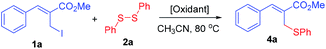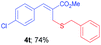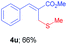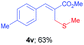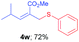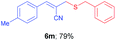DOI:
10.1039/C7RA04817C
(Paper)
RSC Adv., 2017,
7, 30594-30602
Regio- and stereoselective syntheses of allylic thioethers under metal free conditions†
Received
29th April 2017
, Accepted 8th June 2017
First published on 13th June 2017
Abstract
A metal free, regio and stereoselective syntheses of allylic thioethers using allyl iodides and aryl or alkyl disulfides as coupling partners is described. The densely functionalized allyl iodides having different stereochemistry (E & Z) reacted well with a variety of disulfides in a regio and stereoselective manner providing the resulting allyl aryl thioethers in 62–92% yields.
Introduction
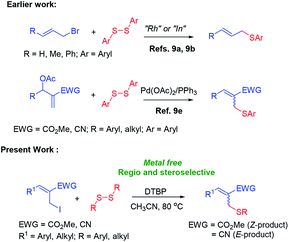
Due to environmental concerns and cost issues, the metal-free organic transformations are in great need. Enough progress has been made in this direction by using various catalytic systems in recent years;1 the peroxide alone or with additives has emerged as the perfect substitute to the traditional transition metal catalysis for several organic transformations.2 Meanwhile, aryl thioethers have been found to play important roles in organic synthesis, the pharmaceutical industry and materials science.3,4 Various transition metals such as Pd,5a–d Cu,5e–g Fe,5h,i Ni,5j,k In,5l Co,5m Au,5n Ag,5o Mg5p etc. have been used so far for the syntheses of thioethers via C–S bond formation between aryl halides or pseudo halides and thiols.6 Recently, the syntheses of aryl thioethers and thioesters have been reported under metal-free conditions via C–H functionalization using a variety of sulphur surrogates.7,8 Various catalyst or catalytic systems such as DTBP,7a,c,d,f,g TBHP,7h,i K2S2O8,7j–l AcOOH,8c etc. have been used so far for the syntheses of thioethers via C–H functionalization. In the case of syntheses of allyl aryl thioethers, various metals such as Rh,9a In,9b Co,9c Ni,9d etc. were used for the C–S coupling between allyl halides/acetates and disulfides (Scheme 1). A palladium acetate catalyzed synthesis of allyl aryl thioethers via cross-coupling reaction between Baylis–Hillman acetates and diphenyl disulfides was reported by Sreedhar and co-workers (Scheme 1).9e The syntheses of allyl aryl thioethers via the C–S bond formation between allyl halides and sulphur surrogate under metal free conditions is not well studied. Therefore, we have decided to find out suitable methodology for the synthesis of allylic thioethers and herein report the first regio- and stereoselective syntheses of allylic thioethers via C–S bond formation between densely functionalized allyl iodides and disulfides under metal free conditions (Scheme 2).
 |
| | Scheme 1 Syntheses of allylic thioethers. | |
 |
| | Scheme 2 Synthesis of allyl aryl thioethers 4a. | |
Results and discussion
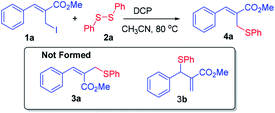
Accordingly, we have selected the allyl iodide 1a (2.0 mmol) and diphenyl disulfide 2a (1.0 mmol) as model substrate and treated them under the influence of DCP (dicumyl peroxide, 10.0 mmol) using CH3CN as solvent at 80 °C for 48 h. The data collection of isolated product revealed the formation of only allyl phenyl thioether 4a in 42% yield (Table 1, entry 1). There was no formation of the thioethers 3a and 3b which were confirmed by GCMS of crude reaction mixture. It is to note here that complete retention in stereochemistry across the double bond was obtained in this reaction i.e. Z-selectivity.
Table 1 Optimization of the reaction conditionsa
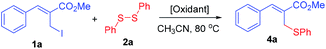
|
| Entry |
Oxidant (equiv.) |
Time (h) |
Yieldb (%) |
| Reaction conditions: allyl iodide 1a (2.0 mmol), diphenyl disulfide 2a (1.0 mmol) and oxidant (10.0 mmol) were reacted in CH3CN (2.0 mL) at 80 °C for 12 h. Isolated yields are based on 1a. TBHP solution in water. 2.0 mmol of 2a was used. 120 °C. 1.0 mL CH3CN was used. 2.0 equivalent of DTBP was used. |
| 1 |
DCP (5.0) |
12 |
42 |
| 2 |
BPO (5.0) |
12 |
36 |
| 3 |
K2S2O8 (5.0) |
12 |
30 |
| 4 |
H2O2 (5.0) |
12 |
51 |
| 5c |
TBHP (5.0) |
12 |
58 |
| 6 |
TBPB (5.0) |
12 |
55 |
| 7 |
DTBP (5.0) |
12 |
60 |
| 8 |
DTBP (5.0) |
48 |
68 |
| 9d |
DTBP (5.0) |
48 |
88 |
| 10e |
DTBP (5.0) |
48 |
82 |
| 11f |
DTBP (5.0) |
48 |
76 |
| 12g |
DTBP (2.0) |
48 |
48 |
Encouraged by these results, we decided to optimize the reaction conditions for this fascinating C–S bond formation strategy. Accordingly, a variety of oxidants were employed to catalyze the reaction between 1a and 2a (Table 1). Oxidant such as BPO (benzoyl peroxide), K2S2O8 could not provide the encouraging results (entries 2 & 3). H2O2, TBHP (tert-butyl hydroperoxide) and TBPB (tert-butyl peroxybenzoate) provided slightly better results (entries 4–6). The DTBP (di-tert-butyl peroxide) provided the desired product in 60% yield after 12 h (entry 7). When the same reaction was carried out for 48 h under the influence of DTBP, provided the thioether 4a in 68% yield (entry 8). Interestingly, when the amount of disulfide was doubled, thioether 4a was obtained in 88% yield (entry 9). Enhancement in the reaction temperature could not increase the yield significantly (entry 10). Diminishing the CH3CN and DTBP amounts were not found favourable for this coupling between 1a and 2a (entry 11 & 12).
Once we have optimized reaction conditions in hand (Table 1, entry 9), we then studied the substrate scope for this interesting C–S bond formation. Accordingly, a variety of allyl iodides 1 and 5 were synthesized from the Baylis–Hillman alcohols following the literature procedures.10a,b It is worth mentioning here that Baylis–Hillman adducts or their derivatives possessing ester functionality (obtained from alkyl acrylates) and nitrile functionality (obtained from acrylonitrile) be evidence for remarkable opposite stereochemical directions in various organic transformations.10a,b,11 This effect of reversibility might be attributed to the steric difference between the nitrile (smaller) and ester (larger) functionalities. The alcohols possessing ester functionality provided the allyl iodides as Z-isomer12 only whereas the alcohols possessing nitrile functionality provided allyl iodides as E-isomer. Therefore, Z-isomer of allyl iodides 1 and E-isomer of allyl iodide 5 was used as coupling partner for C–S bond formation with disulfides.
Firstly, the various allyl iodides possessing ester functionality with Z stereochemistry were treated with different disulfides 2 under the influence of DTBP following the optimized reaction conditions, provided the resulting allylic thioethers 4 in 62–85% isolated yields. The structures of these allylic thioethers 4 were determined from their spectral (1H NMR, 13C NMR and MS) data which suggested complete retention in stereochemistry across the double bond in the products i.e. Z-stereochemistry were observed (in 1H NMR the olefinic proton appeared at δ 7.64–7.81 range confirm Z-stereochemistry).10a,b,11 Allyl iodides possessing substitutes at o/m/p-position underwent regio- and stereo selective C–S coupling reaction with both aryl and alkyl disulfides to provide the desired allyl aryl thioethers (4a–d & 4f–r) and allyl alkyl thioethers (4e & 4s–v) in good to excellent yields. We have also employed the allyl iodides 1 (E-isomer) possessing alkyl group instead that of aryl group for the C–S bond formation with disulfides under the optimized reaction conditions, provided the desired allyl aryl thioethers 4w and 4x in 72% and 70% yields respectively. In these case also complete retention in stereochemistry across the double bond in the products i.e. E-stereochemistry were observed.12
Subsequently, we have employed the densely functionalized E-allyl iodides 5 possessing nitrile functionality for the C–S coupling with variety of disulfides 2 under the influence of DTBP following the optimized reaction conditions. Both the aryl and alkyl disulfide coupled well with allyl iodides 5 to provide the resulting allylic thioethers 6 in 72–92% yield. Substrates possessing substituent at o, m, p position of phenyl ring coupled well under the reaction conditions employed. The structures of these allylic thioethers 6 were determined from their spectral (1H NMR, 13C NMR and MS) data which suggest that a complete retention in stereochemistry across the double bond in the products i.e. E-stereochemistry were observed (in 1H NMR the olefinic proton appeared at δ 6.47–6.76 range confirm E-stereochemistry).10a,b,11
To establish a possible reaction pathway for this methodology, we have performed few control experiments as shown in Scheme 3. Initially, we have performed the C–S coupling reaction between allyl iodide 1a and disulfide 2a in absence of DTBP under optimized reaction conditions and observed that no reaction took place (Path A, Scheme 3). Next, the same reaction was carried out in presence of TEMPO (2,2,6,6-tetramethylpiperidine-N-oxyl) using optimized reaction conditions which provided the allylic thioether 4a in 76% isolated yield (Path B, Scheme 3). It was found that the TEMPO coupled well with allyl iodide 1a under same reaction conditions to provide coupled product 7 in 82% isolated yield (Path C, Scheme 3). On the basis of these control experiments we proposed plausible mechanism which follows the radical pathway (Scheme 4).
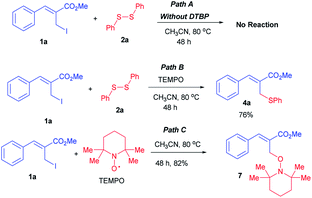 |
| | Scheme 3 Control experiments. | |
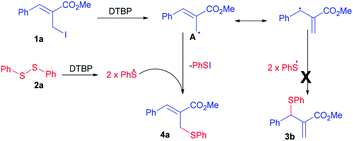 |
| | Scheme 4 Plausible mechanism for the synthesis of thioether 4a. | |
A plausible mechanism for the syntheses of allylic thioethers is presented in the Scheme 4 by taking 4a as model case. In the presence of DTBP, the allyl iodide 1a generated allyl radical A. At the same time disulfide converted into phenyl sulphide radical. The coupling of allyl radical A with phenyl sulphide radical yielded into the resulting thioether 4a.
Conclusions
In conclusions, we have developed a methodology for the synthesis of allylic thioethers via C–S bond formation between allyl iodides and disulfides under metal free conditions for the first time. A variety of densely functionalized allyl iodides and disulfides coupled well under the influence of DTBP provided the thioethers in 62–92% yield. A complete stereo- and regioselectivity were observed in these transformations.
Experimental
General information
All chemicals were purchased from commercial suppliers and used without further purification. NMR spectra were recorded on a Jeol resonance-400 instrument using CDCl3 as solvent. Chemical shifts are reported in parts per million (ppm) and referenced to the residual solvent resonance. Coupling constant (J) are reported in hertz (Hz). Standard abbreviations indicating multiplicity were used as follows: s = singlet, d = doublet, t = triplet, dd = double doublet, q = quartet, m = multiplet. HRMS data were collected on Waters – Xevo G2S QTof with UPLC H-Class Ultra Performance Liquid chromatography-mass spectrometry (LC-MS) facility.
General procedure for Table 1
To a stirred solution of allyl iodide 1a i.e. methyl-(Z)-2-(iodomethyl)-3-phenylacrylate (2.0 mmol) and diphenyl disulfide (2.0 mmol, 0.436 g) in CH3CN (2.0 mL) was added oxidant (10.0 mmol) and then the reaction mixture was stirred for 80 °C under nitrogen atmosphere for 48 h. The solvent was then removed under reduced pressure and the crude product thus obtained was purified by column chromatography (silica gel, 1% EtOAc in hexanes) to provide the allyl thioether 4a as pale yellow colour liquid.
Representative example of Table 1: methyl-(Z)-3-phenyl-2-((phenylthio)methyl)acrylate (entry 9, 4a)10c
The title compound was prepared following the general procedure for Table 1, using allyl iodide 1a i.e. methyl-(Z)-2-(iodomethyl)-3-phenylacrylate (2.0 mmol, 0.604 g), diphenyl disulfide (2.0 mmol, 0.436 g), DTBP (10 mmol, 1.46 g, 1.8 mL) and CH3CN (2.0 mL), providing 4a as pale yellow liquid. Yield: 0.499 g, 88%; 1H NMR (400 MHz, CDCl3): δ 3.77 (s, 3H), 4.05 (s, 2H), 7.20–7.32 (m, 3H), 7.38–7.42 (m, 7H), 7.79 (s, 1H); 13C NMR (100 MHz, CDCl3): δ 32.3, 52.3, 126.8, 128.2, 128.7, 129.0, 129.1, 129.6, 130.7, 134.8, 136.0, 141.6, 167.6.
General procedure for Table 2
To a stirred solution of allyl iodide 1 (2.0 mmol) and disulfide (2.0 mmol) in CH3CN (2.0 mL) was added DTBP (10.0 mmol), then the reaction mixture was stirred for 48 h at 80 °C under nitrogen atmosphere. The solvent was then removed under reduced pressure and the crude product thus obtained was purified by column chromatography (silica gel, 1% EtOAc in hexanes) to provide the allyl thioether 4.
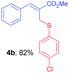
Methyl-(Z)-2-(((4-chlorophenyl)thio)methyl)-3-phenylacrylate (4b). The title compound was prepared following the general procedure for Table 2, using allyl iodide 1a i.e. methyl-(Z)-2-(iodomethyl)-3-phenylacrylate (2.0 mmol, 0.604 g), bis(4-chlorophenyl)disulfide (2.0 mmol, 0.574 g), DTBP (10 mmol, 1.46 g, 1.8 mL) and CH3CN (2.0 mL), providing 4b as pale yellow liquid. Yield: 0.522 g, 82%; 1H NMR (400 MHz, CDCl3): δ 3.77 (s, 3H), 3.98 (s, 2H), 7.11 (d, J = 8.8 Hz, 2H), 7.21 (d, J = 8.8 Hz, 2H), 7.30–7.32 (m, 5H), 7.73 (s, 1H); 13C NMR (100 MHz, CDCl3): δ 32.5, 52.3, 128.0, 128.7, 129.0, 129.1, 129.4, 132.4, 132.9, 134.3, 134.7, 141.6, 167.4; HRMS (ESI) exact mass calcd for C17H15ClO2S + K (M + K), 357.0118; found: 357.0127.
Table 2 DTBP-promoted C–S bond formation between allyl iodides 1 and disulfides 2a,b
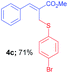
Methyl-(Z)-2-(((4-bromophenyl)thio)methyl)-3-phenylacrylate (4c). The title compound was prepared following the general procedure for Table 2, using allyl iodide 1a i.e. methyl-(Z)-2-(iodomethyl)-3-phenylacrylate (2.0 mmol, 0.604 g), bis(4-bromophenyl)disulfide (2.0 mmol, 0.752 g), DTBP (10 mmol, 1.46 g, 1.8 mL) and CH3CN (2.0 mL), providing 4c as pale yellow liquid. Yield: 0.515 g, 71%; 1H NMR (400 MHz, CDCl3): δ 3.80 (s, 3H), 4.00 (s, 2H), 7.16 (d, J = 8.8 Hz, 2H), 7.29 (d, J = 8.8 Hz, 2H), 7.31–7.33 (m, 5H), 7.75 (s, 1H); 13C NMR (100 MHz, CDCl3): δ 32.3, 52.4, 120.9, 127.9, 128.7, 129.1, 129.4, 131.9, 132.5, 141.7, 167.5; HRMS (ESI) exact mass calcd for C17H15BrO2S + Na (M + Na), 384.9874; found: 384.9881.
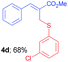
Methyl-(Z)-2-(((3-chlorophenyl)thio)methyl)-3-phenylacrylate (4d). The title compound was prepared following the general procedure for Table 2, using allyl iodide 1a i.e. methyl-(Z)-2-(iodomethyl)-3-phenylacrylate (2.0 mmol, 0.604 g), bis(3-chlorophenyl)disulfide (2.0 mmol, 0.574 g), DTBP (10 mmol, 1.46 g, 1.8 mL) and CH3CN (2.0 mL), providing 4d as pale yellow liquid. Yield: 0.433 g, 68%; 1H NMR (400 MHz, CDCl3): δ 3.82 (s, 3H), 4.05 (s, 2H), 7.08–7.24 (m, 3H), 7.26–7.42 (m, 6H), 7.79 (s, 1H); 13C NMR (100 MHz, CDCl3): δ 31.9, 52.4, 126.7, 127.7, 128.3, 128.8, 129.2, 129.4, 129.8, 129.9, 134.61, 134.65, 138.2, 142.0, 167.5; HRMS (ESI) exact mass calcd for C17H15ClO2S + Na (M + Na), 341.0379; found: 341.0367.
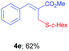
Methyl-(Z)-2-((cyclohexylthio)methyl)-3-phenylacrylate (4e). The title compound was prepared following the general procedure for Table 2, using allyl iodide 1a i.e. methyl-(Z)-2-(iodomethyl)-3-phenylacrylate (2.0 mmol, 0.604 g), dicyclohexyl disulfide (2.0 mmol, 0.44 mL), DTBP (10 mmol, 1.46 g, 1.8 mL) and CH3CN (2.0 mL), providing 4e as pale yellow liquid. Yield: 0.359 g, 62%; 1H NMR (400 MHz, CDCl3): δ 1.06–1.38 (m, 6H), 1.62–1.75 (m, 2H), 1.78–1.93 (m, 2H), 2.61–2.66 (m, 1H), 3.61 (s, 2H), 3.79 (s, 3H), 7.25–7.40 (m, 3H), 7.41–7.52 (m, 2H), 7.68 (s, 1H); 13C NMR (100 MHz, CDCl3): δ 25.9, 26.1, 26.9, 33.5, 44.5, 52.2, 128.6, 128.9, 129.5, 129.7, 135.1, 140.3, 167.9; HRMS (ESI) exact mass calcd for C17H22O2S + Na (M + Na), 313.1238; found: 313.1059.
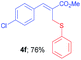
Methyl-(Z)-3-(4-chlorophenyl)-2-((phenylthio)methyl)acrylate (4f)10c. The title compound was prepared following the general procedure for Table 2, using allyl iodide 1b i.e. methyl-(Z)-3-(4-chlorophenyl)-2-(iodomethyl)acrylate (2.0 mmol, 0.673 g), diphenyl disulfide (2.0 mmol, 0.436 g), DTBP (10 mmol, 1.46 g, 1.8 mL) and CH3CN (2.0 mL), providing 4f as pale yellow liquid. Yield: 0.484 g, 76%; 1H NMR (400 MHz, CDCl3): δ 3.78 (s, 3H), 3.98 (s, 2H), 7.20–7.282 (m, 3H), 7.34 (s, 4H), 7.36 (d, J = 8.4 Hz, 2H), 7.67 (s, 1H); 13C NMR (100 MHz, CDCl3): δ 32.3, 52.4, 127.0, 128.8, 128.9, 129.0, 130.8, 131.1, 133.2, 135.0, 135.6, 140.1, 167.4.
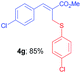
Methyl-(Z)-3-(4-chlorophenyl)-2-(((4-chlorophenyl)thio)methyl) acrylate (4g). The title compound was prepared following the general procedure for Table 2, using allyl iodide 1b i.e. methyl-(Z)-3-(4-chlorophenyl)-2-(iodomethyl)acrylate (2.0 mmol, 0.673 g), bis(4-chlorophenyl)disulfide (2.0 mmol, 0.574 g), DTBP (10 mmol, 1.46 g, 1.8 mL) and CH3CN (2.0 mL), providing 4g as pale yellow liquid. Yield: 0.600 g, 85%; 1H NMR (400 MHz, CDCl3): δ 3.77 (s, 3H), 3.93 (s, 2H), 7.14 (d, J = 8.8 Hz, 2H), 7.222 (d, J = 8.8 Hz, 2H), 7.228 (d, J = 8.8 Hz, 2H), 7.28 (d, J = 8.8 Hz, 2H), 7.64 (s, 1H); 13C NMR (100 MHz, CDCl3): δ 32.5, 52.4, 128.5, 128.9, 129.0, 130.6, 132.6, 133.0, 133.2, 133.9, 135.1, 140.2, 167.2; HRMS (ESI) exact mass calcd for C17H14Cl2O2S + Na (M + Na), 374.9989; found: 374.9922.
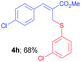
Methyl-(Z)-3-(4-chlorophenyl)-2-(((3-chlorophenyl)thio)methyl) acrylate (4h). The title compound was prepared following the general procedure for Table 2, using allyl iodide 1b i.e. methyl-(Z)-3-(4-chlorophenyl)-2-(iodomethyl)acrylate (2.0 mmol, 0.673 g), bis(3-chlorophenyl)disulfide (2.0 mmol, 0.574 g), DTBP (10 mmol, 1.46 g, 1.8 mL) and CH3CN (2.0 mL), providing 4h as pale yellow liquid. Yield: 0.480 g, 68%; 1H NMR (400 MHz, CDCl3): δ 3.77 (s, 3H), 3.96 (s, 2H), 7.05–7.20 (m, 3H), 7.22–7.36 (m, 5H), 7.66 (s, 1H); 13C NMR (100 MHz, CDCl3): δ 31.8, 52.5, 126.9, 128.2, 128.5, 129.0, 129.9, 130.0, 130.7, 133.0, 134.6, 135.2, 137.8, 140.5, 167.1; HRMS (ESI) exact mass calcd for C17H14Cl2O2S + Na (M + Na), 374.9989; found: 374.9978.
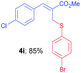
Methyl-(Z)-2-(((4-bromophenyl)thio)methyl)-3-(4-chlorophenyl) acrylate (4i). The title compound was prepared following the general procedure for Table 2, using allyl iodide 1b i.e. methyl-(Z)-3-(4-chlorophenyl)-2-(iodomethyl)acrylate (2.0 mmol, 0.673 g), bis(4-bromophenyl)disulfide (2.0 mmol, 0.752 g), DTBP (10 mmol, 1.46 g, 1.8 mL) and CH3CN (2.0 mL), providing 4i as yellow solid. Mp: 63 °C; yield: 0.675 g, 85%; 1H NMR (400 MHz, CDCl3): δ 3.77 (s, 3H), 3.93 (s, 2H), 7.14 (d, J = 8.4 Hz, 2H), 7.22 (d, J = 8.4 Hz, 2H), 7.27–7.29 (m, 4H), 7.64 (s, 1H); 13C NMR (100 MHz, CDCl3): δ 32.3, 52.5, 121.2, 128.4, 128.9, 130.6, 131.9, 132.7, 133.0, 134.6, 135.1, 140.2, 167.2; HRMS (ESI) exact mass calcd for C17H14BrClO2S + K (M + K), 434.9223; found: 434.9227.
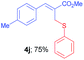
Methyl-(Z)-2-((phenylthio)methyl)-3-(p-tolyl)acrylate (4j). The title compound was prepared following the general procedure for Table 2, using allyl iodide 1c i.e. methyl-(Z)-2-(iodomethyl)-3-(p-tolyl)acrylate (2.0 mmol, 0.632 g), diphenyl disulfide (2.0 mmol, 0.436 g), DTBP (10 mmol, 1.46 g, 1.8 mL) and CH3CN (2.0 mL), providing 4j as pale yellow liquid. Yield: 0.447 g, 75%; 1H NMR (400 MHz, CDCl3): δ 2.37 (s, 3H), 3.81 (s, 3H), 4.09 (s, 2H), 7.18 (d, J = 8.4 Hz, 2H), 7.21–7.29 (m, 3H), 7.38 (d, J = 8.4 Hz, 2H), 7.40–7.42 (m, 2H), 7.80 (s, 1H); 13C NMR (100 MHz, CDCl3): δ 21.5, 32.3, 52.3, 126.7, 127.2, 129.0, 129.5, 129.8, 130.6, 132.0, 136.3, 139.4, 141.9, 167.8; HRMS (ESI) exact mass calcd for C18H18O2S + K (M + K), 337.0665; found: 337.0652.
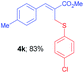
Methyl-(Z)-2-(((4-chlorophenyl)thio)methyl)-3-(p-tolyl)acrylate (4k). The title compound was prepared following the general procedure for Table 2, using allyl iodide 1c i.e. methyl-(Z)-2-(iodomethyl)-3-(p-tolyl)acrylate (2.0 mmol, 0.632 g), bis(4-chlorophenyl)disulfide (2.0 mmol, 0.574 g), DTBP (10 mmol, 1.46 g, 1.8 mL) and CH3CN (2.0 mL), providing 4k as pale yellow liquid. Yield: 0.551 g, 83%; 1H NMR (400 MHz, CDCl3): δ 2.35 (s, 3H), 3.79 (s, 3H), 4.03 (s, 2H), 7.15 (d, J = 8.4 Hz, 2H), 7.16 (d, J = 8.4 Hz, 2H), 7.26 (d, J = 8.8 Hz, 2H), 7.29 (d, J = 8.8 Hz, 2H), 7.75 (s, 1H); 13C NMR (100 MHz, CDCl3): δ 21.4, 32.5, 52.2, 127.0, 128.9, 129.4, 129.6, 131.8, 132.2, 132.9, 134.5, 139.4, 141.9, 167.5; HRMS (ESI) exact mass calcd for C18H17ClO2S + K (M + K), 371.0275; found: 371.0271.
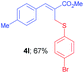
Methyl-(Z)-2-(((4-bromophenyl)thio)methyl)-3-(p-tolyl)acrylate (4l). The title compound was prepared following the general procedure for Table 2, using allyl iodide 1c i.e. methyl-(Z)-2-(iodomethyl)-3-(p-tolyl)acrylate (2.0 mmol, 0.632 g), bis(4-bromophenyl)disulfide (2.0 mmol, 0.752 g), DTBP (10 mmol, 1.46 g, 1.8 mL) and CH3CN (2.0 mL), providing 4l as yellow solid. Mp: 61 °C; yield: 0.505 g, 67%; 1H NMR (400 MHz, CDCl3): δ 2.35 (s, 3H), 3.79 (s, 3H), 4.02 (s, 2H), 7.14–7.19 (m, 4H), 7.27–7.31 (m, 4H), 7.74 (s, 1H); 13C NMR (100 MHz, CDCl3): δ 21.5, 32.3, 52.3, 120.8, 126.9, 129.5, 129.6, 131.8, 131.9, 132.3, 135.2, 139.5, 142.0, 167.7; HRMS (ESI) exact mass calcd for C18H17BrO2S + Na (M + Na), 399.0030; found: 398.9935.
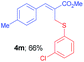
Methyl-(Z)-2-(((3-chlorophenyl)thio)methyl)-3-(p-tolyl)acrylate (4m). The title compound was prepared following the general procedure for Table 2, using allyl iodide 1c i.e. methyl-(Z)-2-(iodomethyl)-3-(p-tolyl)acrylate (2.0 mmol, 0.632 g), bis(3-chlorophenyl)disulfide (2.0 mmol, 0.574 g), DTBP (10 mmol, 1.46 g, 1.8 mL) and CH3CN (2.0 mL), providing 4m as pale yellow liquid. Yield: 0.438 g, 66%; 1H NMR (400 MHz, CDCl3): δ 2.35 (s, 3H), 3.81 (s, 3H), 4.07 (s, 2H), 7.05–7.24 (m, 5H), 7.25–7.40 (m, 3H), 7.78 (s, 1H); 13C NMR (100 MHz, CDCl3): δ 21.5, 31.9, 52.3, 126.6, 128.1, 129.60, 129.65, 129.67, 129.9, 131.8, 134.6, 138.4, 139.6, 142.2, 167.5; HRMS (ESI) exact mass calcd for C18H17ClO2S + Na (M + Na), 355.0535; found: 355.0531.
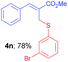
Methyl-(Z)-2-(((3-bromophenyl)thio)methyl)-3-phenylacrylate (4n). The title compound was prepared following the general procedure for Table 2, using allyl iodide 1a i.e. methyl-(Z)-2-(iodomethyl)-3-phenylacrylate (2.0 mmol, 0.604 g), bis(3-bromophenyl)disulfide (2.0 mmol, 0.752 g), DTBP (10 mmol, 1.46 g, 1.8 mL) and CH3CN (2.0 mL), providing 4n as pale yellow liquid. Yield: 0.566, 78%; 1H NMR (400 MHz, CDCl3): δ 3.81 (s, 3H), 4.04 (s, 2H), 7.07 (t, J = 8.0 Hz, 1H), 7.23 (d, J = 8.0 Hz, 1H), 7.29 (d, J = 8.0 Hz, 1H), 7.33–7.44 (m, 5H), 7.46 (s, 1H), 7.79 (s, 1H); 13C NMR (100 MHz, CDCl3): δ 32.0, 52.4, 122.7, 127.7, 128.8, 128.9, 129.2, 129.4, 129.7, 130.2, 132.7, 134.6, 138.4, 142.0, 167.5; HRMS (ESI) exact mass calcd for C17H15BrO2S + Na (M + Na), 384.9874; found: 384.9698.
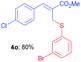
Methyl-(Z)-2-(((3-bromophenyl)thio)methyl)-3-(4-chlorophenyl) acrylate (4o). The title compound was prepared following the general procedure for Table 2, using allyl iodide 1b i.e. methyl-(Z)-3-(4-chlorophenyl)-2-(iodomethyl)acrylate (2.0 mmol, 0.673 g), bis(3-bromophenyl)disulfide (2.0 mmol, 0.752 g), DTBP (10 mmol, 1.46 g, 1.8 mL) and CH3CN (2.0 mL), providing 4o as pale yellow liquid. Yield: 0.635 g, 80%; 1H NMR (400 MHz, CDCl3): δ 3.80 (s, 3H), 3.98 (s, 2H), 7.07 (t, J = 8.0 Hz, 1H), 7.21–7.23 (m, 1H), 7.26–7.32 (m, 5H), 7.43 (t, J = 1.6 Hz, 1H), 7.69 (s, 1H); 13C NMR (100 MHz, CDCl3): δ 32.0, 52.5, 122.7, 128.3, 129.0, 129.2, 129.9, 130.2, 130.7, 133.0, 135.2, 138.0, 140.5, 167.2; HRMS (ESI) exact mass calcd for C17H14BrClO2S + K (M + K), 434.9223; found: 434.9226.
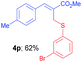
Methyl-(Z)-2-(((3-bromophenyl)thio)methyl)-3-(p-tolyl)acrylate (4p). The title compound was prepared following the general procedure for Table 2, using allyl iodide 1c i.e. methyl-(Z)-2-(iodomethyl)-3-(p-tolyl)acrylate (2.0 mmol, 0.632 g), bis(3-bromophenyl)disulfide (2.0 mmol, 0.752 g), DTBP (10 mmol, 1.46 g, 1.8 mL) and CH3CN (2.0 mL), providing 4p as pale yellow liquid. Yield: 0.467 g, 62%; 1H NMR (400 MHz, CDCl3): δ 2.36 (s, 3H), 3.81 (s, 3H), 4.06 (s, 2H), 7.08 (t, J = 8.0 Hz, 1H), 7.17 (d, J = 8.0 Hz, 2H), 7.24–7.32 (m, 4H), 7.46 (t, J = 1.6 Hz, 1H), 7.77 (s, 1H); 13C NMR (100 MHz, CDCl3): δ 21.5, 32.0, 52.4, 122.7, 126.6, 128.7, 129.5, 129.6, 130.1, 131.7, 132.5, 138.6, 139.6, 142.2, 167.6; HRMS (ESI) exact mass calcd for C18H17BrO2S + Na (M + Na), 399.0030; found: 399.6734.
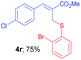
Methyl-(Z)-2-(((2-bromophenyl)thio)methyl)-3-phenylacrylate (4q). The title compound was prepared following the general procedure for Table 2, using allyl iodide 1a i.e. methyl-(Z)-2-(iodomethyl)-3-phenylacrylate (2.0 mmol, 0.604 g), bis(2-bromophenyl)disulfide (2.0 mmol, 0.752 g), DTBP (10 mmol, 1.46 g, 1.8 mL) and CH3CN (2.0 mL), providing 4q as pale yellow liquid. Yield: 0.537 g, 74%; 1H NMR (400 MHz, CDCl3): δ 3.81 (s, 3H), 4.04 (s, 2H), 7.02 (td, J = 8.0 Hz & 1.6 Hz, 1H), 7.19 (td, J = 8.4 Hz & 1.2 Hz, 1H), 7.27 (dd, J = 6.4 Hz & 1.6 Hz, 1H), 7.32–7.38 (m, 3H), 7.41–7.45 (m, 2H), 7.50 (dd, J = 6.4 Hz & 1.6 Hz, 1H), 7.81 (s, 1H); 13C NMR (100 MHz, CDCl3): δ 31.4, 52.4, 125.1, 127.2, 127.6, 127.8, 128.8, 129.2, 129.5, 130.6, 133.0, 134.6, 137.3, 142.4, 167.5; HRMS (ESI) exact mass calcd for C17H15BrO2S + K (M + K), 400.9613; found: 400.9681.
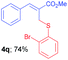
Methyl-(Z)-2-(((2-bromophenyl)thio)methyl)-3-(4-chlorophenyl)acrylate (4r). The title compound was prepared following the general procedure for Table 2, using allyl iodide 1b i.e. methyl-(Z)-3-(4-chlorophenyl)-2-(iodomethyl)acrylate (2.0 mmol, 0.673 g), bis(2-bromophenyl)disulfide (2.0 mmol, 0.752 g), DTBP (10 mmol, 1.46 g, 1.8 mL) and CH3CN (2.0 mL), providing 4p as yellow solid. Mp: 74 °C; yield: 0.595 g, 75%; 1H NMR (400 MHz, CDCl3): δ 3.80 (s, 3H), 3.99 (s, 2H), 7.03 (t, J = 7.2 Hz, 1H), 7.19 (t, J = 7.6 Hz, 1H), 7.26–7.38 (m, 5H), 7.50 (d, J = 8.0 Hz, 1H), 7.72 (s, 1H); 13C NMR (100 MHz, CDCl3): δ 31.4, 52.5, 125.4, 127.7, 127.922, 127.928, 129.03, 130.9, 131.0, 133.0, 133.1, 135.2, 136.9, 141.0, 167.2; HRMS (ESI) exact mass calcd for C17H14BrClO2S + K (M + K), 434.9223; found: 434.9214.
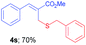
Methyl-(Z)-2-((benzylthio)methyl)-3-phenylacrylate (4s). The title compound was prepared following the general procedure for Table 2, using allyl iodide 1a i.e. methyl-(Z)-2-(iodomethyl)-3-phenylacrylate (2.0 mmol, 0.604 g), dibenzyl disulfide (2.0 mmol, 0.492 g), DTBP (10 mmol, 1.46 g, 1.8 mL) and CH3CN (2.0 mL), providing 4s as pale yellow liquid. Yield: 0.417 g, 70%; 1H NMR (400 MHz, CDCl3): δ 3.46 (s, 2H), 3.64 (s, 2H), 3.72 (s, 3H), 7.10–7.16 (m, 5H), 7.21–7.22 (m, 3H), 7.28–7.30 (m, 2H), 7.63 (s, 1H); 13C NMR (100 MHz, CDCl3): δ 28.5, 37.4, 52.3, 127.0, 128.5, 128.7, 129.0, 129.1, 129.8, 134.9, 138.3, 140.8, 168.0; HRMS (ESI) exact mass calcd for C18H18O2S + Na (M + Na), 321.0925; found: 321.0920.
Methyl-(Z)-2-((benzylthio)methyl)-3-(4-chlorophenyl)acrylate (4t). The title compound was prepared following the general procedure for Table 2, using allyl iodide 1b i.e. methyl-(Z)-3-(4-chlorophenyl)-2-(iodomethyl)acrylate (2.0 mmol, 0.673 g), dibenzyl disulfide (2.0 mmol, 0.492 g), DTBP (10 mmol, 1.46 g, 1.8 mL) and CH3CN (2.0 mL), providing 4t as pale yellow liquid. Yield: 0.493 g, 74%; 1H NMR (400 MHz, CDCl3): δ 3.52 (s, 2H), 3.74 (s, 2H), 3.82 (s, 3H), 7.22–7.32 (m, 9H), 7.65 (s, 1H); 13C NMR (100 MHz, CDCl3): δ 28.3, 37.4, 52.4, 127.1, 128.5, 128.94, 128.99, 129.0, 129.5, 131.0, 133.2, 135.0, 138.1, 139.5, 167.7; HRMS (ESI) exact mass calcd for C18H17ClO2S + Na (M + Na), 355.0535; found: 355.0529.
Methyl-(Z)-2-((methylthio)methyl)-3-phenylacrylate (4u)13. The title compound was prepared following the general procedure for Table 2, using allyl iodide 1a i.e. methyl-(Z)-2-(iodomethyl)-3-phenylacrylate (2.0 mmol, 0.604 g), dimethyl disulfide (2.0 mmol, 0.188 g), DTBP (10 mmol, 1.46 g, 1.8 mL) and CH3CN (2.0 mL), providing 4u as colourless oil. Yield: 0.293 g, 66%; 1H NMR (400 MHz, CDCl3): δ 2.01 (s, 3H), 3.55 (s, 2H), 3.76 (s, 3H), 7.25–7.34 (m, 3H), 7.40 (d, J = 7.6 Hz, 2H), 7.68 (s, 1H); 13C NMR (100 MHz, CDCl3): δ 16.1, 30.4, 52.2, 128.6, 128.8, 129.3, 129.5, 134.9, 140.6, 167.9.
Methyl-(Z)-2-((methylthio)methyl)-3-(p-tolyl)acrylate (4v). The title compound was prepared following the general procedure for Table 2, using allyl iodide 1c i.e. methyl-(Z)-2-(iodomethyl)-3-(p-tolyl)acrylate (2.0 mmol, 0.632 g), dimethyl disulfide (2.0 mmol, 0.188 g), DTBP (10 mmol, 1.46 g, 1.8 mL) and CH3CN (2.0 mL), providing 4v as colourless oil. Yield: 0.297 g, 63%; 1H NMR (400 MHz, CDCl3): δ 2.09 (s, 3H), 2.35 (s, 3H), 3.63 (s, 2H), 3.82 (s, 3H), 7.19 (d, J = 8.0 Hz, 2H), 7.38 (d, J = 8.0 Hz, 2H), 7.72 (s, 1H); 13C NMR (100 MHz, CDCl3): δ 16.1, 21.3, 30.5, 52.1, 128.3, 129.3, 129.6, 132.0, 139.0, 140.7, 168.0; HRMS (ESI) exact mass calcd for C13H16O2S + K (M + K), 275.0508; found: 275.0501.
Methyl-(E)-4-methyl-2-((phenylthio)methyl)pent-2-enoate (4w). The title compound was prepared following the general procedure for Table 2, using allyl iodide 1d i.e. methyl-(E)-2-(iodomethyl)-4-methylpent-2-enoate (2.0 mmol, 0.536 g), diphenyl disulfide (2.0 mmol, 0.436 g), DTBP (10 mmol, 1.46 g, 1.8 mL) and CH3CN (2.0 mL), providing 4w as pale yellow liquid. Yield: 0.360 g, 72%; 1H NMR (400 MHz, CDCl3): δ 0.84 (s, 3H), 0.85 (s, 3H), 2.39–2.45 (m, 1H), 3.71 (s, 3H), 3.79 (s, 2H), 6.60 (d, J = 10.4 Hz, 1H), 7.18–7.26 (m, 3H), 739–7.41 (m, 2H); 13C NMR (100 MHz, CDCl3): δ 19.1, 22.1, 28.4, 31.4, 52.0, 125.9, 127.1, 128.8, 128.9, 131.9, 135.9, 152.0, 167.4; HRMS (ESI) exact mass calcd for C14H18O2S + Na (M + Na), 273.0925; found: 273.0920.
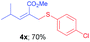
Methyl-(E)-2-(((4-chlorophenyl)thio)methyl)-4-methylpent-2-enoate (4x). The title compound was prepared following the general procedure for Table 2, using allyl iodide 1d i.e. methyl-(E)-2-(iodomethyl)-4-methylpent-2-enoate (2.0 mmol, 0.536 g), bis(3-chlorophenyl)disulfide (2.0 mmol, 0.574 g), DTBP (10 mmol, 1.46 g, 1.8 mL) and CH3CN (2.0 mL), providing 4x as pale yellow liquid. Yield: 0.398 g, 70%; 1H NMR (400 MHz, CDCl3): δ 0.87 (s, 3H), 0.88 (s, 3H), 2.39–2.45 (m, 1H), 3.72 (s, 3H), 3.76 (s, 2H), 6.62 (d, J = 10.4 Hz, 1H), 7.22 (dd, J = 8.4 Hz & 2.0 Hz, 2H), 7.32 (dd, J = 8.4 Hz & 2.0 Hz, 2H); 13C NMR (100 MHz, CDCl3): δ 22.1, 28.4, 31.6, 52.0, 125.6, 128.9, 133.2, 134.4, 152.3, 167.2; HRMS (ESI) exact mass calcd for C14H17ClO2S + Na (M + Na), 307.0535; found: 307.0539.
General procedure for Table 3
To a stirred solution of allyl bromide 5 (2.0 mmol) and disulfide (2.0 mmol) in CH3CN (2.0 mL) was added DTBP (10.0 mmol), then the reaction mixture was stirred for 48 h at 80 °C under nitrogen atmosphere. The solvent was then removed under reduced pressure and the crude product thus obtained was purified by column chromatography (silica gel, 1% EtOAc in hexanes) to provide the allyl thioether 6.
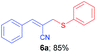
(E)-3-Phenyl-2-((phenylthio)methyl)acrylonitrile (6a)10c. The title compound was prepared following the general procedure for Table 3, using allyl iodide 5a i.e. (E)-2-(iodomethyl)-3-phenylacrylonitrile (2.0 mmol, 0.538 g), diphenyl disulfide (2.0 mmol, 0.436 g), DTBP (10 mmol, 1.46 g, 1.8 mL) and CH3CN (2.0 mL), providing 6a as pale yellow liquid. Yield: 0.426 g, 85%; 1H NMR (400 MHz, CDCl3): δ 3.73 (s, 2H), 6.65 (s, 1H), 7.27–7.35 (m, 6H), 7.44–7.46 (m, 2H), 7.58–7.59 (m, 2H); 13C NMR (100 MHz, CDCl3): δ 41.1, 107.7, 118.2, 128.1, 128.8, 128.9, 129.3, 130.5, 132.9, 133.1, 133.5, 144.9.
Table 3 DTBP-promoted C–S bond formation between allyl iodides 5 and disulfides 2a,b
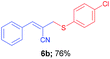
(E)-2-(((4-Chlorophenyl)thio)methyl)-3-phenylacrylonitrile (6b). The title compound was prepared following the general procedure for Table 3, using allyl iodide 5a i.e. (E)-2-(iodomethyl)-3-phenylacrylonitrile (2.0 mmol, 0.538 g), bis(4-chlorophenyl)disulfide (2.0 mmol, 0.574 g), DTBP (10 mmol, 1.46 g, 1.8 mL) and CH3CN (2.0 mL), providing 6b as pale yellow liquid. Yield: 0.433 g, 76%; 1H NMR (400 MHz, CDCl3): δ 3.71 (s, 2H), 6.68 (s, 1H), 7.24 (dd, J = 8.8 Hz & 2.0 Hz, 2H), 7.35–7.37 (m, 5H; multiplet conations one doublet at δ 7.36, J = 8.4 Hz, 2H and multiplet for 3H), 7.58–7.61 (m, 2H); 13C NMR (100 MHz, CDCl3): δ 41.2, 107.5, 118.0, 128.8, 128.9, 129.4, 130.7, 132.0, 132.9, 134.2, 134.3, 145.1, HRMS (ESI) exact mass calcd for C16H12ClNS + Na (M + Na), 308.0277; found: 308.0092.
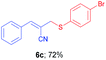
(E)-2-(((4-Bromophenyl)thio)methyl)-3-phenylacrylonitrile (6c). The title compound was prepared following the general procedure for Table 3, using allyl iodide 5a i.e. (E)-2-(iodomethyl)-3-phenylacrylonitrile (2.0 mmol, 0.538 g), bis(4-bromophenyl)disulfide (2.0 mmol, 0.752 g), DTBP (10 mmol, 1.46 g, 1.8 mL) and CH3CN (2.0 mL), providing 6c as yellow solid. Mp: 83 °C; yield: 0.475 g, 72%; 1H NMR (400 MHz, CDCl3): δ 3.72 (s, 2H), 6.70 (s, 1H), 7.28 (d, J = 8.4 Hz, 2H), 7.36–7.40 (m, 5H, multiplet contains one doublet at δ 7.37, J = 8.4 Hz, 2H and one multiplet for 3H), 7.59–7.61 (m, 2H); 13C NMR (100 MHz, CDCl3): δ 41.0, 107.4, 118.0, 122.3, 128.8, 129.0, 130.7, 132.3, 132.7, 132.9, 134.3, 145.1; HRMS (ESI) exact mass calcd for C16H12BrNS + Na (M + Na), 351.9772; found: 351.9749.
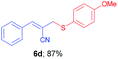
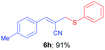
(E)-2-(((4-Methoxyphenyl)thio)methyl)-3-phenylacrylonitrile (6d). The title compound was prepared following the general procedure for Table 3, using allyl iodide 5a i.e. (E)-2-(iodomethyl)-3-phenylacrylonitrile (2.0 mmol, 0.538 g), bis(4-methoxyphenyl)disulfide (2.0 mmol, 0.556 g), DTBP (10 mmol, 1.46 g, 1.8 mL) and CH3CN (2.0 mL), providing 6d as pale yellow liquid. Yield: 0.488 g, 87%; 1H NMR (400 MHz, CDCl3): δ 3.60 (s, 2H), 3.71 (s, 3H), 6.47 (s, 1H), 6.79 (d, J = 8.8 Hz, 2H), 7.32–7.34 (m, 3H), 7.38 (d, J = 8.8 Hz, 2H), 7.53–7.55 (m, 2H); 13C NMR (100 MHz, CDCl3): δ 42.7, 55.4, 108.0, 114.8, 118.2, 123.5, 128.7, 128.9, 130.4, 133.1, 136.3, 144.7, 160.1; HRMS (ESI) exact mass calcd for C17H15NOS + K (M + K), 320.0511; found: 320.0517.
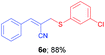
(E)-2-(((3-Chlorophenyl)thio)methyl)-3-phenylacrylonitrile (6e). The title compound was prepared following the general procedure for Table 3, using allyl iodide 5a i.e. (E)-2-(iodomethyl)-3-phenylacrylonitrile (2.0 mmol, 0.538 g), bis(3-chlorophenyl)disulfide (2.0 mmol, 0.574 g), DTBP (10 mmol, 1.46 g, 1.8 mL) and CH3CN (2.0 mL), providing 6e as pale yellow liquid. Yield: 0.502 g, 88%; 1H NMR (400 MHz, CDCl3): δ 3.75 (s, 2H), 6.76 (s, 1H), 7.07–7.22 (m, 2H), 7.24–7.44 (m, 5H), 7.55–7.65 (m, 2H); 13C NMR (100 MHz, CDCl3): δ 40.5, 107.2, 118.0, 128.0, 128.9, 129.0, 130.2, 130.3, 130.7, 131.7, 132.9, 134.8, 135.7, 145.3; HRMS (ESI) exact mass calcd for C16H12ClNS + Na (M + Na), 308.0277; found: 308.0091.
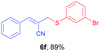
(E)-2-(((3-Bromophenyl)thio)methyl)-3-phenylacrylonitrile (6f). The title compound was prepared following the general procedure for Table 3, using allyl iodide 5a i.e. (E)-2-(iodomethyl)-3-phenylacrylonitrile (2.0 mmol, 0.538 g), bis(3-bromophenyl)disulfide (2.0 mmol, 0.752 g), DTBP (10 mmol, 1.46 g, 1.8 mL) and CH3CN (2.0 mL), providing 6f as pale yellow liquid. Yield: 0.587 g, 89%; 1H NMR (400 MHz, CDCl3): δ 3.74 (s, 2H), 6.76 (s, 1H), 7.11 (t, J = 8.0 Hz, 1H), 7.21–7.41 (m, 5H), 7.56 (s, 1H), 7.61–7.66 (m, 2H); 13C NMR (100 MHz, CDCl3): δ 40.6, 107.2, 118.0, 122.9, 128.9, 129.05, 129.06, 130.7, 130.8, 130.9, 132.9, 134.5, 136.1, 145.4; HRMS (ESI) exact mass calcd for C16H12BrNS + Na (M + Na), 351.9772; found: 351.9625.
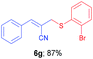
(E)-2-(((2-Bromophenyl)thio)methyl)-3-phenylacrylonitrile (6g). The title compound was prepared following the general procedure for Table 3, using allyl iodide 5a i.e. (E)-2-(iodomethyl)-3-phenylacrylonitrile (2.0 mmol, 0.538 g), bis(2-bromophenyl)disulfide (2.0 mmol, 0.752 g), DTBP (10 mmol, 1.46 g, 1.8 mL) and CH3CN (2.0 mL), providing 6g as yellow solid. Mp: 79 °C; yield: 0.574 g, 87%; 1H NMR (400 MHz, CDCl3): δ 3.81 (s, 2H), 6.74 (s, 1H), 7.10 (td, J = 7.6 Hz & 1.6 Hz, 1H), 7.21 (td, J = 7.6 Hz & 1.2 Hz, 1H), 7.31–7.37 (m, 3H), 7.45 (dd, J = 8.0 Hz & 1.6 Hz, 2H), 7.53–7.62 (m, 3H); 13C NMR (100 MHz, CDCl3): δ 39.5, 106.8, 118.1, 127.3, 128.2, 128.8, 128.9, 129.3, 130.7, 133.0, 133.5, 133.6, 134.5, 145.4; HRMS (ESI) exact mass calcd for C16H12BrNS + Na (M + Na), 351.9772; found: 351.9713.
(E)-2-((Phenylthio)methyl)-3-(p-tolyl)acrylonitrile (6h)10c. The title compound was prepared following the general procedure for Table 3, using allyl iodide 5b i.e. (E)-2-(iodomethyl)-3-(p-tolyl)acrylonitrile (2.0 mmol, 0.566 g), diphenyl disulfide (2.0 mmol, 0436 g), DTBP (10 mmol, 1.46 g, 1.8 mL) and CH3CN (2.0 mL), providing 6h as pale yellow liquid. Yield: 0.482 g, 91%; 1H NMR (400 MHz, CDCl3): δ 2.33 (s, 3H), 3.71 (s, 2H), 6.63 (s, 1H), 7.14 (d, J = 8.0 Hz, 2H), 7.26–7.29 (m, 3H), 7.44 (d, J = 8.0 Hz, 2H), 7.52 (d, J = 8.0 Hz, 2H); 13C NMR (100 MHz, CDCl3): δ 21.6, 41.1, 106.3, 118.4, 128.0, 128.9, 129.3, 129.6, 130.4, 132.8, 133.7, 141.0, 144.9.
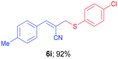
(E)-2-(((4-Chlorophenyl)thio)methyl)-3-(p-tolyl)acrylonitrile (6i). The title compound was prepared following the general procedure for Table 3, using allyl iodide 5b i.e. (E)-2-(iodomethyl)-3-(p-tolyl)acrylonitrile (2.0 mmol, 0.566 g), bis(4-chlorophenyl)disulfide (2.0 mmol, 0.574 g), DTBP (10 mmol, 1.46 g, 1.8 mL) and CH3CN (2.0 mL), providing 6i as yellow solid. Mp: 73 °C; yield: 0.551 g, 92%; 1H NMR (400 MHz, CDCl3): δ 2.35 (s, 3H), 3.71 (s, 2H), 6.65 (s, 1H), 7.17 (d, J = 8.0 Hz, 2H), 7.24 (d, J = 8.4 Hz, 2H), 7.36 (d, J = 8.8 Hz, 2H), 7.52 (d, J = 8.4 Hz, 2H); 13C NMR (100 MHz, CDCl3): δ 22.7, 41.3, 106.1, 118.1, 128.8, 129.3, 129.6, 130.2, 132.1, 134.20, 134.29, 141.2, 145.0; HRMS (ESI) exact mass calcd for C17H14ClNS + K (M + K), 338.0173; found: 338.0179.
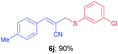
(E)-2-(((3-Chlorophenyl)thio)methyl)-3-(p-tolyl)acrylonitrile (6j). The title compound was prepared following the general procedure for Table 3, using allyl iodide 5b i.e. (E)-2-(iodomethyl)-3-(p-tolyl)acrylonitrile (2.0 mmol, 0.566 g), bis(3-chlorophenyl)disulfide (2.0 mmol, 0.574 g), DTBP (10 mmol, 1.46 g, 1.8 mL) and CH3CN (2.0 mL), providing 6j as yellow solid. Mp: 70 °C; yield: 0.539 g, 90%; 1H NMR (400 MHz, CDCl3): δ 2.31 (s, 3H), 3.74 (s, 2H), 6.75 (s, 1H), 7.13 (d, J = 7.6 Hz, 2H), 7.16–7.17 (m, 2H), 7.22–7.31 (m, 1H), 7.39 (s, 1H), 7.46–7.56 (m, 2H); 13C NMR (100 MHz, CDCl3): δ 21.6, 40.5, 105.8, 118.3, 127.8, 128.9, 129.7, 130.0, 130.2, 130.3, 131.4, 134.7, 136.0, 141.2, 145.4; HRMS (ESI) exact mass calcd for C17H14ClNS + Na (M + Na), 322.0433; found: 322.0439.
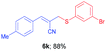
(E)-2-(((3-Bromophenyl)thio)methyl)-3-(p-tolyl)acrylonitrile (6k). The title compound was prepared following the general procedure for Table 3, using allyl iodide 5b i.e. (E)-2-(iodomethyl)-3-(p-tolyl)acrylonitrile (2.0 mmol, 0.566 g), bis(3-bromophenyl)disulfide (2.0 mmol, 0.752 g), DTBP (10 mmol, 1.46 g, 1.8 mL) and CH3CN (2.0 mL), providing 6k as yellow solid. Mp: 79 °C; yield: 0.605 g, 88%; 1H NMR (400 MHz, CDCl3): δ 2.35 (s, 3H), 3.75 (s, 2H), 6.73 (s, 1H), 7.10–7.20 (m, 3H), 7.30–7.39 (m, 2H), 7.52–7.58 (m, 3H); 13C NMR (100 MHz, CDCl3): δ 21.6, 40.7, 105.8, 118.2, 122.8, 128.9, 129.7, 130.1, 130.5, 130.7, 130.8, 134.5, 136.1, 141.3, 145.4; HRMS (ESI) exact mass calcd for C17H14BrNS + K (M + K), 381.9667; found: 381.9666.
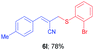
(E)-2-(((2-Bromophenyl)thio)methyl)-3-(p-tolyl)acrylonitrile (6l). The title compound was prepared following the general procedure for Table 3, using allyl iodide 5b i.e. (E)-2-(iodomethyl)-3-(p-tolyl)acrylonitrile (2.0 mmol, 0.566 g), bis(2-bromophenyl)disulfide (2.0 mmol, 0.752 g), DTBP (10 mmol, 1.46 g, 1.8 mL) and CH3CN (2.0 mL), providing 6l as yellow solid. Mp: 69 °C; yield: 0.537 g, 78%; 1H NMR (400 MHz, CDCl3): δ 2.34 (s, 3H), 3.80 (s, 2H), 6.71 (s, 1H), 7.10 (td, J = 7.6 Hz & 1.6 Hz, 1H), 7.15 (d, J = 8.0 Hz, 2H), 7.21 (td, J = 7.6 Hz, & 1.2 Hz, 1H), 7.43 (dd, J = 8.0 Hz & 1.6 Hz, 1H), 7.49 (d, J = 8.0 Hz, 2H), 7.58 (dd, J = 8.0 Hz & 1.6 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 21.6, 39.5, 105.5, 118.3, 127.5, 128.1, 128.8, 129.3, 129.6, 130.2, 133.4, 133.9, 141.2, 145.4; HRMS (ESI) exact mass calcd for C17H14BrNS + K (M + K), 381.9667; found: 381.9673.
(E)-2-((benzylthio)methyl)-3-(p-tolyl)acrylonitrile (6m). The title compound was prepared following the general procedure for Table 3, using allyl iodide 5b i.e. (E)-2-(iodomethyl)-3-(p-tolyl)acrylonitrile (2.0 mmol, 0.566 g), dibenzyl disulfide (2.0 mmol, 0.492 g), DTBP (10 mmol, 1.46 g, 1.8 mL) and CH3CN (2.0 mL), providing 6m as yellow liquid; yield: 0.441 g, 79%; 1H NMR (400 MHz, CDCl3): δ 2.40 (s, 3H), 3.31 (s, 2H), 3.76 (s, 2H), 6.83 (s, 1H), 7.24 (d, J = 8.0 Hz, 2H), 7.31–7.35 (m, 5H), 7.67 (d, J = 8.0 Hz, 2H); 13C NMR (100 MHz, CDCl3): δ 21.6, 35.4, 36.2, 106.7, 118.5, 127.4, 128.8, 129.0, 129.1, 129.7, 130.3, 137.2, 141.2, 144.6; HRMS (ESI) exact mass calcd for C18H17NS + K (M + K), 318.0719; found: 318.0726.
Methyl (E)-3-phenyl-2-(((2,2,6,6-tetramethylpiperidin-1-yl)oxy)methyl)acrylate (7). To a stirred solution of allyl iodide 1a i.e. methyl-(Z)-2-(iodomethyl)-3-phenylacrylate (2.0 mmol, 0.604 g) in CH3CN (2.0 mL) was added TEMPO (2.0 mmol, 0.312 g) and then the reaction mixture was stirred for 80 °C under nitrogen atmosphere for 48 h. The solvent was then removed under reduced pressure and the crude product thus obtained was purified by column chromatography (silica gel, 1% EtOAc in hexanes) to provide the allyl thioether 7 as pale yellow colour liquid. Yield: 0.543 g, 82%; 1H NMR (400 MHz, CDCl3): δ 1.06 (s, 6H), 1.09 (s, 6H), 1.40–1.42 (m, 6H), 3.82 (s, 3H), 4.67 (s, 2H), 7.34–7.68 (m, 3H), 7.47–7.49 (m, 2H), 7.83 (s, 1H); 13C NMR (100 MHz, CDCl3): δ 17.1, 20.2, 32.9, 40.0, 52.0, 59.9, 70.8, 128.3, 128.9, 129.5, 135.1, 143.6, 168.5; HRMS (ESI) exact mass calcd for C20H29NO3 + K (M + K), 370.1785; found: 370.1778.
Acknowledgements
SERB-DST, New Delhi (YSS/2015/001870) and UGC-India (Start-up grant) are gratefully acknowledged for financial support. P. S. and R. C. thanks the UGC and R. B. thanks the SERB-DST for their fellowships. S. S. B. is DST INSPIRE Faculty (IFA-2014/CH-167), DST-India. We thank MRC-MNIT Jaipur for NMR and USIC University of Rajasthan, Jaipur for HRMS & GCMS data collection.
Notes and references
-
(a) C.-L. Sun and Z.-J. Shi, Chem. Rev., 2014, 114, 9219 CrossRef CAS PubMed and reference cited therein;
(b) C. R. Reddy and M. D. Reddy, J. Org. Chem., 2014, 79, 106 CrossRef CAS PubMed;
(c) M. Ochiai, Y. Takeuchi, T. Katayama, T. Sueda and K. Miyamoto, J. Am. Chem. Soc., 2005, 127, 12244 CrossRef CAS PubMed;
(d) T. Dohi, A. Maruyama, M. Yoshimura, K. Morimoto, H. Tohma and Y. Kita, Angew. Chem., Int. Ed., 2005, 44, 6193 CrossRef CAS PubMed;
(e) M. Uyanik, D. Suzuki, T. Yasui and K. Ishihara, Angew. Chem., Int. Ed., 2011, 50, 5331 CrossRef CAS PubMed;
(f) S. K. R. Parumala and R. K. Peddinti, Green Chem., 2015, 17, 4068 RSC;
(g) Y. Siddaraju and K. R. Prabhu, J. Org. Chem., 2016, 81, 7838 CrossRef CAS PubMed.
-
(a) R. N. Reddi, P. K. Prasad and A. Sudalai, Org. Lett., 2014, 16, 5674 CrossRef CAS PubMed;
(b) J. Huang, L. Li, H. Li, E. Husan, P. Wang and B. Wang, Chem. Commun., 2012, 48, 10204 RSC;
(c) G. Songjin, Y. Jin-Tao, D. Qiang, Y. Haitao and C. Jiang, Chem. Commun., 2014, 50, 6240 RSC;
(d) A. Wagner and A. R. Ofial, J. Org. Chem., 2015, 80, 2848 CrossRef CAS PubMed;
(e) J. Meesin, P. Katrun, C. Pareseecharoen, M. Pohmakotr, V. Reutrakul, D. Soorukram and C. Kuhakarn, J. Org. Chem., 2016, 81, 2744 CrossRef CAS PubMed.
-
(a) S. V. Ley and A. W. Thomas, Angew. Chem., Int. Ed., 2003, 42, 5400 CrossRef CAS PubMed;
(b) T. Kondo and T.-a. Mitsudo, Chem. Rev., 2000, 100, 3205 CrossRef CAS PubMed;
(c) I. P. Beletskaya and V. P. Ananikov, Chem. Rev., 2011, 111, 1596 CrossRef CAS PubMed;
(d) A. Gangjee, Y. Zeng, T. Talreja, J. J. McGuire, R. L. Kisliuk and S. F. Queener, J. Med. Chem., 2007, 50, 3046 CrossRef CAS PubMed;
(e) G. Liu, J. R. Huth, E. T. Olejniczak, F. Mendoza, S. W. Fesik and T. W. von Geldern, J. Med. Chem., 2001, 44, 1202 CrossRef CAS PubMed;
(f) G. De Martino, G. La Regina, A. Coluccia, M. C. Edler, M. C. Barbera, A. Brancale, E. Wilcox, E. Hamel, M. Artico and R. Silvestri, J. Med. Chem., 2004, 47, 6120 CrossRef CAS PubMed;
(g) J. Hutton, A. D. Jones, S. A. Lee, D. M. G. Martin, B. R. Meyrick, I. Patel, R. F. Peardon and L. Powell, Org. Process Res. Dev., 1997, 1, 61 CrossRef CAS;
(h) S. W. Kaldor, V. J. Kalish, J. F. Davies, B. V. Shetty, J. E. Fritz, K. Appelt, J. A. Burgess, K. M. Campanale, N. Y. Chirgadze, D. K. Clawson, B. A. Dressman, S. D. Hatch, D. A. Khalil, M. B. Kosa, P. P. Lubbehusen, M. A. Muesing, A. K. Patick, S. H. Reich, K. S. Su and J. H. Tatlock, J. Med. Chem., 1997, 40, 3979 CrossRef CAS PubMed;
(i) A. Dondoni, Angew. Chem., Int. Ed., 2008, 47, 8995 CrossRef CAS PubMed;
(j) A. B. Lowe, Polym. Chem., 2010, 1, 17 RSC;
(k) J. Liu, J. Yang, Q. Yang, G. Wang and Y. Li, Adv. Funct. Mater., 2005, 15, 1297 CrossRef CAS.
-
(a) M.-L. Alcaraz, S. Atkinson, P. Cornwall, A. C. Foster, D. M. Gill, L. A. Humphries, P. S. Keegan, R. Kemp, E. Merifield, R. A. Nixon, A. J. Noble, D. O'Beirne, Z. M. Patel, J. Perkins, P. Rowan, P. Sadler, J. T. Singleton, J. Tornos, A. J. Watts and I. A. Woodland, Org. Process Res. Dev., 2005, 9, 555 CrossRef CAS;
(b) J. C. Sheehan and K. R. HeneryLogan, J. Am. Chem. Soc., 1959, 81, 3089 CrossRef CAS;
(c) S. Raghavan and B. Sridhar, J. Org. Chem., 2010, 75, 498 CrossRef CAS PubMed;
(d) C. Bharathi, K. J. Prabahar, C. S. Prasad, M. S. Rao, G. N. Trinadhachary, V. K. Handa, R. Dandala and A. Naidu, Pharmazie, 2008, 63, 14 CAS.
-
(a) T. Migita, T. Shimizu, Y. Asami, J. Shiobara, Y. Kato and M. Kosugi, Bull. Chem. Soc. Jpn., 1980, 53, 1385 CrossRef CAS;
(b) M. Kosugi, T. Ogata, M. Terada, H. Sano and T. Migita, Bull. Chem. Soc. Jpn., 1985, 58, 3657 CrossRef CAS;
(c) C. S. Bryan, J. A. Braunger and M. Lautens, Angew. Chem., Int. Ed., 2009, 48, 7064 CrossRef CAS PubMed;
(d) M. Kuhn, F. C. Falk and J. Paradies, Org. Lett., 2011, 13, 4100 CrossRef CAS PubMed;
(e) Y.-J. Chen and H. H. Chen, Org. Lett., 2006, 8, 5609 CrossRef CAS PubMed;
(f) J.-H. Cheng, C.-L. Yi, T.-J. Liu and C.-F. Lee, Chem. Commun., 2012, 48, 8440 RSC;
(g) Y.-A. Chen, S. S. Badsara, W.-T. Tsai and C.-F. Lee, Synthesis, 2015, 47, 181 CAS;
(h) J.-R. Wu, C.-H. Lin and C.-F. Lee, Chem. Commun., 2009, 4450 RSC;
(i) A. Correa, M. Carril and C. Bolm, Angew. Chem., Int. Ed., 2008, 47, 2880 CrossRef CAS PubMed;
(j) V. Percec, J.-Y. Bae and D. H. Hill, J. Org. Chem., 1995, 60, 6895 CrossRef CAS;
(k) Y. Zhang, K. C. Ngeow and J. Y. Ying, Org. Lett., 2007, 9, 3495 CrossRef CAS PubMed;
(l) V. P. Reddy, A. V. Kumar, K. Swapna and K. R. Rao, Org. Lett., 2009, 11, 1697 CrossRef CAS PubMed;
(m) Y.-C. Wong, T. T. Jayanth and C.-H. Cheng, Org. Lett., 2006, 8, 5613 CrossRef CAS PubMed;
(n) N. Morita and N. Krause, Angew. Chem., Int. Ed., 2006, 45, 1897 CrossRef CAS PubMed;
(o) R. Das and D. Chakraborty, Tetrahedron Lett., 2012, 53, 7023 CrossRef CAS;
(p) T.-J. Liu, C.-L. Yi, C.-C. Chan and C.-F. Lee, Chem.–Asian J., 2013, 8, 1029 CrossRef CAS PubMed.
-
(a) C.-F. Lee, Y.-C. Liu and S. S. Badsara, Chem.–Asian J., 2014, 9, 706 CrossRef CAS PubMed;
(b) D. J. Procter, J. Chem. Soc., Perkin Trans. 1, 2001, 335 RSC;
(c) H. Liu and X. Jiang, Chem.–Asian J., 2013, 8, 2546 CrossRef CAS PubMed.
-
(a) J. Feng, G. Lu, M. Lv and C. Cai, Synlett, 2015, 26, 915 CrossRef CAS;
(b) J. Feng, M.-F. Lv, G.-P. Lu and C. Cai, Org. Biomol. Chem., 2015, 13, 677 RSC;
(c) S.-r. Guo, Y.-q. Yuan and J.-n. Xiang, Org. Lett., 2013, 15, 4654 CrossRef CAS PubMed;
(d) B. Du, B. Jin and P. Sun, Org. Lett., 2014, 16, 3032 CrossRef CAS PubMed;
(e) J.-W. Zeng, Y.-C. Liu, P.-A. Hsieh, Y.-T. Huang, C.-L. Yi, S. S. Badsara and C.-F. Lee, Green Chem., 2014, 16, 2644 RSC;
(f) S. S. Badsara, Y.-C. Liu, P.-A. Hsieh, J.-W. Zeng, S.-Y. Lu, Y.-W. Liu and C.-F. Lee, Chem. Commun., 2014, 50, 11374 RSC;
(g) J. Yuan, X. Ma, H. Yi, C. Liu and A. Lei, Chem. Commun., 2014, 50, 14386 RSC;
(h) R.-Y. Tang, Y.-X. Xie, Y.-L. Xie, J.-N. Xiang and J.-H. Li, Chem. Commun., 2011, 47, 12867 RSC;
(i) X. Zhu, X. Xie, P. Li, J. Guo and L. Wang, Org. Lett., 2016, 18, 1546 CrossRef CAS PubMed;
(j) B. V. Varun and K. R. Prabhu, J. Org. Chem., 2014, 79, 9655 CrossRef CAS PubMed;
(k) Y.-W. Liu, S. S. Badsara, Y.-C. Liu and C.-F. Lee, RSC Adv., 2015, 5, 44299 RSC;
(l) C. D. Prasad, S. J. Balkrishna, A. Kumar, B. S. Bhakuni, K. Shrimali, S. Biswas and S. Kumar, J. Org. Chem., 2013, 78, 1434 CrossRef CAS PubMed.
-
(a) Z. Yang, W.-J. Hao, S.-L. Wang, J.-P. Zhang, B. Jiang, G. Li and S.-J. Tu, J. Org. Chem., 2015, 80, 9224 CrossRef CAS PubMed;
(b) Y. He, J. Li, S. Luo, J. Huang and Q. Zhu, Chem. Commun., 2016, 52, 8444 RSC;
(c) P.-A. Hsieh, S. S. Badsara, C.-H. Tsai, D. M. Reddy and C.-F. Lee, Synlett, 2016, 27, 1557 CrossRef CAS.
-
(a) K. Ajiki, M. Hirano and K. Tanaka, Org. Lett., 2005, 7, 4193 CrossRef CAS PubMed;
(b) B. C. Ranu and T. Mandal, J. Org. Chem., 2004, 69, 5793 CrossRef CAS PubMed;
(c) S. Chowdhury and S. Roy, Tetrahedron Lett., 1997, 38, 2149 CrossRef CAS;
(d) Y. Yatsumonji, Y. Ishida, A. Tsubouchi and T. Takeda, Org. Lett., 2007, 9, 4603 CrossRef CAS PubMed;
(e) P. S. Reddy, M. A. Reddy, B. Sreedhar and M. V. B. Rao, Synth. Commun., 2010, 40, 2075 CrossRef CAS.
-
(a) D. Basavaiah, K. R. Reddy and N. Kumaragurubaran, Nat. Protoc., 2007, 2, 2665 CrossRef CAS PubMed;
(b) D. Basavaiah, B. S. Reddy and S. S. Badsara, Chem. Rev., 2010, 110, 5447 CrossRef CAS PubMed;
(c) K. Karnakar, K. Ramesh, S. N. Murty and Y. P. D. Nageswar, Helv. Chim. Acta, 2013, 96, 2276 CrossRef CAS.
-
(a) A. Gruiec and A. Foucaud, New J. Chem., 1991, 15, 943 CAS;
(b) D. Basavaiah, P. K. S. Sarma and A. K. D. Bhavani, J. Chem. Soc., Chem. Commun., 1994, 1091 RSC;
(c) D. Basavaiah and P. K. S. Sarma, J. Chem. Soc., Chem. Commun., 1992, 955 RSC;
(d) D. Basavaiah, A. K. D. Bhavani, S. Pandiaraju and P. K. S. Sarma, Synlett, 1995, 2434 Search PubMed;
(e) D. Basavaiah and S. Pandiaraju, Tetrahedron, 1996, 52, 2261 CrossRef CAS;
(f) H. J. Lee, M. R. Seong and J. N. Kim, Tetrahedron Lett., 1998, 39, 6223 CrossRef CAS;
(g) G. W. Kabalka, B. Venkataiah and G. Dong, Org. Lett., 2003, 5, 3803 CrossRef CAS PubMed;
(h) G. W. Kabalka, G. Dong, B. Venkataiah and C. Chen, J. Org. Chem., 2005, 70, 9207 CrossRef CAS PubMed;
(i) B. C. Ranu, K. Chattopadhyay and R. Jana, Tetrahedron Lett., 2007, 48, 3847 CrossRef CAS;
(j) M. L. Kantam, K. B. S. Kumar and B. Sreedhar, J. Org. Chem., 2008, 73, 320 CrossRef CAS PubMed;
(k) D. Basavaiah, S. S. Badsara and B. C. Sahu, Chem.–Eur. J., 2013, 19, 2961 CrossRef CAS PubMed.
- The allyl iodide 1d was obtained as E-isomer. Accordingly, the corresponding allylic thioethers 4w and 4x were also obtained as E-isomer.
- P. O. Deane, J. J. Guthrie-Strachan, P. T. Kaye and R. E. Whittaker, Synth. Commun., 1998, 28, 2601 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra04817c |
| ‡ Authors contributed equally. |
|
| This journal is © The Royal Society of Chemistry 2017 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *
*