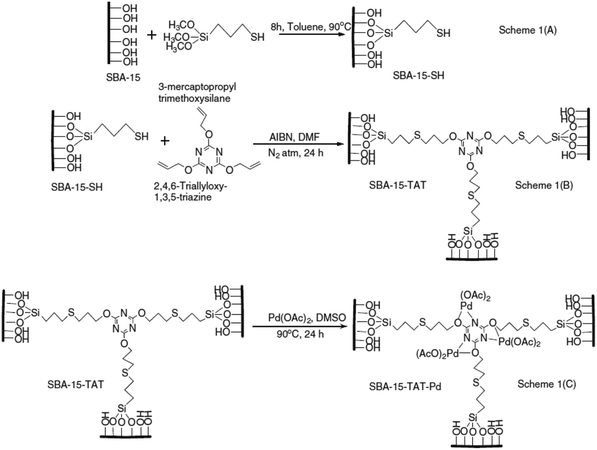 | ||
| Fig. 14 Synthesis of SBA-15–TAT–Pd(II) catalyst. Reprinted from ref. 108 with permission of Elsevier. | ||
This catalyst was also used for promoting hydrogenation reaction.109 The results established excellent catalytic activity of the hybrid system (selectivity 100% and conversion >99%) which was superior compared with the commercial catalyst, 10 wt% Pd/C. Noteworthy, the catalyst was reusable and could be recovered and reused up to five runs with negligible loss of performance.
Rostamnia et al. designed and synthesized a hybrid system composed of SBA-15, carbene complexes of Pd(II) and ionic liquid. The procedure for preparing the catalyst is depicted in Fig. 15. Using ICP-AES analysis the content of Pd was estimated to be 0.81%. The hybrid, NHC–Pd@SBA-15/IL system was used for promoting Hiyama coupling reaction of aryl halides and trimethoxyphenyl silane in the presence of tetra-n-butylammonium fluoride, TBAF. It was found that Cs2CO3 could improve the yield of reaction and reduce the required amount of Pd mol% due to synergic effect with TBAF for activation of C–Si bond. The authors studied the reusability of the catalyst and proved that it could be recovered and reused for 5 reaction times with insignificant loss of catalytic performance.110 However, upon reusing for the sixth run the catalytic activity decreased dramatically which was attributed to the collapse of the silica support. ICP-AES analysis established no Pd leaching upon reusing the catalyst for 5 times.
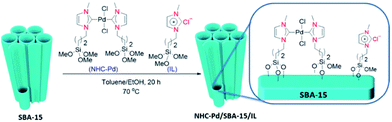 | ||
| Fig. 15 Schematic synthesis pathway of NHC–Pd/SBA-15/IL. Reprinted from ref. 110 with permission of Elsevier. | ||
This research group also reported a green and efficient protocol for S-arylation of benzylic and aromatic thiols and various aryl halides in the presence of K2CO3 under the catalysis of a novel heterogeneous catalyst based on immobilized Pd ions on porous periodic mesoporous organosilica with ionic liquid pore walls (Fig. 16).111 The authors studied the reaction variables such as solvent and temperature and found that performing the reaction in water under reflux was the optimum reaction condition. The catalyst was reusable and could be recovered and reused for 10 reaction runs with negligible loss of the activity. Broad substrate scope, using low amount of Pd (0.054 mol%) and aqueous reaction media were other merits of that procedure.
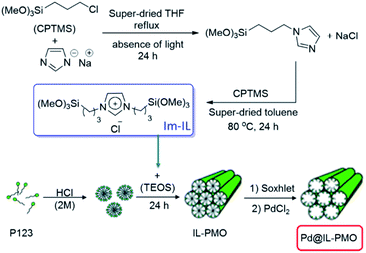 | ||
| Fig. 16 Procedures for the synthesis of the Pd@IL-PMO. Reprinted from ref. 111 with permission of Elsevier. | ||
A new heterogeneous Pd catalyst was developed by functionalization of SBA-15 with (3-mercaptopropyl)trimethoxysilane followed by reaction with (CH3CN)2PdCl2. The catalyst was found as an efficient and reusable catalyst for promoting Sonogashira and Suzuki–Miyaura cross-coupling reactions of aryl halides.112 The Sonogashira reaction could proceed at 75 °C using piperidine as a base and in the absence of copper under solvent-free condition to furnish the corresponding products in high yields (89–98%) after 1 h. The Suzuki reaction proceeded at 90 °C in the presence of K2CO3 after 6 h to furnish the desired product in moderate to high yields (51–97%). Moreover, the catalyst could be reused for five reaction runs with only slight loss of the catalytic activity.
Ghorbani-Vaghei et al. deposited PdCl2 on amidoxime functionalized SBA-15, SBA-15/AO, to afford SBA-15/AO/Pd(II) nanocatalyst. The authors also reduced the Pd species by hydrazine to afford a novel catalyst contains Pd(0) species (Fig. 17). The catalytic activity of SBA-15/AO/Pd(II) was studied for Suzuki coupling reaction between phenylboronic acid and aryl halides. The reaction could proceed in the presence of very low amount of Pd loading (∼0.2 mol%) to furnish the desired products in high yields. The reusability of the catalyst, broad substrate scope, mild reaction condition were the merits of this phosphine-free catalyst.113
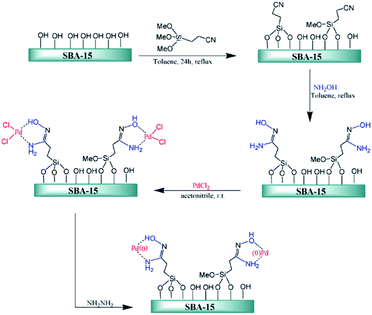 | ||
| Fig. 17 Schematic diagram of SBA-15/AO/Pd(II) and SBA-15/AO/Pd(0) fabrications. Reprinted from ref. 113 with permission of Elsevier. | ||
Veisi et al. disclose a novel functionalized SBA-15 in which pyridine groups containing melamine were attached on SBA-15. This organic–inorganic system was then used for immobilization of Pd(II) and Pd(0) nanoparticles (Fig. 18). The obtained hybrid catalyst was employed as an efficient catalyst for promoting Suzuki cross-coupling reaction between phenylboronic acid and wide range of aryl chloride, bromide and iodide in the presence of K2CO3 in the mixture of H2O/EtOH at 50 °C. This procedure furnished the desired biaryls with use of very low amount of Pd (∼0.3 mol%) under mild reaction condition in high yields with no need to additional ligand or additive. The reusability of the SBA-15/CCPy/Pd(II) was also demonstrated by employing the reused catalyst for consecutive reaction runs (up to 7 runs).114 Moreover, no Pd ion was detected in the liquid reaction mixtures by atomic absorption spectroscopy. This result indicated the key role of functionality on anchoring the Pd species.
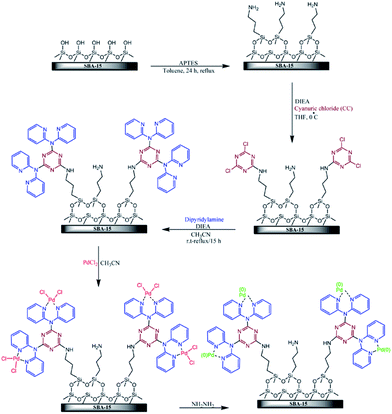 | ||
| Fig. 18 Schematic diagram of SBA-15/CCPy/Pd(II) and SBA-15/CCPy/Pd(0) fabrications. Reprinted from ref. 114 with permission of Elsevier. | ||
Manirul Islam and Bhaumik reacted amine functionalized SBA-15 with 2-pyridinecarboxaldehyde to afford pyridine-imine functionalized SBA-15 which could be served for coordinating Cu(II) (Fig. 19). The novel catalyst, Cu@PyIm–SBA-15, was successfully used as an efficient and reusable catalyst for promoting synthesis of 1,4-di-substituted 1,2,3-triazoles through click reaction of azides, derived in situ from reaction of amines and NaN3, and acetylenes under mild and green condition (aqueous media and 0 °C-ambient temperature).115 Various reagents with different electron densities and steric hindrance could be used and lead to the corresponding products in relatively short reaction times (6–8 h) in excellent yields (90–98).
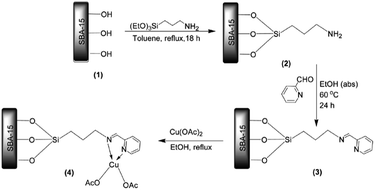 | ||
| Fig. 19 Synthesis of Cu@PyIm–SBA-15 catalyst. Reprinted from ref. 115 with permission of Elsevier. | ||
The recycling test established that the catalyst could be recovered and reused for five reaction runs. Notably, the EPR spectra of the fresh and reused catalysts indicated the identical oxidation state of copper, Cu2+, for both of the catalysts. Furthermore, the leaching of the active catalytic species was negligible. High yields, preventing the formation of dimer of alkyne were other advantages of this protocol.
Sadeghzadeh functionalized SBA-15 with 2,2′-(chloromethylene)dipyridine and subsequently reacted the hybrid with sodium tetrachloroaurate(III)hydrate to prepare a novel SBA-15 supported gold dipyridine complex. The system was successfully used as an efficient and reusable catalyst for promoting the four-component reaction of α-enolic dithioesters, cysteamine, aldehyde and cyclohexane-1,3-dione under ultrasonic irradiation to afford corresponding thiazoloquinolines.116 The comparison of the synthesized catalysts with the previously reported ones demonstrated that the novel catalyst could promote the reaction under milder and greener reaction condition and shorter reaction time. Moreover, the author studied the catalytic activity of the catalysts derived from incorporation of various metal ions. The results established that the catalytic activities varied in the order of Au(III) > Cr(III) > Cu(II) > Ni(II) > Zn(II) > Mn(II) > Co(II) > Cd(II) > Hg(II).
As obvious from the abovementioned examples, the encapsulation of complexes within the pores of SBA can lead to the heterogeneous catalysts with high reusability. The SBA-encapsulated catalysts could be recovered easily and reused for several reaction runs with only slight loss of their catalytic activity. Notably, in most cases, the leaching of the catalytically active species was highly supressed. It was also shown that encapsulation of the complex not only did not diminish the catalytic activity, but also could result in higher catalytic activity which could be attributed to the protecting the catalyst, concentration of substrate and synergetic effects between SBA and the complex. In most case, the nature of the catalysis with SBA-encapsulated catalysts was proved to be heterogeneous. Noteworthy, functionalized SBA can more effectively anchor the catalytic species and prevent the catalyst leaching. In this context various functionalities could be introduced on SBA surface via post-synthesis approach. The catalytic performance of the final catalyst can depend on the nature of complex and metallic centre, the strength of the interactions between the catalytic species and the functionalized SBA and the size of the pore of SBA structure.
3. Functionalized SBA for incorporation of porphyrin complexes
Encapsulation of (metallo) porphyrins in porous compounds such as SBA not only protect them from harsh reaction condition, but also results in catalysts that mimic the features of natural enzymes. In this way, the reaction can proceed with high efficiency and stereochemical selectivities.1 To incorporate the porphyrin catalyst within the cavity of SBA more efficiently, the SBA could be modified via post or pre-synthesized method.A photocatalyst based on immobilization of V-doped mesoporous TiO2/tetrakis(4-carboxyphenyl)porphyrin (V–TiO2/TCPP) onto amine-functionalized SBA-15 was developed and used for photo-degradation of 2,4-dichlorophenol. To elucidate the role of SBA-15, the catalytic activities of V–TiO2/TCPP/SBA and V–TiO2/TCPP were compared.117 It was found that not only the V–TiO2/TCPP/SBA was more efficient but also it exhibited good reusability and could be recovered and reused for four times with negligible loss of activity.117 It is worth mentioning that degradation of 2,4-dichlorophenol was of first-order kinetic model. It was proposed that the incorporation of V–TiO2/TCPP in SBA-15 led to its dispersion and prevented from particle–particle aggregation and light scattering. Studying the effect of the initial concentration of 2,4-dichlorophenol proved an inverse relationship between this parameter and photodegradation percentage.
Nakagaki et al. developed two methodologies for immobilizing Mn porphyrins (MnP) on SBA-15 including (1) using electrostatic interaction for supporting three cationic isomers of Mn(III) N-methylpyridiniumporphyrins (MnTM-X-PyPCl5, X = 2, 3, 4) on SBA-15 and (2) employing Cl-functionalized SBA-15 for embedding three neutral isomers of Mn(III) N-pyridylporphyrins (MnT-X-PyPCl, X = 2, 3, 4) through covalent bonding (Fig. 20). The loading amount of MnP was 0.3%. The catalytic activities of the two catalysts were investigated for oxidation of cyclohexane employing iodosylbenzene as oxygen donor. The results established the superior catalytic activities of the novel catalysts compared to homogeneous counterparts. Moreover, the SBA-15–Cl/MnT-X-PyPCl proved to be slightly more efficient than MnTM-X-PyPCl5. Low leaching of the MnP which was attributed to interaction of MnP and SBA, high yield and selectivity to cyclohexanol as well as the reusability of the catalyst were the advantageous of this protocol.118
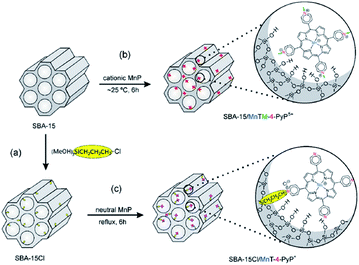 | ||
| Fig. 20 Schematic representation of the synthetic routes for (a) preparation of support SBA-15Cl by the silanization of SBA-15 with (3-chloropropyl)trimethoxysilane, (b) immobilization of MnTM-4-PyPCl5 on SBA-15 by electrostatic interaction, and (c) anchorage of MnT-4-PyPCl on SBA-15Cl by covalent bonding. The two classes of heterogenized catalysts are illustrated with the para MnP isomers. Reprinted from ref. 118 with permission of Elsevier. | ||
In another attempt, Iamamoto et al. attached 5,10,15,20-tetrakis(pentafluorophenyl)porphyrin iron(III) chloride (FeP) on amine-functionalized SBA-15. The obtained hybrid system, FeP–APSBA, was successfully used for oxidation of hydrocarbons. The authors also developed another catalyst, FeP–APSBA–TMS, by introducing non-polar Si(CH3)3 moieties by using 1,1,1,3,3,3-hexamethyldisilazane (HMDS) (Fig. 21). Comparing the catalytic activities of the catalysts, which were considered as P-450 model systems, it was found that the end-capped with TMS groups had detrimental effect on the catalytic activity and the presence of Si-OH groups promoted adsorption of iodosylbenzene molecules, used as oxygen donors, during the oxidation reactions. This increased the probability that the intermediate species collided with the substrate.119
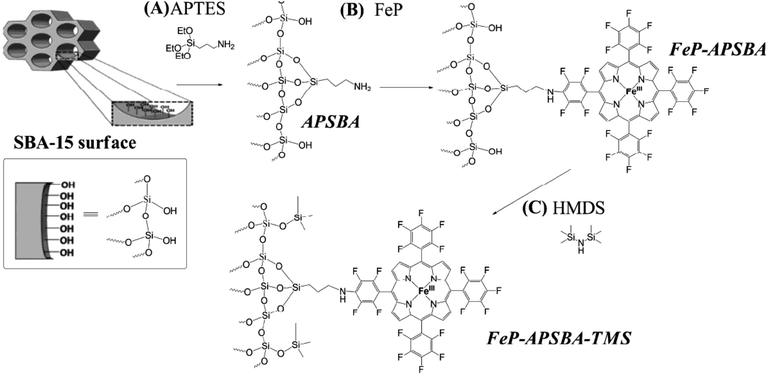 | ||
| Fig. 21 (A) Preparation of the hybrid material APSBA from the starting SBA-15 structure; (B) FeP immobilization on the hybrid APSBA, to give FeP–APSBA; and (C) silylation of the FeP–APSBA surface with HMDS, to yield FeP–APSBA–TMS. Reprinted from ref. 119 with permission of Royal Society of Chemistry. | ||
SBA-16 was also employed for immobilization of copper(II) meso-tetrakis(4-chlorophenyl)porphyrin (CuTClPP) through ship in bottle approach. The hybrid catalyst, (CuTClPP@SBA-16) was successfully used as a heterogeneous and reusable catalyst for the oxidation of cyclohexene with t-butyl hydroperoxide (TBHP) and hydrogen peroxide as oxidants to epoxide, ketone and alcohol (Fig. 22). It was reported that using the bare SBA-16 and amine-functionalized SBA-16 did not lead to oxidation products indicating the dominant role of metalloporphyrin in catalysis. It was found that each 1 mol metalloporphyrin converted the 174![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 311 mole of cyclohexene molecules within the 90 min. The investigation of visible light effect on the efficiency of the catalyst proved the positive effect of the visible light.120 The catalyst could be recovered and reused for four reaction runs with only slight loss of the catalytic activity.
311 mole of cyclohexene molecules within the 90 min. The investigation of visible light effect on the efficiency of the catalyst proved the positive effect of the visible light.120 The catalyst could be recovered and reused for four reaction runs with only slight loss of the catalytic activity.
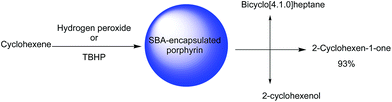 | ||
| Fig. 22 The oxidation of cyclohexene. Adapted from ref. 120. | ||
In conclusion, encapsulation of porphyrin which can be achieved through ship-in-bottle approach can be considered as an effective strategy for developing heterogeneous and reusable catalysts. Encapsulation can also improve dispersion and prevent from particle–particle aggregation. In the case of photocatalysis and photodegradation, SBA can improve adsorption of the substrates and enhance the photocatalytic activity through increasing the possibility of collision of the substrates.
4. Functionalized SBA for incorporation of nanoparticles
The interconnected cavities of SBA mesoporous materials can serve as nanoreactors for embedding nanoparticles. In this way, not only the aggregation of nanoparticles is prevented and their particle size and dispersion improved, the catalytic nanoparticles could be protected in the course of catalysis. The encapsulation of the nanoparticles can occur through simple physical entrapment or via electrostatic and non-covalent interactions. In the latter case, the surface of SBA is usually functionalized with ligands containing one or more heteroatoms (oxygen, nitrogen, phosphorous or sulphur).1 These ligands can range from simple heteroatom containing aliphatic or heterocyclic compounds to complicated ligands such as dendrimers. From the above discussed examples, the SBA-encapsulated nanoparticles can be considered as efficient and reusable catalysts with low leaching of nanoparticles for promoting diverse range of the organic transformations.Suzuki–Miyaura cross-coupling reaction was promoted under mild condition, i.e. aqueous media and atmospheric condition in DMF in the presence of Pd nanoparticles supported on 1,3-dicyclohexylguanidine functionalized mesoporous silica SBA-15. The novel catalyst, SBA-15/1,3-DCG/Pd, was prepared by introducing 1,3-dicyclohexylguanidine (1,3-DCG) moieties on SBA-15 and subsequent deposition of Pd nanoparticles (Fig. 23).121 Notably, the dispersion of the fine Pd nanoparticles (size ∼ 10 nm) was uniform. Using ICP analysis the content of Pd nanoparticles was estimated to be 0.3 mmol g−1. Additionally, the formation of Pd(0) was confirmed by XPS analysis. The reaction condition was optimized by varying the reaction variables such as reaction temperature, solvent and base. Using optimum reaction condition, i.e. using K2CO3 as base and water–ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as solvent at 40 °C the generality of the reaction was studied. The aryl halides reacted in the order of R–I > R–Br > R–Cl. The broad substrate scope, high selectivity and reactivity, excellent reusability (up to five reaction runs) simplicity of catalyst preparation and moisture stability of the catalyst were the merits of this protocol.
1) as solvent at 40 °C the generality of the reaction was studied. The aryl halides reacted in the order of R–I > R–Br > R–Cl. The broad substrate scope, high selectivity and reactivity, excellent reusability (up to five reaction runs) simplicity of catalyst preparation and moisture stability of the catalyst were the merits of this protocol.
 | ||
| Fig. 23 Preparation of SBA-15/1,3-DCG/Pd. Reprinted from ref. 121 with permission of Royal Society of Chemistry. | ||
Xin and Rostamnia developed a series of heterogeneous Pd catalysts [(PdX2@SBA-15/SO3H or Pd(PrSO3)2@SBA-15) and (Pd-NPs@SBA-15/SO3H or Pd-NPs(PrSO3)@SBA-15)] by preparing SO3H-functionalized SBA-15 followed by ion exchange method and subsequently reduction of the Pd ions (Fig. 24). The authors investigated the catalytic activities of the catalysts for phosphine-free Suzuki cross-coupling reaction in the presence of K2CO3 in DMF/H2O at 80 °C.122 They established that the Pd(MeCN)2Cl2@SBA-15/SO3H and Pd-NPs@SBA-15/SO3H possessed the best catalytic performances in terms of activity and selectivity.
 | ||
| Fig. 24 Schematic representation of the catalysts preparation. Reprinted from ref. 122 with permission of Elsevier. | ||
The excellent reusability of the catalysts (up to 11 reaction runs) as well as good substrate tolerance and commercially availability of the starting materials were other merits of this protocol.
This research group also reported phosphine-free Heck arylation reaction of aryl halide derivatives and conjugate alkenes with Pd incorporated into sulfonate–SBA-15, SBA-15/PrSO3Pd and SBA-15/PrSO3PdNP.123 Low Pd loading, reusability of the catalyst and high yields were the advantageous of that protocol.
Pd nanoparticles (particle size of ∼2 nm) were confined in various thiol-functionalized silica materials including Aerosil-380, plugged SBA-15, SBA-15, and m-MCF. The obtained hybrid systems were used as catalysts for Heck and the Suzuki reactions. It was found that in the case of Suzuki reaction, stronger basic condition was needed and the catalyst obtained from plugged SBA-15 exhibited better stability (the SBA-15 was fragile and collapsed in the first reaction run) while the leached palladium was recognized as the main factor for promoting the Heck reaction.124 Furthermore, the catalysts derived from ordered mesoporous silica could limit the growth of Pd nanoparticles and consequently improve the stability.
The amidoxime-functionalized mesoporous SBA-15 was used for immobilizing Pd(0) nanoparticles (Fig. 25). The catalytic activity of the obtained catalyst, SBA-15/AO/Pd(0) was investigated for the synthesis of N-arylindole derivatives via the Ullmann-type coupling reaction of indoles with aryl iodides in the presence of Et3N at 110 °C. The results established that the low amount of the catalyst (ca. 0.3 mol%) could furnish the corresponding products in good to high yields. The protocol showed broad substrate scope. Notably, the catalyst was reusable and could be recovered and reused for five reaction runs with no remarkable loss of activity.125 To elucidate the nature of the catalysis, the hot filtration method was employed. The results established the preservation of Pd on the SBA at elevated temperature and consequently the heterogeneous entity of the catalysis. Comparing the efficiency of this catalyst with previously reported ones such as polystyrene supported Cu(II), MCM-41-2N–Cu, CuI, metformine demonstrated the superior catalytic activity of the novel catalyst.
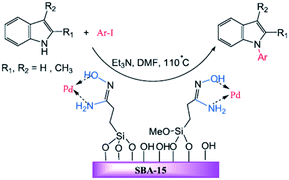 | ||
| Fig. 25 Palladium-catalysed N-arylation of indoles. Reprinted from ref. 125 with permission of Wiley. | ||
Ultrafine Pd nanoparticles were also supported on SBA-15 functionalized with APTES. The obtained catalyst was successfully used as a reusable catalyst for hydrogen evolution through formic acid dehydrogenation of at ambient temperature with TOF of 293 h−1.126
Using co-reduction method, Wan et al. supported bimetallic AgPd nanoparticles on graphitic carbon nitride (g-C3N4) functionalized SBA-15. The hybrid system was successfully used for hydrogen formation from decomposition of formic acid. The authors demonstrated that the content of carbon nitride as well as the composition of AgPd affected the process and Ag10Pd90/0.2CND/SBA-15 was obtained as the catalyst of the choice for dehydrogenation of formic acid with TOFinitial = 893 h−1 at 323 K.127
Novel ligand-functionalized SBA-15 mesoporous silica compounds were obtained by incorporation of 2-aminothiazole groups, AMT–SBA-15, and aminopropyl triazole groups, Tr–SBA-15, and subsequently used for immobilization of silver nanoparticles. The procedure for the synthesis of AMT–SBA-15, depicted in Fig. 26, included reaction with (3-glycidyloxypropyl) trimethoxysilane (GPTMS) followed by coupling of 2-aminothiazole. Tr–SBA-15 was obtained through reaction of G–SBA-15 with sodium azide and click coupling reaction with alkyne group from propargylamine. The Ag doped catalysts, Ag/AMT–SBA-15 and Ag/Tr–SBA-15 were found to be highly active for reduction of methylene blue. Reduction rate constants of 14.3 × 10−3 and 9.4 × 10−3 s−1 for Ag/Tr–SBA-15 and Ag/AMT–SBA-15, respectively.128
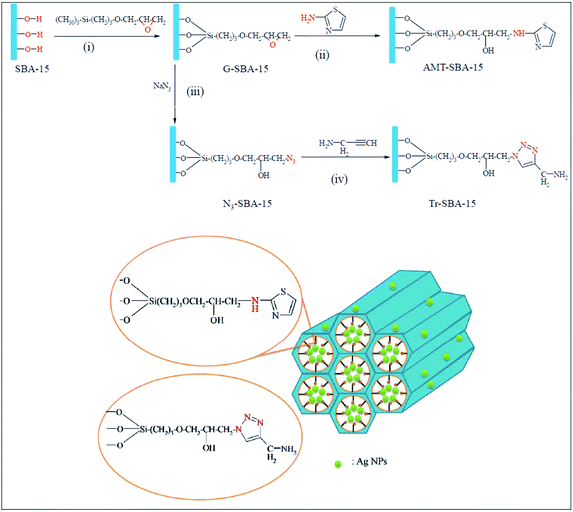 | ||
| Fig. 26 Schematic representation of the preparation of AMT–SBA-15 and Tr–SBA-15 hybrid supports. Reprinted from ref. 128 with permission of Royal Society of Chemistry. | ||
Using carboxylic acid functionalized SBA-15 Kao et al. reported the synthesis of nano-sized Ag nanoparticles (∼3 nm). The authors demonstrated that in the absence of carboxylic acid functionalities, the larger Ag nanoparticles (∼20 nm) would be formed in the exterior of SBA-15 (Fig. 27). The authors believed that the interaction between Ag+ ions and –COO− moieties facilitate the formation of Ag nano particles. The catalytic activity of the catalyst was studied for the reduction of 4-nitrophenol to 4-aminophenol. It was established that the particle size of the Ag nanoparticles played an important role in catalytic performance and inverse relationship was observed between catalytic activity and Ag particle size. Noteworthy, the catalyst could be recovered and reused for 5 reaction runs.129
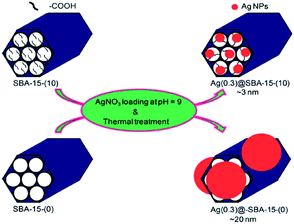 | ||
| Fig. 27 Schematic illustration of the synthesis of Ag(y)@SBA-15-(x) nanocatalysts. Reprinted from ref. 129 with permission of Royal Society of Chemistry. | ||
Benzyl alcohol oxidation under aqueous media was promoted using copper(II) complexes, copper and copper oxide nanoparticles supported on functionalized SBA-15. 1-Methyl-3-[(triethoxysilyl)propyl]imidazolium chloride (IMIL) ionic liquid derived from reaction of 1-methylimidazole and (3-chloropropyl)triethoxysilane as well as 3-[bis[(2-hydroxyethyl)amino]propyl-triethoxysilane] (PADOH) were employed for preparing functionalized SBA-15 (Fig. 28). To obtain copper nanoparticles stabilized by immobilized imidazole ionic liquid or poly(ethylene glycol), chemical reduction was used. The copper oxide samples were prepared using calcinations. The best catalytic activity, conversion (73%), selectivity of benzaldehyde (54%) was obtained using the copper catalyst prepared from incorporation of Cu(II)–IMIL complex in SBA-15 followed by chemical reduction Cu/IMIL–SBA-15-G1.130
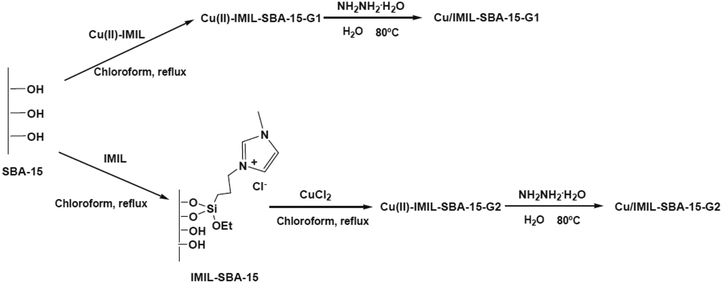 | ||
| Fig. 28 Preparation of immobilized copper ion-containing ionic liquid and copper nanoparticles stabilized by ionic liquid over SBA-15. Reprinted from ref. 130 with permission of Elsevier. | ||
Small Au or Pd nanoparticles as well as AuPd nanoalloys with non passivated surfaces were incorporated in SBA-15 modified with an ionic liquid-like alkoxysilane (Fig. 29). It was found that the mesoporous framework of SBA-15 did not collapse upon functionalization or reduction processes. Moreover, the results obtained from EDS and DRS measurement ruled out the formation of core–shell structure but proved the alloy formation. The obtained bimetallic catalyst was used for the reduction of 4-nitrophenol. The higher the Pd content, the higher the velocity constant and catalytic activity was observed.131
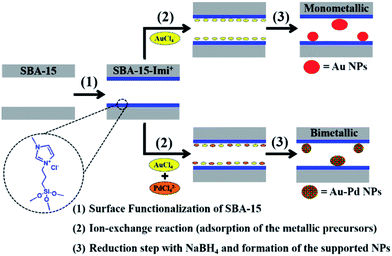 | ||
| Fig. 29 Schematic illustration of the procedure carried out for the in situ synthesis of the supported mono- and bimetallic nanoparticles. Reprinted from ref. 131 with permission of Royal Society of Chemistry. | ||
Au nanoparticles were also deposited on propylamine-functionalized SBA-15.132 Initially the SBA-15 was functionalized via post synthesis approach and subsequently reacted with AuCl3. The nanoparticles were furnished upon reduction with NaBH4. The loading of Au was estimated to be 2 wt%. The catalytic activity of the obtained catalyst, AuNPs/SBA–NH2 was confirmed for oxidative coupling of amines. The results established that various amines could tolerate the reaction to furnish the corresponding mines in high yields in the presence of O2 as oxidant under mild and green reaction condition, i.e. atmospheric pressure with no need to any additive or base. Notably, the leaching of Au was insignificant and the catalyst was reusable and the reused catalyst could be applied for four reaction runs with only slight loss of the catalytic activity.
SH-Functionalized SBA-15 was employed by Li et al. for immobilizing Au nanoparticles.133 The synthetic approach included preparation of SH-functionalized SBA-15 through co-condensation in the presence of (3-mercaptopropyl) trimethoxysilane followed by reaction with HAuCl4 to afford Au–SH@HSO3–SBA-15. The sulfonic acid groups were generated via oxidation by Au3+. The Au–SH@SO3H–SBA-15 was employed as an efficient and reusable catalyst for alkyne hydration in aqueous media. Various alkynes with different electron densities could be used successfully in this protocol to afford the corresponding products in relatively short reaction times (1.5–5 h) in excellent yields (75 to >99%). The catalyst could be simply recovered and reused for 9 reaction runs with preserving its catalytic activity. The ICP analysis also confirmed the suppressed Au leaching. This observation was assigned to the Au–SH coordination bond. The authors compared the catalytic activity of the catalyst with those of SO3H–SBA-15, Au@SH–SBA-15, Au–SH@SO3H–SBA-15, and Au(PPh3)Cl and proved the superior catalytic activity of the former.
Nickel nanoparticles were also incorporated into hyperbranched polyamidoamine–polyvinylamine/SBA-15. To obtain the catalyst, initially SBA-15 was functionalized with polyvinyl amine, PVAm/SBA-15, and subsequently were used for growing hyperbranched polyamidoamine, PAMAM. The incorporation of Ni was preceded through formation of complex between Ni2+ ions and PAMAM followed by reduction by NaBH4 (Fig. 30). The utility of the catalyst, PAMAM–PVAm/SBA-15, was studied for reduction of various nitro containing aromatics under mild reaction condition (aqueous media at ambient temperature). The results proved that the hybrid system could act as a heterogeneous catalyst with ability to reduce various nitro aromatic compounds in excellent yields in short reaction time.134 The reusability tests confirmed the excellent reusability (up to ten times) of the catalyst. Moreover, the comparison of the catalytic activity of Ni–PAMAM–PVAm/SBA-15 and Ni–PVAm/SBA-15 proved the superior catalytic performance of the former in terms of reusability, reaction time and TOF. Noteworthy, the nature of the catalysis was heterogeneous.
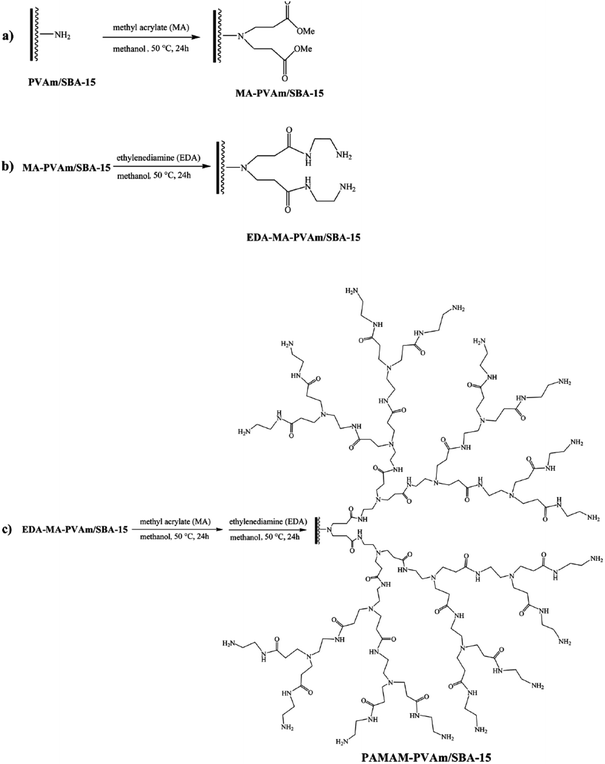 | ||
| Fig. 30 Systematic procedures for the preparation of hyperbranched PAMAM polymer supported on SBA-15. Reprinted form ref. 134 with permission of Royal Society of Chemistry. | ||
Zhang et al. developed a series of novel visible-light-driven photocatalysts, Cr–PFMS-X%, based on supporting Cr on spacious mesoporous channels of phenyl-functionalized mesoporous silica with cubic structure and large pore size (>5 nm). The catalyst was prepared through assembling by co-condensation of tetraethoxysilane and various amounts of phenyltriethoxysilane using P123 as the template followed by phenyl groups-assisted loading with Cr species. The catalytic activity of the photocatalyst was studied for the cyclohexane oxidation with O2 at ambient temperature and visible light irradiation. The authors believed that the presence of the phenyl moiety and consequently large pores could improve the Cr loading and resulted in superior catalytic activity compared to Cr–SBA-15 or samples with hexagonal structure.135 It was also reported that the solvent could dramatically affect the catalytic activity and dichloromethane was the solvent of the choice. The best TON for oxidation of cyclohexane was reported for Cr–PFMS-5%.
It can be concluded that various nanoparticles such as Ag, Pd, Cr, Cu, Ni etc. can be encapsulated within the cavity of SBA to develop hybrid catalysts which in most cases exhibited excellent catalytic activity and reusability. Mostly, this class of catalysts could be prepared via synthesis of SBA, post-functionalization of the SBA, introduction of the metal precursor and finally reduction of metal precursor to zero valent metallic nanoparticles. Notably, bimetallic nanoparticles and nanoalloy could also encapsulated in SBA. Mostly, the nanoparticles encapsulated in SBA are of small sizes and high distribution. This can be attributed to the role of SBA in controlling the particle growth and preventing the aggregation. Moreover, in the case of using functionalized SBA, the leaching of the nanoparticles in the course of the reaction is highly reduced. It is worth noting that the incorporation of nanoparticles usually did not lead to the collapse of SBA framework and the SBA preserved its ordered porous structure.
5. Functionalized SBA for embedding heteropolyacids
Heteropolyacids are nontoxic and non-corrosive catalysts with both redox potentiality and Bronsted acidity. They have been widely used for catalyzing various organic reactions136,137 including electrochemical,138 petrochemical,139 and photochemical reactions.140–142 The main drawbacks of this class of catalysts are low surface area and high solubility in water and conventional organic solvents. To circumvent these issues, immobilization of heteropolyacids on inorganic supports such as clays143,144 have been suggested. More sophisticatedly, heteropolyacids can be encapsulated within a porous support.145In this regard, SBA has received great attention heteropolyacid immobilization onto mesoporous silica can transform it into a heterogeneous catalyst and improve its catalytic activity through synergistic effects between silica support and heteropolyacid as well as increasing surface area.69 Moreover, in comparison of some reported porous supports/nanoreactors such as MOFs, SBA exhibited high thermal, mechanical and chemical stability. Notably, the synthesis and modification of SBA are relatively simple and cost-effective. In the following some recent advances in the field of the utility of SBA for immobilization of heteropolyacids are discussed.
Zhou et al. immobilized Keggin type heteropolyacid, H3PW12O40, onto sulfonate-functionalized ionic liquid modified SBA-15 via ion exchange and used the hybrid system as an efficient and reusable catalyst for alkylation of o-xylene with styrene. The optimum amount of heteropolyacid loading was found to be 30%, 30% HPW–PMIMPS–SBA-15. The authors compared the catalytic activity of the catalyst with basic ionic liquid 1-(propyl-3-sulfonate) 3-methyl-imidazolium phosphotungstate ([MIMPS]3PW12O40), pure heteropolyacid and pure SBA-15 (Fig. 31). The results demonstrated that although heteropolyacid exhibited high catalytic activity, its homogeneous nature made the work-up procedure and reusing of the catalyst difficult. Moreover, bare SBA-15 was not catalytically active. Comparing with ([MIMPS]3PW12O40), 30% HPW–PMIMPS–SBA-15 exhibited superior catalytic activity.146 The novel catalyst reduced the use of ionic liquid significantly. Moreover, it could be recovered and reused for 6 reaction runs with only slight loss of the catalytic activity.
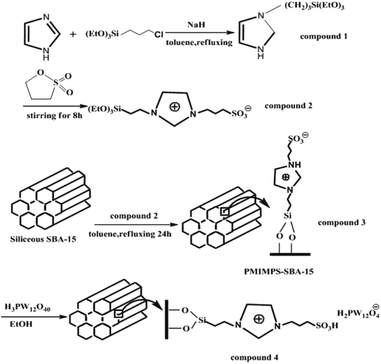 | ||
| Fig. 31 Schematic procedures for the synthesis of immobilized HPW–PMIMPS–SBA-15 materials. Reprinted from ref. 146 with permission of Royal Society of Chemistry. | ||
Zhu, Li and et al. supported phosphotungstic acid on ionic liquid-modified SBA-15 and used it for oxidative desulfurization147 in the presence of hydrogen peroxide. It was found that upon immobilization of heteropolyacid the structure of functionalized SBA did not destroyed. The synthetic method for the preparation of the catalyst, HPW–IL/SBA-15, is illustrated Fig. 32. The introduction of ionic liquid resulted in good wettability. The catalyst exhibited high catalytic activity for removal of dibenzothiophene and 4,6-dimethyldibenzothiophene and benzothiophene under mild reaction condition and short reaction time. Comparing the catalytic activity of HPW–IL/SBA-15 and HPW–SBA-15 confirmed the superior catalytic activity of the former. The authors studied the effect of reaction variables such as temperature, heteropolyacid loading and, peroxide amount. The optimized H2O2/DBT molar ratio was estimated to be 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. A linear relationship between the catalytic activity and temperature was detected and 60 °C was selected as the best temperature. Moreover, 0.1 g loading of HPW led to the best results. The reusability of the catalyst for four reaction runs was also confirmed.
1. A linear relationship between the catalytic activity and temperature was detected and 60 °C was selected as the best temperature. Moreover, 0.1 g loading of HPW led to the best results. The reusability of the catalyst for four reaction runs was also confirmed.
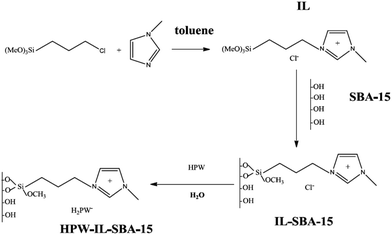 | ||
| Fig. 32 Synthesis of HPW–IL/SBA-15 catalyst. Reprinted from ref. 147 with permission of American Chemical Society. | ||
Covalent immobilization of polyoxometalates (PMO) on different silica supports functionalized with amine or carboxylic acid, SBA-15 and mesocellular foams (MCF) (POM-CO2H@SBA-NH2, POMCO2H@MCF-NH2, and POM-NH2@SBA-CO2H) was studied and compared in the epoxidation of cyclooctene and cyclohexene in the presence of hydrogen peroxide by Villanneau, Launay and their co-workers. The authors believed that using amine-functionalized SBA-15, the best dispersion and loading of polyoxometalate could be achieved.148 Moreover, the POM-CO2H@SBA-NH2 was proved to be a truly anchored catalyst and significant leaching of the catalytic species did no observed.
Amino-functionalized SBA-15 was prepared by using APTES and then applied for immobilization of a series of transition metal-substituted phosphomolybdic acids (PMo11M, M = Fe, Co or Cu) or un-substituted phosphomolybdic acid (PMo12). High dispersion of PMo12 on the support was confirmed. Moreover, the oxidation states of Mo in all the catalysts were identical. The catalytic activities of the catalysts were investigated for the epoxidation of olefins with molecular oxygen as oxidant and isobutyraldehyde as co-reagent. The results proved the efficiency of PMo11Co/SBA and PMo11Cu/SBA for the epoxidation of cyclooctene. Moreover, the proper solvent (CH3CN) could effectively prevent from deactivation of the catalyst and consequently improve the reusability of the catalyst.149
Amine-functionalized SBA-15 was used for supporting the tricopper(II)-substituted polytungstates, [(Cu(H2O))3(α-XW9O33)2]12− (Cu3X2W18, X = BiIII, SbIII). The obtained catalyst Cu3X2W18–NH2–SBA-15 exhibited highly dispersed heteropolyanions and successfully used for oxidation reactions including selective oxidation of thiophene in the presence of H2O2 and oxidation of benzyl alcohol to the benzaldehyde. In the case of oxidation of benzylalcohol, the authors studied the effects of reaction time, temperature, catalyst amount and the ratio of hydrogen peroxide to benzyl alcohol. The best catalytic performance was achieved by 0.05 g 4.7% Cu3X2W18–NH2–SBA-15 (X = Sb, Bi) at 80 °C after 8 h with hydrogen peroxide to benzyl alcohol ratio of 3. In the case of thiophene oxidation, the optimized reaction variables were found to be 2 mL 30% hydrogen peroxide, 5 mL acetonitrile, 10 mg of the catalyst at 65 °C for 70 min. Noteworthy, the catalyst could be reused for five reaction runs with only negligible loss of the catalytic performance.150
From the examples discussed above, it can be concluded that the incorporation of heteropolyacids in SBA can highly improve its reusability and the heterogeneous catalysts can be simply recovered and reused with only negligibly loss of the catalytic activity. In this regard, functionalization of the SBA surface is imperative. Various functionalities from simple amino functionality to ionic liquids can be introduced on SBA. The incorporation of heteropolyacid can be achieved through a simple impregnation method. According to the results, encapsulation of heteropolyacids in SBA did not diminish their catalytic activities and the hybrid catalysts with low loading of heteropolyacids could effectively catalyse the reactions under mild and green conditions.
6. Functionalized SBA for incorporation of DABCO
There are several reports in the literature concerning incorporation of DABCO into SBA-15.151–154 In this regard, Davarpanah et al. immobilized DABCO on Cl–SBA-15 to afford a novel catalyst (SBA–DABCO) with utility for catalyzing the multi component reaction of ammonium acetate, aldehydes, and ethyl acetoacetate under mild reaction condition (Fig. 33). Noteworthy the reaction did not proceed well in the absence of the catalyst. Broad range of substrate, high yields, reusability of the catalyst, solvent-less condition and simplicity of the procedure were the merits of this protocol.155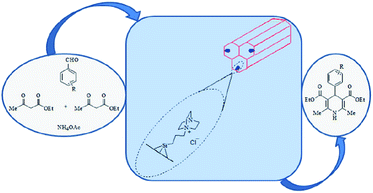 | ||
| Fig. 33 Application of SBA–DABCO as basic mesoporous catalyst for the synthesis of DHP derivatives. Reprinted from ref. 155 with permission of Elsevier. | ||
In another attempt, double-charged DABCO was grafted on Cl–SBA-15.156 The authors used the system for immobilizing Pd species (Fig. 34). The obtained catalyst, Pd@SBA-15/ILDABCO was used for promoting the Suzuki–Miyaura coupling reaction of aryl halide and arylboronic acid. The optimum reaction condition was using water as solvent in the presence of K2CO3 at 80 °C. Using aryl iodides the reactions could precede in short reaction times (1.5–2 h) to afford the desired biaryles in high yields (96–98%). In the case of aryl bromide, however, lower activity was observed and longer reaction times were required to furnish the desired products. Moreover, the catalyst was reusable and could catalyze at least 9 reaction cycles with only slight loss of catalytic activity.
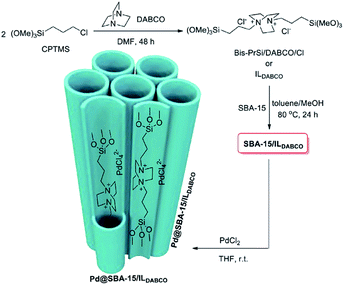 | ||
| Fig. 34 Schematic synthesis of Pd@SBA-15/ILDABCO. Reprinted from ref. 156 with permission of Elsevier. | ||
7. Functionalized SBA for incorporation of polymer
Using “grafting from” approach, Kustrowski et al. deposited poly(vinylamine) (PVAm) on various mesoporous silicas including (SBA-16, SBA-15 and MCF).157 To synthesis the catalyst, the vinyltriethoxysilane-functionalized silicas were copolymerized with N-vinylformamide followed by hydrolization and neutralization (Fig. 35). The authors believed that the porosity of the support affect the content and distribution of introduced poly(vinylamine). The smaller the pore size, the more difficult the dispersion of poly(vinylamine) would be. Investigating the catalytic activities of the catalysts in Knoevenagel condensation proved that the catalyst obtained from MCF possessed the best poly(vinylamine) dispersion and was the most efficient one.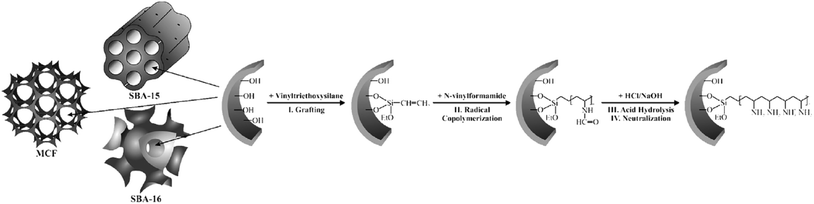 | ||
| Fig. 35 Functionalization of mesoporous silica supports (SBA-16, SBA-15 and MCF) with poly(vinylamine) by the “grafting from” method. Reprinted from ref. 157 with permission of Elsevier. | ||
8. Functionalized SBA for developing catalysts with hydrophobic and lipophilic properties
Using surface functionalization, the hydrophilicity/hydrophobicity of the SBA-based catalysts can be adjusted. This is especially important when the catalyst is water-sensitive and can be poisoned with water. In this case, incorporation of a hydrophobic moiety in SBA can furnish a solution. However, it must be taken into account that the steric features of the functionality can reduce the pore size of the SBA. In the following the recent advances in the field of controlling the hydrophobic and lipophilic properties of SBA-based catalysts is discussed.Diphenyldichlorosilane (DPCS) was used for functionalization of mesoporous SBA-15 silica. The functionalized SBA-15 was subsequently silylated and then sulfonated to afford a novel heterogeneous catalyst, (SBA-15–Ph-SO3H) (Fig. 36) for preparation of 2H-indazolo[2,1-b]phthalazine-trione and triazolo [1,2-a]indazole-trione derivatives.158 It was demonstrated that the sulfonic acid moieties of the hydrophobic nanoreactor were leaching-resistant in both aqueous and organic solvents. Moreover, the catalyst exhibited high reusability. Comparing the catalytic activity of this catalyst with those of some previously reported catalysts such as SBA-15–Ph-SO3H, N-bromosulfonamide, [bmim]BF4 and PEG–OSO3H established the superior performance of the novel catalyst. Noteworthy, the stability of the grafted sulfonic acid surface toward water was higher than that of silica sulfonic acid (SSA) and sulfonated SBA-15.
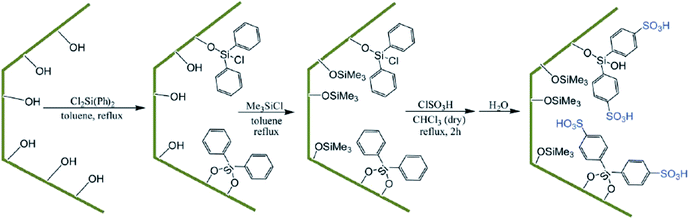 | ||
| Fig. 36 Preparation of SBA-15–Ph-SO3H. Reprinted from ref. 158 with permission of Royal Society of Chemistry. | ||
To address one of the most important challenges in the field of sulfonated mesoporous silica, Rostamnia et al. confined fluoroalkyl-chain alcohols (RFOH) such as trifluoroethanol or hexafluoroisopropanol as a moiety with both hydrophobic and lipophilic properties within SBA-15 to afford a novel water-tolerant catalyst (RFOH/SBA-15) with improved mass transfer for the synthesis of multi-functionalized imidazoles through multicomponent reaction (Fig. 37).159 Various amines and aldehydes could be successfully used in his protocol to furnish the corresponding imidazoles after 50 min in high yields (88–95%). The simplicity of the process, reusability of the catalyst and low amount of catalyst were the merits of this protocol.
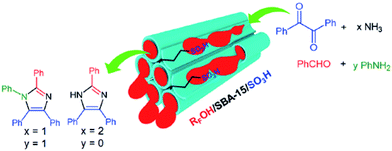 | ||
| Fig. 37 RFOH/SBA-15–Pr-SO3H for the synthesis of imidazoles. Reprinted from ref. 159 with permission of Thieme. | ||
Uracil-fused spirooxindole derivative were efficiently synthesized via three component reaction of isatins, 1,3-dimethyl-6-amino uracil, cyclic 1,3-diketones in the presence of a water-tolerant solid acid hybrid catalyst, SBA-15–PhSO3H, obtained from functionalization of SBA-15 with sulfanilic acid.160 The simplicity of the reaction, mild reaction condition (80 °C in the mixture of water and ethanol), low amount of the catalyst (10 mol%) and high yields were the merits of this atom economical methodology. Moreover, the catalyst was reusable and could be recovered and reused up to five times with only slight loss of the catalytic activity.
In summary, the development of water-tolerant SBA-based catalyst with high mass transport can be achieved by proper functionalization of SBA with functional groups with hydrophobic properties. These catalysts exhibited high performance in various organic transformations under mild and green condition.
9. Functionalized SBA for developing chiral catalysts
One of the main challenges in immobilization of chiral catalysts is preserving their enantioselectivity and activity.161,162 In this context, cage-like SBA can be considered as an effective nanoreactor for encapsulation of this class of catalyst. One of the advantageous of SBA mesoporous silica is the possibility of tuning their characteristics such as surface polarity, electrostatic features and hydrophobicity/hydrophilicity. Furthermore, the ingress and egress of the substrate and product through the 3D interconnected pores of SBA could readily occur. To date, various heterogeneous catalysts with utilities for promoting asymmetric reactions have been developed by confining catalytic species such as metal complexes within the cavity of SBA. In some case, the observed catalytic activity was comparative to the homogeneous catalyst.163 Herein, some recent advances are explained in more detail to disclose the merit of use of SBA as a nanoreactor.In an initiative research Wang, Ma et al. developed a chiral and metal-free catalyst based on functionalization of cage-like mesoporous material SBA-16 with L-4-hydroxyproline (Fig. 38). The hybrid system was used as an efficient and reusable catalyst for asymmetric aldol reaction of aldehyde acceptors and ketone donors. High diastereoselectivities and enantioselectivities, good yield, broad substrate scope, avoiding heavy metal pollution; reusability (up to 5 reaction runs) and mechanical strength of the catalyst were the merits of this protocol.164
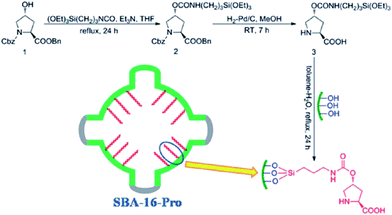 | ||
| Fig. 38 Preparation of the catalyst SBA-16-pro. Reprinted from ref. 164 with permission of Royal Society of Chemistry. | ||
CuI was immobilized on functionalized 3D-hexagonally ordered SBA-16, the obtained hybrid system was used as an efficient catalyst, SBA-16–pro-Cu(I), for direct oxidative synthesis of various α-ketoamides with no need to any additive. The catalyst preparation procedure is depicted in Fig. 39. The catalyst could effectively lead to formation of the desired products under mild reaction condition in high yields. Noteworthy, the air was used as the oxygen source. The catalyst could be easily recovered and reused with preserving its catalytic activity.165
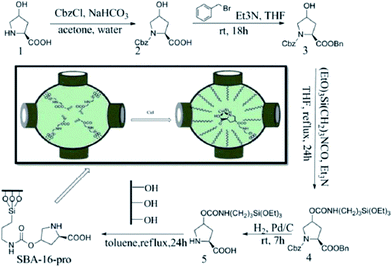 | ||
| Fig. 39 The preparation of the SBA-16–pro-Cu(I). Reprinted from ref. 165 with permission of Royal Society of Chemistry. | ||
To achieve chiral catalysts, chiral ligands can be incorporated in the cavity of SBA. These chiral functionalized SBA can also be used for incorporation of metallic species. The results established that this class of catalysts could promote the reactions with high diastereoselectivities and enantioselectivities. The presence of SBA could improve the mechanical strength of the catalyst and protect it from hash reaction condition. Notably, the reusability of this class of chiral catalyst was high and they could be reused for several consecutive reaction runs.
10. Miscellaneous catalysts
Besides the discussed issues, other catalytic species such as enzymes166,167 have been also incorporated into the functionalized SBA.Mesoporous silica SBA-15 was also functionalized with 8-hydroxyquinoline-5-sulfonic acid (HQS–SBA-15) through initial iodo-functionalization of SBA-15 and subsequent reaction with 8-hydroxyquinoline-5-sulphonic acid. A catalytic amount of the novel catalyst (0.02 g) was used for promoting reaction of amino acid methylesters and isothiocyanates to afford 2-thiohydantoins under solvent-less condition (Fig. 40).168 The proposed reaction mechanism included activation of thiocyanate with the catalyst followed by nucleophilic addition of the α-amino ester and formation of an intermediate. The desired product could be obtained by intramolecular cyclization. Noteworthy, the catalyst was reusable and could be recovered and reused without significant loss of activity.
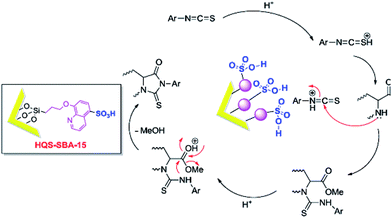 | ||
| Fig. 40 A plausible mechanism for the synthesis of 2-thiohydantoin in the presence of HQS–SBA-15. Reprinted from ref. 168 with permission of Elsevier. | ||
Kiasat et al. designed a novel nanocomposite based on synthesis of chloro-functionalized SBA-15, SBA–Cl, through co-condensation of TEOS and CPTMS precursors and subsequent nucleophilic substitution with imidazole and then quaternization with 2-bromo ethylamine hydrobromide to afford SBA–Im-NH2. The hybrid system was used as an efficient catalyst for ultrasonic-assisted synthesis of wide range of benzopyranopyrimidines through one-pot pseudo four-components reaction of salicylaldehydes, malononitrile and secondary amines under solvent-less condition at ambient temperature.169 It is worth noting that the catalyst could be easily recovered and reused with negligible loss of catalytic activity. Rostamnia et al. used amine-functionalized SBA-15 for immobilizing SO3H moiety. The obtained catalyst, SBA-15/NHSO3H (Fig. 41) served as ionic liquid type catalyst for promoting synthesis of polyhydroquinolines through four-component reaction of dimedone, benzaldehyde, NH4OAc, and methyl acetylacetonate under solvent-free condition and dihydropyridines via reaction of aldehydes, ammonium acetate and β-dicarbonyl compounds. The results established that the desired products could be furnished in high yields and relatively short reaction time. Broad substrate scope as well as catalyst reusability were the merit of this protocol.170
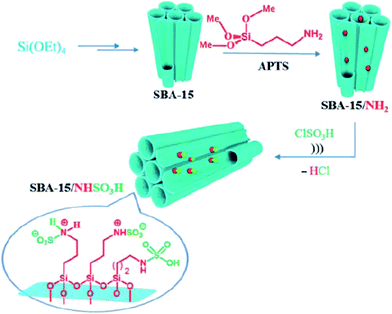 | ||
| Fig. 41 Schematic representation of prepared SBA-15/NHSO3H. Reprinted from ref. 170 with permission of Thieme. | ||
In an initiative research, glycidoxypropyl-SBA-15 was coupled with N,N-dihydroxypyromellitimide (NDHPI). Moreover, SBA-15 functionalized with N-hydroxyphthalimide (NHPI) was obtained through impregnation method (Fig. 42).171 To study and compare the catalytic activities of the two catalysts, they were applied together with N,N-bis(salicylidene)ethylenediiminocobalt (Cosalen) complex for oxidation of toluene with molecular oxygen. The results established that the catalytic activity of NDHPI/propyl-2-propanol–SBA-15 was superior to SBA-15-immobilized NHPI. The NDHPI/propyl-2-propanol–SBA-15 was reusable and led to a favorable product distribution with total selectivity of benzaldehyde and benzyl alcohol to 46.0% with the conversion of toluene 14.8% at 90 °C for 7 h.
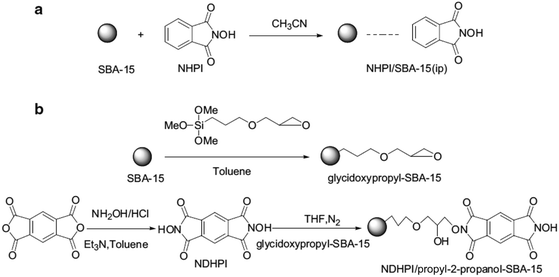 | ||
| Fig. 42 The synthesis routes, (a) for NHPI/SBA-15 (ip) and (b) for NDHPI/propyl-2-propanol–SBA-15. Reprinted from ref. 171 with permission of Springer. | ||
Using a two-step pore surface-confined polymerization technique Kleitz et al. developed novel composites based on mesoporous amine-functionalized polymer–silica and studied their base catalytic properties in model aldol-type reactions including Michael addition, Knoevenagel reaction, Henry reaction and Claisen–Schmidt condensations. The authors demonstrated the superior catalytic activity of the composites compared to references samples when comparison was made based on the amine content.172
The synthesis of aromatic linear polyester amides was catalyzed by a novel catalyst prepared through incorporation of Pd into the ionic liquid modified SBA-15 (Fig. 43). The carbonylation–polycondensation reaction proceeded with no need to phosphine ligand. Moreover, the catalyst was reusable and could be applied for four reaction runs with slight loss of activity.173 This observation was justified by TEM and XPS analyses of the fresh and reused catalysts. The results established that the Pd2+ species did not reduced in the course of the process. Moreover, in TEM mages Pd0 black particles were not detected ruling out the possibility of reduction and aggregation of Pd2+ species.
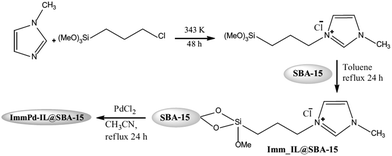 | ||
| Fig. 43 Synthetic steps for the ImmPd-IL@SBA-15 catalyst. Reprinted from ref. 173 with permission of Springer. | ||
11. Challenges
To date various hybrid catalysts based on functionalized SBA have been introduced and successfully used for promoting various organic reactions. In this regard, some issues must be taken into account for a successful synthesis of the hybrid catalysts.(1) The pore size of the SBA must be suitable and large enough to accommodate a definite catalytic species. This factor can be controlled by incorporation of specific functionalities. Moreover, engross and ingress of the products and substrates through the cavities of SBA must be feasible.
(2) The hydrophobicity/hydrophilicity of SBA must be adjusted according to the catalytic purposes. This can be achieved by using specific functionalities. Notably, the functionalization should be done in a way that does not affect the pore size and mass transport.
(3) Sometimes, the functionalization of SBA must be performed via a multi-step process. This may make the synthetic process time-consuming and costly. Moreover, the purification must be performed in each step.
(4) The functionalities must be efficient enough for anchoring the catalytic species via non-covalent interactions and reduce the leaching of the catalytic species.
(5) The incorporation of the catalytic species must be performed in a way that does not lead to the collapse of SBA structure. Moreover, the encapsulation should not block the pores of SBA.
(6) In the case of encapsulation of chiral catalysts/ligands within SBA structure, the process should be accomplished in the way that does not diminish the diastereoselectivities and enantioselectivities.
Notably, the catalytic activity of the SBA-based hybrid catalyst depends on various factors such as the nature of the encapsulated species, the accessibility of substrate to the encapsulated catalyst, product inhibition, the efficiency of the SBA surface functional groups on immobilization of the catalytic activity, the loading of the catalytic species, the nature of SBA, the synergistic effects between SBA and the encapsulated catalytic species, the synthetic route of the catalyst etc. Hence, for successful design of SBA-based catalysts, one should consider all these factors.
12. Conclusions
In this review article, the utility of functionalized SBA for designing novel and more sophisticated encapsulated catalysts through incorporation of an additional catalytic species such as nanoparticles, heteropolyacids and complexes or functionality has been disclosed. The results established high potential of functionalized SBA for developing state of the art catalysts. In most cases low leaching of the immobilized catalytic species and high reusability of the catalyst were reported which can emerged from the interaction of catalytic species with the functionalities immobilized on the surface of SBA. Moreover, using functionalized SBA as support, improved dispersion and less aggregation of nanoparticles were observed. As the pore size of the SBA can be controlled by incorporating various functionalities, the access of the reagents to the confined catalytic species can be achieved. Hope this review article motivates more research on this research field.Acknowledgements
The authors appreciate partial financial supports from Alzahra University and Iran Polymer and Petrochemical Institute. MMH is also thankful to Iran National Science Foundation for the Individual given grant.Notes and references
- S. Sadjadi, Encapsulated Catalyst, Elsevier, 2017 Search PubMed.
- C. Deraedt, L. Salmon and D. Astruc, Adv. Synth. Catal., 2014, 356, 2525 CrossRef CAS.
- L. Chen, B. Huang, X. Qiu, X. Wang, R. Luque and Y. Li, Chem. Sci., 2016, 7, 228 RSC.
- D. A. Gomez-Gualdron, S. T. Dix, R. B. Getman and R. Q. Snurr, Phys. Chem. Chem. Phys., 2015, 17, 27596 RSC.
- T. Baie, H. A. Oskooie, T. Hosseinnejad and M. M. Heravi, J. Coord. Chem., 2017 DOI:10.1080/00958972.2017.1321742.
- T. Hossiennejad, M. Darai, M. M. Heravi and N. N. Tajoddin, J. Inorg. Organomet. Polym. Mater., 2017 DOI:10.1007/s10904-017-0530-z.
- S. Sadjadi, M. M. Heravi and M. Daraie, Res. Chem. Intermed., 2017, 43, 843 CrossRef CAS.
- M. M. Heravi, E. Hashemi, Y. Shirazi Beheshtiha, S. Ahmadi and T. Hosseinnejad, J. Mol. Catal., 2014, 394, 74 CrossRef CAS.
- S. Sadjadi, M. M. Heravi and M. Daraie, J. Mol. Liq., 2017, 231, 98 CrossRef CAS.
- E. Hashemi, Y. S. Beheshtiha, S. Ahmadi and M. M. Heravi, Transition Met. Chem., 2014, 39, 593 CrossRef CAS.
- S. Sadjadi, M. M. Heravi and M. Daraie, Res. Chem. Intermed., 2017, 43, 2201 CrossRef CAS.
- F. Wang, J. Ren, Y. Cai, L. Sun, C. Chen, S. Liang and X. Jiang, Chem. Eng. J., 2016, 283, 922 CrossRef CAS.
- S. Sadjadi and M. M. Heravi, RSC Adv., 2016, 6, 88588 RSC.
- A. Chirieac, B. Dragoi, A. Ungureanu, C. Ciotonea, I. Mazilu, S. Royer, A. S. Mamede, E. Rombi, I. Ferino and E. Dumitriu, J. Catal., 2016, 339, 270 CrossRef CAS.
- Q. Yang, F. Gu, Y. Tang, H. Zhang, Q. Liu, Z. Zhong and F. Su, RSC Adv., 2015, 5, 26815 RSC.
- P. Cruz, Y. Pérez, I. del Hierro, R. Fernández-Galán and M. Fajardo, RSC Adv., 2016, 6, 19723 RSC.
- G. Mohammadi Ziarani, P. Hajiabbasi and A. Badiei, J. Iran. Chem. Soc., 2015, 12, 1649 CrossRef CAS.
- B. Karimi, S. Vahdati and H. Vali, RSC Adv., 2016, 6, 63717 RSC.
- Y. Noda, K. Li, A. M. Engler, W. Elliot and R. M. Rioux, Catal. Sci. Technol., 2016, 6, 5961 CAS.
- F. Rajabi, D. Schaffner, S. Follmann, C. Wilhelm, S. Ernst and W. R. Thiel, ChemCatChem, 2015, 7, 3513 CrossRef CAS.
- H. Albuquerque, L. Carneiro, A. P. Carvalho, J. Pires and A. R. Silva, Polyhedron, 2014, 79, 315 CrossRef CAS.
- B. Karimi, E. Farhangi, H. Vali and S. Vahdati, ChemSusChem, 2014, 7, 2735 CrossRef CAS PubMed.
- T. Wang, X. Yuan, S. Li, L. Zeng and J. Gong, Nanoscale, 2015, 7, 7593 RSC.
- T. Baskaran, R. Kumaravel, J. Christopher, T. G. Ajithkumar and A. Sakthivel, New J. Chem., 2015, 39, 3758 RSC.
- P.-Y. Hoo and A. Z. Abdullah, Chem. Eng. J., 2014, 250, 274 CrossRef CAS.
- M. Rocquin, M. Henrion, M.-G. Willinger, P. Bertani, M. J. Chetcuti, B. Louis and V. Ritleng, Dalton Trans., 2014, 43, 3722 RSC.
- G. Mohammadi Ziarani, N. Lashgari and A. Badiei, J. Mol. Catal., 2015, 397, 166 CrossRef CAS.
- J. Liu, Y. Liu, W. Yang, H. Guo, F. Fang and Z. Tang, J. Mol. Catal. A: Chem., 2014, 393, 1 CrossRef CAS.
- I. Kim, J. Kim and D. Lee, Appl. Catal., B, 2014, 148–149, 295 CrossRef CAS.
- H. Veisi, A. Naeimi, B. Maleki, S. Sedigh Ashrafi and A. Sedrpoushan, Org. Prep. Proced. Int., 2015, 47, 1 CrossRef.
- W. Sun and J. Hu, React. Kinet., Mech. Catal., 2016, 119, 305 CrossRef CAS.
- R. H. Vekariya and H. D. Patel, Synth. Commun., 2015, 45, 1031 CrossRef CAS.
- G. Mohammadi Ziarani, N. Hosseini Mohtasham, N. Lashgari and A. Badiei, Res. Chem. Intermed., 2015, 41, 7581 CrossRef CAS.
- G. Mohammadi Ziarani, M. Azizi, P. Hajiabbasi and A. Badiei, Quim. Nova, 2015, 38, 466 Search PubMed.
- G. Mohammadi Ziarani, A. Badiei, Z. Aslani and N. Lashgari, Arabian J. Chem., 2015, 8, 54 CrossRef CAS.
- L. Ma'mani, E. Hajihosseini, M. Saeedi, M. Mahdavi, A. Asadipour, L. Firoozpour, A. Shafiee and A. Foroumadi, Synth. React. Inorg. Met.-Org. Chem., 2016, 46, 306–310 CrossRef.
- L. Frattini, M. A. Isaacs, C. M. A. Parlett, K. Wilson, G. Kyriakou and A. F. Lee, Appl. Catal., B, 2017, 200, 10 CrossRef CAS.
- A. Taguchi, Y. Kato, R. Akai and Y. Torikai, Fusion Eng. Des., 2016, 111, 255–259 CrossRef.
- M. Popova, I. Trendafilova, A. Szegedi, J. Mihaly, P. Nemeth, S. G. Marinova, H. A. Aleksandrov and G. N. Vayssilov, Microporous Mesoporous Mater., 2016, 228, 256 CrossRef CAS.
- W. Xie and C. Zhang, Food Chem., 2016, 211, 74 CrossRef CAS PubMed.
- S.-Y. Chen, S. Lao-ubol, T. Mochizuki, Y. Abe, M. Toba and Y. Yoshimura, Appl. Catal., A, 2014, 485, 28 CrossRef CAS.
- S.-Y. Chen, S. Lao-ubol, T. Mochizuki, Y. Abe, M. Toba and Y. Yoshimura, Bioresour. Technol., 2014, 157, 346 CrossRef CAS PubMed.
- W. Xie, X. Yang and X. Zang, J. Am. Oil Chem. Soc., 2015, 92, 915–925 CrossRef CAS.
- G. Morales, M. Paniagua, J. A. Melero and J. Iglesias, Catal. Today, 2017, 279, 305–316 CrossRef CAS.
- R. Fazaeli and H. Aliyan, Russ. J. Appl. Chem., 2015, 88, 676–681 CrossRef CAS.
- Ö. D. Bozkurt, F. M. Tunç, N. Bağlar, S. Çelebi, İ. D. Günbaş and A. Uzun, Fuel Process. Technol., 2015, 138, 780 CrossRef.
- S. A. Ganiyu, K. Alhooshani and S. A. Ali, Appl. Catal., B, 2017, 203, 428 CrossRef CAS.
- M. Tao, Z. Xin, X. Meng, Z. Bian and Y. Lv, Fuel, 2017, 188, 267 CrossRef CAS.
- W. Chen, X. Li, Z. Pan, S. Ma and L. Li, Sep. Purif. Technol., 2017, 173, 99 CrossRef CAS.
- L. Konga, X. Zhoua, Y. Yaoa, P. Jiana and G. Diaoa, Environ. Technol., 2016, 37, 422 CrossRef PubMed.
- B. O. Dalla Costa, M. S. Legnoverde, C. Lago, H. P. Decolatti and C. A. Querini, Microporous Mesoporous Mater., 2016, 230, 66 CrossRef CAS.
- V. Calvino-Casilda, M. Olejniczak, R. M. Martín-Aranda and M. Ziolek, Microporous Mesoporous Mater., 2016, 224, 201 CrossRef CAS.
- A. Zuhairi Abdullah, Z. Gholami, M. Ayoub and F. Gholami, Chem. Eng. Commun., 2016, 203, 496–504 CrossRef.
- P. Hajiabbasi, G. Mohammadi Ziarani, A. Badiei and A. Abolhasani Soorki, J. Iran. Chem. Soc., 2015, 12, 57 CrossRef CAS.
- G. Mohammadi Ziarani, N. Hosseini Mohtasham, A. Badiei and N. Lashgari, J. Chin. Chem. Soc., 2014, 61, 990 CrossRef.
- W. Xie, C. Qi, H. Wang and Y. Liu, Fuel Process. Technol., 2014, 119, 98 CrossRef CAS.
- K. Liu, Z. Chen, Z. Hou, Y. Wang and L. Dai, Catal. Lett., 2014, 144, 935 CrossRef CAS.
- K. A. Shah, J. K. Parikh and K. C. Maheria, Catal. Today, 2014, 237, 29 CrossRef CAS.
- G. Mohammadi Ziarani, S. Faramarzi, N. Lashgari and A. Badiei, J. Iran. Chem. Soc., 2014, 11, 701 CrossRef CAS.
- M. Trejda, B. Pokora and M. Ziolek, Catal. Today, 2015, 254, 104 CrossRef CAS.
- J. A. Melero, L. F. Bautista, J. Iglesias, G. Morales, R. Sánchez-Vázquez, K. Wilson and A. F. Lee, Appl. Catal., A, 2014, 488, 111 CrossRef CAS.
- N. Lashgari, G. Mohammadi Ziarani, A. Badiei and M. Zarezadeh-Mehrizi, J. Heterocycl. Chem., 2014, 51, 628 CrossRef.
- V. Gascón, C. Márquez-Álvarez and R. M. Blanco, Appl. Catal., A, 2014, 482, 116 CrossRef.
- E. S. Sanz-Pérez, T. C. M. Dantas, A. Arencibi, G. Calleja, A. P. M. A. Guedes, A. S. Araujo and R. Sanz, Chem. Eng. J., 2017, 308, 1021 CrossRef.
- R. L. Oliveira, J. B. F. Hooijmans, P. E. de Jongh, R. J. M. Klein Gebbink and K. P. de Jong, ChemCatChem, 2014, 6, 1 CrossRef.
- W. Xie, X. Yang and M. Fan, Renewable Energy, 2015, 80, 230 CrossRef CAS.
- G. R. Wilson and A. Dubey, J. Chem. Sci., 2016, 128, 1285 CrossRef CAS.
- B. Castanheira, F. de Jesus Trindade, L. dos Santos Andrade, I. Lourenço Nantes, M. José Politi, E. Rezende Triboni and S. Brochsztain, J. Photochem. Photobiol., A, 2017, 332, 316 CrossRef CAS.
- S. Sadjadi, M. M. Heravi, V. Zadsirjan and M. Ebrahimzadeh, Res. Chem. Intermed. Search PubMed , in press..
- F. Rajabi, A. Feiz and R. Luque, Catal. Lett., 2015, 145, 1621 CrossRef CAS.
- S. Ravi, R. Roshan, J. Tharun, A. C. Kathalikkattil and D. W. Park, J. CO2 Util., 2015, 10, 88 CrossRef CAS.
- J. Davarpanah, P. Rezaee and S. Elahi, Res. Chem. Intermed., 2015, 41, 9903 CrossRef CAS.
- N. Mittal, G. M. Nisola, J. G. Seo, S.-P. Lee and W.-J. Chung, J. Mol. Catal. A: Chem., 2015, 404–405, 106 CrossRef CAS.
- W. Xie and C. Qi, J. Agric. Food Chem., 2014, 62, 3348–3355 CrossRef CAS PubMed.
- F. Rajabi, S. De and R. Luque, Catal. Lett., 2015, 145, 1566 CrossRef CAS.
- C. Lavenn, A. Demessence and A. Tuel, J. Catal., 2015, 322, 130 CrossRef CAS.
- S.-H. Huang, C.-H. Liu and C.-M. Yang, Green Chem., 2014, 16, 2706 RSC.
- C. K. P. Neeli, R. K. Marella, S. Ganji, K. S. R. Rao and D. R. Burri, J. Chem. Technol. Biotechnol., 2015, 90, 1657–1664 CrossRef CAS.
- S. Ganji, S. Sankar Enumula, R. Kumar Marella, K. Seetha Rama Rao and D. Raju Burri, Catal. Sci. Technol., 2014, 4, 1813 CAS.
- S. H. Kim and Y. S. Ko, J. Nanosci. Nanotechnol., 2014, 14, 3073 CrossRef CAS PubMed.
- M. Bagherzadeh, M. Zare, M. Amini, T. Salemnoush, S. Akbayrak and S. Özkar, J. Mol. Catal. A: Chem., 2014, 395, 470 CrossRef CAS.
- X. Liu, J. Xiao, H. Ding, W. Zhong, Q. Xu, S. Su and D. Yin, Chem. Eng. J., 2016, 283, 1315 CrossRef CAS.
- J. Mondal, P. Borah, A. Modak, Y. Zhao and A. Bhaumik, Org. Process Res. Dev., 2014, 18, 257–265 CrossRef CAS.
- J. Mondal, S. Sreejith, P. Borah and Y. Zhao, ACS Sustainable Chem. Eng., 2014, 2, 934 CrossRef CAS.
- M. Ahmed and A. Sakthivel, J. Mol. Catal. A: Chem., 2016, 424, 85 CrossRef CAS.
- M. Mohammadi, G. Rezanejade Bardajee and N. Noroozi Pesyan, RSC Adv., 2014, 4, 62888 RSC.
- S. Rostamnia and E. Doustkhah, RSC Adv., 2014, 4, 28238 RSC.
- B. Karimi, F. Mansouri and M. Khorasani, Curr. Org. Chem., 2016, 20, 349 CrossRef CAS.
- G. M. Ziarani, N. L. Alireza and A. Badiei, J. Mol. Catal. A: Chem., 2015, 397, 166 CrossRef.
- M. M. Heravi, T. Hosseinnejad and N. Nazari, Can. J. Chem., 2017 DOI:10.1139/cjc-2016-0507.
- R. Mirsafaei, M. M. Heravi, S. Ahmadi, M. H. Moslemin and T. Hosseinnejad, J. Mol. Catal., 2015, 402, 100 CrossRef CAS.
- J. Zhang, P. Jiang, Y. Shen, G. Zhao, W. Zhang and G. Bian, Aust. J. Chem., 2016, 69, 817 CrossRef CAS.
- A. Lazar, W. R. Thiel and A. P. Singh, RSC Adv., 2014, 4, 14063 RSC.
- M. Zare, Z. Moradi-Shoeili, F. Ashouri and M. Bagherzadeh, Catal. Commun., 2017, 88, 9 CrossRef CAS.
- V. Mahdavi and M. Mardani, Res. Chem. Intermed., 2015, 41, 8907 CrossRef CAS.
- H. Mahmoudi and R. Malakooti, React. Kinet., Mech. Catal., 2014, 113, 241 CrossRef CAS.
- Y. Bao, H. Jiang, W. Xing, R. Chen and Y. Fan, React. Kinet., Mech. Catal., 2015, 116, 535 CrossRef CAS.
- P. Bhanja, R. Gomes, L. Satyanarayana and A. Bhaumik, J. Mol. Catal. A: Chem., 2016, 415, 104 CrossRef CAS.
- L. Paul, B. Banerjee, A. Bhaumik and M. Ali, J. Solid State Chem., 2016, 237, 105 CrossRef CAS.
- T. Baskaran, R. Kumaravel, J. Christopher, S. Radhakrishnan and A. Sakthivel, Catal. Lett., 2015, 145, 851 CrossRef CAS.
- L. Qu, J. Cai and Q. Chen, RSC Adv., 2016, 6, 14416 RSC.
- J. Zhang, P. Jiang, Y. Shen, W. Zhang and G. Bian, J. Porous Mater., 2016, 23, 431 CrossRef CAS.
- S. Rayati, Z. Sheybanifard, M. M. Amini and A. Aliakbari, J. Mol. Catal., 2016, 423, 105 CrossRef CAS.
- G. Rezanejade Bardajee, M. Mohammadi and N. Kakavand, Appl. Organomet. Chem., 2016, 30, 51 CrossRef.
- P. Kalita, A. V. Baskar, J.-H. Choy, K. S. Lakhi, M. El-Newehy, G. Lawrence, S. S. Al-deyab, V. V. Balasubramanian and A. Vinu, ChemCatChem, 2016, 8, 336 CrossRef CAS.
- A. Pathak and A. P. Singh, J. Porous Mater., 2017, 24, 327–340 CrossRef CAS.
- P. Sharma and A. P. Singh, Catal. Sci. Technol., 2014, 4, 2978 CAS.
- C. Singh, K. Jawade, P. Sharma, A. P. Singh and P. Kumar, Catal. Commun., 2015, 69, 11 CrossRef CAS.
- P. Sharma and A. P. Singh, RSC Adv., 2014, 4, 58467 RSC.
- S. Rostamnia, H. Golchin Hossieni and E. Doustkhah, J. Organomet. Chem., 2015, 791, 18 CrossRef CAS.
- S. Rostamnia, E. Doustkhah, R. Bulgar and B. Zeynizadeh, Microporous Mesoporous Mater., 2016, 225, 272 CrossRef CAS.
- S. M. Sarkar, M. L. Rahman and M. M. Yusoff, RSC Adv., 2015, 5, 1295 RSC.
- R. Ghorbani-Vaghei, S. Hemmati and H. Veisi, J. Mol. Catal. A: Chem., 2014, 393, 240 CrossRef CAS.
- H. Veisia, M. Hameliana and S. Hemmati, J. Mol. Catal. A: Chem., 2014, 395, 25 CrossRef.
- S. Roy, T. Chatterjee, M. Pramanik, A. Singha Roy, A. Bhaumik and S. Manirul Islam, J. Mol. Catal. A: Chem., 2014, 386, 78 CrossRef CAS.
- S. M. Sadeghzadeh, RSC Adv., 2015, 5, 68947 RSC.
- A. Najafian, R. Rahimi, S. Zargari, M. Mahjoub-Moghaddas and A. Nazemi, Res. Chem. Intermed., 2016, 42, 3441 CrossRef CAS.
- V. H. A. Pinto, J. S. Reboucas, G. M. Ucoski, E. H. de Faria, B. F. Ferreira, R. A. S. San Gil and S. Nakagaki, Appl. Catal., A, 2016, 526, 9 CrossRef CAS.
- L. D. Zanatta, I. A. Barbosa, F. B. Zanardi, P. C. de Sousa Filho, L. B. Bolzon, A. P. Ramos, O. A. Serra and Y. Iamamoto, RSC Adv., 2016, 6, 104886 RSC.
- A. Najafian, M. Rabbani, R. Rahimi, M. Deilamkamar and A. Malek, Solid State Sci., 2015, 46, 7 CrossRef CAS.
- H. Veisi, A. A. Manesh, N. Eivazi and A. R. Faraji, RSC Adv., 2015, 5, 20098 RSC.
- S. Rostamnia, T. Rahmani and H. Xin, J. Ind. Eng. Chem., 2015, 32, 218 CrossRef CAS.
- S. Rostamnia and T. Rahmani, Appl. Organomet. Chem., 2015, 29, 471 CrossRef CAS.
- R. L. Oliveira, W. He, R. J. M. Klein Gebbink and K. P. de Jong, Catal. Sci. Technol., 2015, 5, 1919 CAS.
- R. Ghorbani-Vaghei, S. Hemmati, M. Hamelian and H. Veisi, Appl. Organomet. Chem., 2015, 29, 195 CrossRef CAS.
- K. Koh, J.-E. Seo, J. H. Lee, A. Goswami, C. W. Yoon and T. Asefa, J. Mater. Chem. A, 2014, 2, 20444 CAS.
- L. Xu, B. Jin, J. Zhang, D.-g. Cheng, F. Chen, Y. An, P. Cui and C. Wan, RSC Adv., 2016, 6, 46908 RSC.
- A. Saad, Y. Snoussi, M. Abderrabba and M. M. Chehimi, RSC Adv., 2016, 6, 57672 RSC.
- D. Saikia, Y.-Y. Huang, C.-E. Wu and H.-M. Kao, RSC Adv., 2016, 6, 35167 RSC.
- P. Cruz, Y. Perez, I. del Hierro and M. Fajardo, Microporous Mesoporous Mater., 2016, 220, 136e147 CrossRef.
- J. P. Vita Damasceno, C. Marchetti Maroneze, M. Strauss, F. Aparecido Sigoli and I. Odone Mazali, New J. Chem., 2016, 40, 6636 RSC.
- C. K. Prasad Neeli, S. Ganji, V. S. Prasad Ganjala, S. R. Rao Kamaraju and D. R. Burri, RSC Adv., 2014, 4, 14128 RSC.
- F. Zhu and H. Li, Chin. J. Chem., 2014, 32, 1072 CrossRef CAS.
- R. J. Kalbasi and F. Zamani, RSC Adv., 2014, 4, 7444 RSC.
- H. Wang, Y. Zhang, Y. Guo, L. Zhang, Y. Han and X. Zhao, RSC Adv., 2016, 6, 38176 RSC.
- M. S. Tiwari and G. D. Yadav, Chem. Eng. J., 2015, 266, 64 CrossRef CAS.
- K. Pamin, M. Pronczuk, S. Basąg, W. Kubiak, Z. Sojka and J. Połtowicz, Inorg. Chem. Commun., 2015, 59, 13 CrossRef CAS.
- L. Hong, Y. Gui, J. Lu, J. Hu, J. Yuan and L. Niu, Int. J. Hydrogen Energy, 2013, 38, 11074 CrossRef CAS.
- Y. Zhu, M. Zhu, L. Kang, F. Yu and B. Dai, Ind. Eng. Chem. Res., 2015, 54, 2040–2047 CrossRef CAS.
- J. J. Walsh, A. M. Bond, R. J. Forster and T. E. Keyes, Coord. Chem. Rev., 2016, 306, 217 CrossRef CAS.
- J. H. Choi, T. H. Kang, Y. Bang, J. H. Song and I. K. Song, Catal. Commun., 2014, 55, 29 CrossRef CAS.
- S.-S. Wang and G.-Y. Yang, Chem. Rev., 2015, 115, 4893 CrossRef CAS PubMed.
- G. D. Yadav and D. P. Tekale, Catal. Today, 2014, 237, 54 CrossRef CAS.
- B. A. Dar, A. K. Sahu, P. Patidar, P. R. Sharma, N. Mupparapu, D. Vyas, S. Maity, M. Sharma and B. Singh, J. Ind. Eng. Chem., 2013, 19, 407 CrossRef CAS.
- S. Sadjadi and M. M. Heravi, Curr. Org. Chem., 2016, 20, 1404 CrossRef CAS.
- X. Sheng, Y. Zhou, Y. Yang, Y. Zhang, Z. Zhang, S. Zhou, X. Fu and S. Zhao, RSC Adv., 2014, 4, 30697 RSC.
- J. Xiong, W. Zhu, W. Ding, L. Yang, Y. Chao, H. Li, F. Zhu and H. Li, Ind. Eng. Chem. Res., 2014, 53, 19895 CrossRef CAS.
- F. Bentaleb, O. Makrygenni, D. Brouri, C. Coelho Diogo, A. Mehdi, A. Proust, F. Launay and R. Villanneau, Inorg. Chem., 2015, 54, 7607 CrossRef CAS PubMed.
- X. Song, W. Zhu, K. Li, J. Wang, H. Niu, H. Gao, W. Gao, W. Zhang, J. Yu and M. Jia, Catal. Today, 2016, 259, 59 CrossRef CAS.
- Y. Zhang, L. Wu, X. Dong, P. Wu, H. Hu and G. Xue, Catal. Lett., 2016, 146, 2468 CrossRef CAS.
- J. Davarpanah and A. R. Kiasat, RSC Adv., 2014, 4, 4403 RSC.
- R. Baharfar and R. Azimi, Synth. Commun., 2014, 44, 89–100 CrossRef CAS.
- R. Baharfar, H. Alinezhad and R. Azimi, Res. Chem. Intermed., 2015, 41, 8637 CrossRef CAS.
- R. Azimi and R. Baarfar, Can. J. Chem., 2014, 92, 1163 CrossRef CAS.
- A. R. Kiasat and J. Davarpanah, Catal. Commun., 2015, 69, 179 CrossRef CAS.
- S. Rostamnia, E. Doustkhah and B. Zeynizadeh, Microporous Mesoporous Mater., 2016, 222, 87 CrossRef CAS.
- A. Wach, M. Drozdek, B. Dudek, P. Latka and P. Kustrowski, Microporous Mesoporous Mater., 2016, 226, 433 CrossRef CAS.
- H. Veisi, A. Sedrpoushan, A. R. Faraji, M. Heydari, S. Hemmati and B. Fatahi, RSC Adv., 2015, 5, 68523 RSC.
- S. Rostamnia and E. Doustkhah, Synlett, 2015, 26, 1345 CrossRef CAS.
- R. Baharfar and R. Azimi, J. Chem. Sci., 2015, 127, 1389 CrossRef CAS.
- H. Yang, L. Zhang, W. Su, Q. Yang and C. Li, J. Catal., 2007, 248, 204 CrossRef CAS.
- H. Yang, L. Zhang, P. Wang, Q. Yang and C. Li, Green Chem., 2009, 11, 257 RSC.
- H. Yang, J. Li, J. Yang, Z. Liu, Q. Yang and C. Li, Chem. Commun., 2007, 1086 RSC.
- H. Yang, X. Zhang, S. Li, X. Wang and J. Ma, RSC Adv., 2014, 4, 9292 RSC.
- X. Zhang, H. Yang, Y. Huo, J. Li, J. Ma and J. Ma, Dalton Trans., 2016, 45, 8972 RSC.
- L. F. Bautista, G. Morales and R. Sanz, Chemosphere, 2015, 136, 273 CrossRef CAS PubMed.
- M. Babaki, M. Yousefi, Z. Habibi, M. Mohammadi, P. Yousefi, J. Mohammadi and J. Brask, Renewable Energy, 2016, 91, 196 CrossRef CAS.
- V. Fathi Vavsari, G. Mohammadi Ziarani, S. Balalaie, A. Latifi, M. Karimi and A. Badiei, Tetrahedron, 2016, 72, 5420 CrossRef.
- M. Jafari Nasab and A. R. Kiasat, RSC Adv., 2015, 5, 75491 RSC.
- S. Rostamnia and A. Hassankhani, Synlett, 2014, 25, 2753 CrossRef CAS.
- M. Zhou, X. Li, L. Bao, X. Yuan and H. Luo, Catal. Lett., 2016, 146, 383 CrossRef CAS.
- L. Marcoux, J. Florek and F. Kleitz, Appl. Catal., A, 2015, 504, 493 CrossRef CAS.
- A. Satapathy, S. T. Gadge, E. N. Kusumawati, K. Harada, T. Sasaki, D. Nishio-Hamane and B. M. Bhanage, Catal. Lett., 2015, 145, 824 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |


