Open Access Article
Open Access ArticlePhase transformations of vanadium recovery from refractory stone coal by novel NaOH molten roasting and water leaching technology
Zhenlei Cai *abc and
Yimin Zhang*abcd
*abc and
Yimin Zhang*abcd
aSchool of Resource and Environmental Engineering, Wuhan University of Science and Technology, Wuhan 430081, China. E-mail: caizhenlei@wust.edu.cn; ande559@163.com; zym126135@126.com
bHubei Provincial Engineering Technology Research Center of High Efficient Cleaning Utilization for Shale Vanadium Resource, Wuhan 430081, China
cHubei Collaborative Innovation Center for High Efficient Utilization of Vanadium Resources, Wuhan 430081, China
dSchool of Resource and Environmental Engineering, Wuhan University of Technology, Wuhan 430070, China
First published on 26th July 2017
Abstract
In this study, a novel NaOH molten roasting and water leaching technology, an eco-friendly process with good selectivity and good availability, was investigated for vanadium recovery from refractory stone coal. In addition, the phase transformations during the vanadium recovery process were also studied. During the NaOH molten roasting process, the monoclinic crystalline structure of muscovite (K(Al,V)2[Si3AlO10](OH)2) was converted into the orthorhombic crystalline structure of Na2SiO3 and the tetragonal crystalline structure of gehlenite (Ca2Al2SiO7); this could promote the liberation and recovery of vanadium. During the water leaching process, the tetragonal crystalline structure of gehlenite (Ca2Al2SiO7) was converted into the monoclinic crystalline structure of Ca3Si2O7·H2O. Ca3Si2O7·H2O, a kind of C–S–H gel, produced during the water leaching process probably covered the surface of the roscoelite and thus prevented effective vanadium recovery from roscoelite.
1. Introduction
Vanadium plays significant roles in many fields such as ferrous and nonferrous alloy production,1 catalysts,2 and redox flow batteries.3 More than 87% of the vanadium resources exist as stone coal in China. Moreover, most of them are refractory for vanadium recovery because vanadium in this stone coal usually exists as isomorphism substitution in the mica (muscovite or illite) lattice structure, which is named as roscoelite.4In recent years, many additives were employed for the recovery of vanadium from different vanadium resources, which could be divided into leaching additives and roasting additives. The study on the leaching additives utilized for vanadium slag or spent catalysts was mainly focused on oxalic acid,5 NaClO3,6 Na2SO3,7 etc. Moreover, the existence of leaching additives for vanadium recovery from stone coal is universal. These additives primarily include FeSO4,8 NaClO,9 CaF2,10 H2SiF6,11 etc. Most of these leaching additives are effectively used under acidic conditions, resulting in lower selectivity and increased dissolution of impurities. The increase in impurity concentration probably causes negative effects in the subsequent purification process. Although there have been a few investigations on the basic additives for the vanadium recovery from stone coal, such as on the use of NaOH12 solution for vanadium leaching from roasted stone coal, the efficiency of vanadium recovery is low since high roasting or leaching temperature and extended roasting time are required.
Based on the developments of the roasting additives and technologies for the vanadium recovery, Na2CO3 (ref. 13 and 14) and CaO15 were effective roasting additives. They were not, however, suitable for vanadium recovery from stone coal with high alumino–silicate content because of the requirement for high roasting temperatures. Although the utilization of NaCl16 or Na2SO4 (ref. 17) was relatively effective for vanadium extraction from stone coal, it was gradually restricted in China because of serious poisonous emissions of Cl2, HCl or SO2 gases. The effect of calcified roasting via CaCO3 (ref. 18) or Ca(OH)2 (ref. 19) on vanadium extraction from stone coal was limited because of low crystalline destruction of roscoelite and vanadium oxidation degree. Moreover, composite additives such as NaCl and CaO can also be effective for vanadium extraction from stone coal and remove Cl from fuel gas, but their application has been confined to a fluidized bed roasting reactor.20 Recently, our research group reported a novel BaCO3/CaO composite roasting additive and acid leaching technology for vanadium recovery from stone coal. However, its industrial application and feasibility remains to be further studied.21,22
While the NaOH molten roasting technology exhibits eco-friendliness (no discharge of poisonous gases and wastes), good selectivity (low extraction of impurities), and good availability (low roasting temperature and high recovery efficiency), to date, it has only been applied for the recovery of titanium or chromium from secondary resources.23,24 However, the feasibility and applicability of this technology to vanadium recovery from stone coal, especially for high alumino–silicate-containing refractory stone coal, still lacks sufficient scientific evidence. In addition, the phase transformation and the ion leaching behavior during the recovery process require further investigation for the guidance and reference of this technology.
In this study, the effectiveness of the novel NaOH molten roasting and water leaching technology for vanadium recovery from refractory stone coal was investigated. Many technical conditions were optimized, and the leaching behaviors of vanadium, silicon, aluminum, and potassium were studied. Moreover, the phase transformations were also investigated to reveal the mechanisms responsible for the vanadium recovery process.
2. Materials and methods
2.1. Materials
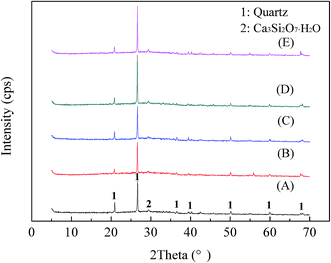
The raw stone coal was obtained from Hubei, China. The raw ore was crushed and ground into powder with the particle size of −0.074 mm accounting for 75% of the sample. The chemical multi-elemental analysis of the raw stone coal is listed in Table 1. The main mineral phase compositions of the raw stone coal, which were determined by XRD (Fig. 1), included quartz, muscovite, phlogopite, calcite, and pyrite. The electron microprobe analysis of the raw stone coal is provided in Table 2. The vanadium-containing minerals mainly consisted of muscovite and illite.| V2O5 | SiO2 | Al2O3 | CaO | Fe2O3 | K2O | MgO | Na2O | SO3 | P2O5 | TC | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Raw stone coal | 0.77 | 51.15 | 9.08 | 8.33 | 2.44 | 1.97 | 1.82 | 0.45 | 3.55 | 1.29 | 17.89 |
| Blank roasted sample | 0.91 | 56.36 | 10.69 | 9.27 | 2.83 | 3.81 | 2.75 | 0.44 | 1.42 | 1.18 | 2.67 |
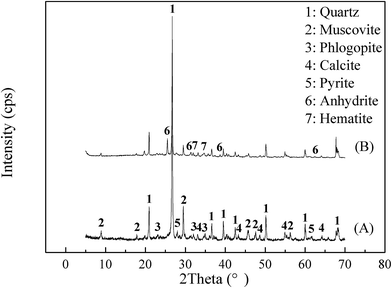 | ||
| Fig. 1 XRD image of raw stone coal and blank roasted sample. ((A) Raw stone coal, (B) blank roasted sample). | ||
| Mineral | SiO2 | V2O3 | Al2O3 | FeO | MgO | CaO | Na2O | K2O |
|---|---|---|---|---|---|---|---|---|
| Pyrite | 0.06 | 0.00 | 0.00 | 59.12 | 0.00 | 0.00 | 0.00 | 0.00 |
| Calcite | 0.03 | 0.00 | 0.00 | 0.07 | 0.55 | 63.44 | 0.00 | 0.00 |
| Quartz | 98.35 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Muscovite | 51.08 | 3.48 | 27.22 | 0.23 | 4.53 | 0.02 | 0.07 | 9.52 |
| Illite | 31.88 | 2.25 | 16.98 | 0.18 | 1.56 | 0.74 | 0.02 | 6.11 |
| Anorthose | 56.94 | 0.00 | 25.25 | 0.00 | 0.10 | 6.51 | 6.82 | 0.98 |
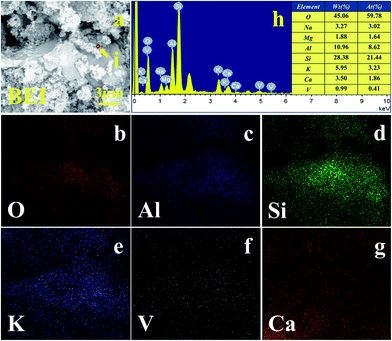
The raw stone coal was subjected to carbon removal pre-treatment by blank roasting in a muffle furnace at 750 °C for 1 h. The chemical multi-elemental analysis and the XRD pattern of the blank roasted sample are shown in Table 1 and Fig. 1, respectively. The results when compared with those of the raw ore indicated that the V2O5 content increased to 0.91% and the crystalline phase diffraction peak of pyrite was converted into that of hematite. Additionally, a new crystalline phase diffraction peak of anhydrite appeared. The BEI and EDS elemental distributions of the blank roasted sample are shown in Fig. 2. The EDS spectra analysis of the (i) point showed that the aluminium content was 11.80%, which was close to the theoretical aluminium content 12.80% of muscovite (K(Al,V)2[Si3AlO10](OH)2) containing vanadium. Moreover, the relevance of V, O, Al, Si, and K in the blank roasted sample indicated that vanadium probably existed in muscovite (K(Al,V)2[Si3AlO10](OH)2).
 | ||
| Fig. 2 (a) BEI of blank roasted sample, EDS elemental distribution: (b) O; (c) Al; (d) Si; (e) K; (f) V and (g) EDS spectra marked from BEI by circle. | ||
2.2. Experimental procedure
Herein, 20 g of blank roasted sample was added to a nickel crucible with a certain mass ratio of NaOH added to it and completely mixed. After the crucible was placed in a muffle furnace, the NaOH molten roasting process was started at the required temperature for a certain period of time. When the roasting process was complete, the roasted product was transferred to a leaching pod containing 100 mL water. The solution was continuously stirred at the required temperature for a certain period. After the leaching solution was filtrated, vanadium, silicon, aluminium, and potassium in the filtrate were analysed by ICP-OES to calculate the leaching efficiency. The leaching efficiency can be calculated as follows:
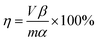 | (1) |
3. Results and discussion
3.1. NaOH molten roasting process
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
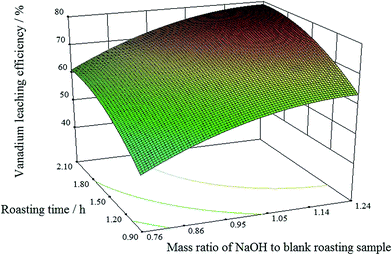
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, when the condition of the roasting temperature was intermediate level (550 °C). The vanadium leaching efficiency increased with the extension of the roasting time. When the roasting time was 1.5 h, the vanadium leaching efficiency was maximized.
1, when the condition of the roasting temperature was intermediate level (550 °C). The vanadium leaching efficiency increased with the extension of the roasting time. When the roasting time was 1.5 h, the vanadium leaching efficiency was maximized.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
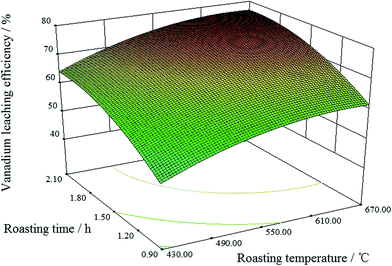
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The vanadium leaching efficiency did not reduce until the roasting temperature was over 550 °C when the condition of the mass ratio was intermediate level (1
1. The vanadium leaching efficiency did not reduce until the roasting temperature was over 550 °C when the condition of the mass ratio was intermediate level (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Longer roasting times benefited the vanadium leaching efficiency. However, when the roasting time was over 1.5 h, further extension of the roasting time only slightly improved the vanadium leaching efficiency.
1). Longer roasting times benefited the vanadium leaching efficiency. However, when the roasting time was over 1.5 h, further extension of the roasting time only slightly improved the vanadium leaching efficiency.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and a roasting temperature of 550 °C were used, with the roasting time ranging from 10 min to 90 min. The XRD patterns are shown in Fig. 5. The results indicated that the muscovite and phlogopite crystalline phases disappeared, and new crystalline phases of Na2SiO3 and gehlenite were present in the roasted sample, indicating that the structure of muscovite or phlogopite was effectively destroyed. The diffraction peaks of the quartz crystalline phase were first enhanced during the first 30 min of roasting and then weakened in the following period. This is mainly because of the selective dissolution of mica during the NaOH molten roasting process in the first period and the dissolution of quartz in the subsequent stage. Moreover, V(III), which was an isomorphic replacement of Al(III) in the octahedral alumina in the mica of stone coal, was liberated from the crystal lattice and oxidized into V(IV) or V(V) for the preparation of the following water leaching process.
1 and a roasting temperature of 550 °C were used, with the roasting time ranging from 10 min to 90 min. The XRD patterns are shown in Fig. 5. The results indicated that the muscovite and phlogopite crystalline phases disappeared, and new crystalline phases of Na2SiO3 and gehlenite were present in the roasted sample, indicating that the structure of muscovite or phlogopite was effectively destroyed. The diffraction peaks of the quartz crystalline phase were first enhanced during the first 30 min of roasting and then weakened in the following period. This is mainly because of the selective dissolution of mica during the NaOH molten roasting process in the first period and the dissolution of quartz in the subsequent stage. Moreover, V(III), which was an isomorphic replacement of Al(III) in the octahedral alumina in the mica of stone coal, was liberated from the crystal lattice and oxidized into V(IV) or V(V) for the preparation of the following water leaching process.
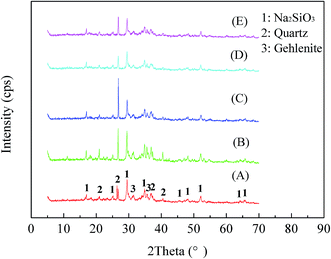 | ||
| Fig. 5 XRD patterns of roasted samples at different roasting times. ((A) roasting for 10 min; (B) roasting for 20 min; (C) roasting for 30 min; (D) roasting for 60 min (E) roasting for 90 min). | ||
The crystal transformation relationship of muscovite (K(Al,V)2[Si3AlO10](OH)2), Na2SiO3, and gehlenite (Ca2Al2SiO7) is shown in Fig. 6. The crystalline structure of muscovite (K(Al,V)2[Si3AlO10](OH)2) is with a monoclinic crystal system, and the unit cell parameters are a = 0.5193 nm, b = 0.9045 nm, c = 2.0044 nm, α = γ = 90°, and β = 95.8°. During the NaOH molten roasting process, Si–O and Al–O bonds were broken. Moreover, the orthorhombic structure of Na2SiO3 and the tetragonal structure of gehlenite (Ca2Al2SiO7) were formed. The crystalline structure of Na2SiO3 is with the CMC21 space group and the unit cell parameters are a = 1.0466 nm, b = 0.603 nm, c = 0.471 nm, and α = β = γ = 90°. The crystalline structure of gehlenite (Ca2Al2SiO7) is with the P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 21m space group and the unit cell parameters are a = b = 0.7693 nm, c = 0.5072 nm, and α = β = γ = 90°. Al3+ ion and the Si4+ ion locate at the same position in the unit cell with different occupancies. Al–Si–O oxides form a double close packed type array with the inclusion of Ca2+ through tetragonal coordination.
21m space group and the unit cell parameters are a = b = 0.7693 nm, c = 0.5072 nm, and α = β = γ = 90°. Al3+ ion and the Si4+ ion locate at the same position in the unit cell with different occupancies. Al–Si–O oxides form a double close packed type array with the inclusion of Ca2+ through tetragonal coordination.
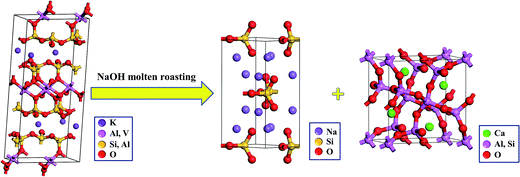 | ||
| Fig. 6 Crystal transformation relationship of muscovite (K(Al,V)2[Si3AlO10](OH)2), Na2SiO3 and gehlenite (Ca2Al2SiO7) in the NaOH molten roasting process. | ||
The crystal transformation usually includes two steps: modification and reconstruction. The modification only refers to the change of cell parameters, but not the chemical bonds. However, reconstruction refers to the original large area breakage of chemical bonds and the rebuilding of new structure. Therefore, according to the abovementioned analysis, the phase transformation in the NaOH molten roasting process belongs to the reconstruction, and vanadium can be liberated during this process. The reaction equation corresponding to the phase transformation in the NaOH molten roasting process is as follows:
| 2K(Al,V)2[Si3AlO10](OH)2 (muscovite) + 6NaOH + 6CaCO3 + 5O2 → 3Na2SiO3 + 3Ca2Al2SiO7 (gehlenite) + 2V2O5 + K2O + 6CO2 + 5H2O | (2) |
3.2. Water leaching process
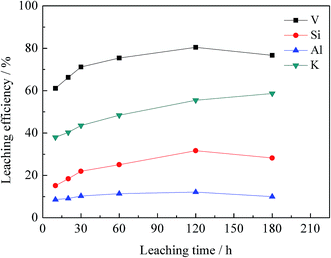 | ||
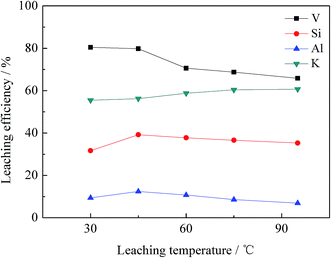
| Fig. 8 Effect of leaching time on leaching efficiency of vanadium, silicon, aluminium and potassium. | ||
The chemical multi-elemental analysis of the leachate under the optimal conditions is shown in Table 3. The V2O5 content was 0.72 g L−1, and the main impurities were SiO2 (22.54 g L−1), K2O (2.28 g L−1), and Al2O3 (1.28 g L−1).
| Element | V2O5 | SiO2 | K2O | Al2O3 | CaO | MgO |
|---|---|---|---|---|---|---|
| Content | 0.72 | 22.54 | 2.28 | 1.28 | 0.50 | 0.08 |
The crystal transformation relationship of gehlenite (Ca2Al2SiO7) and Ca3Si2O7·H2O is shown in Fig. 10. During the water leaching process, the Al–O bond of gehlenite (Ca2Al2SiO7) was broken. Moreover, the Ca–Si and Ca–O bonds were shaped, and the monoclinic structure of Ca3Si2O7·H2O was formed. The crystalline structure of Ca3Si2O7·H2O is with the P21/M space group and the unit cell parameters are a = 0.6824 nm, b = 1.5465 nm, c = 0.6839 nm, α = γ = 90°, and β = 97.692°. The reaction equation corresponding to the phase transformation during the water leaching process is as follows:
| 3Ca2Al2SiO7 (gehlenite) + Na2SiO3 + 4NaOH → 2Ca3Si2O7·H2O + 6NaAlO2 | (3) |
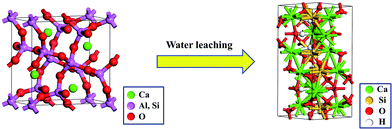 | ||
| Fig. 10 Crystal transformation relationship of gehlenite (Ca2Al2SiO7) and Ca3Si2O7·H2O in the water leaching process. | ||
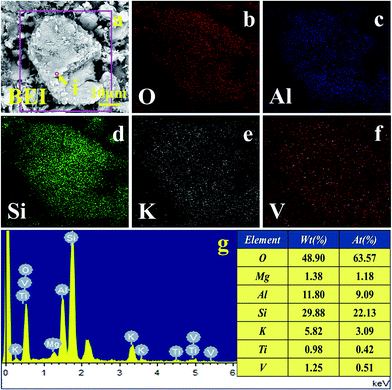
The BEI and EDS elemental distributions of water leaching residual are shown in Fig. 11. The results indicate that the element distribution of O, Si, and Ca have obvious relevance. Combined with the XRD analysis of the water leaching residual, Ca3Si2O7·H2O indeed existed after the NaOH molten roasting and water leaching process. The EDS spectra analysis of the (i) point showed that the aluminium content was 10.96%, which was close to the theoretical aluminium content 12.80% of muscovite (K(Al,V)2[Si3AlO10](OH)2) containing vanadium. Moreover, the relevance of V, O, Al, Si, and K in the leaching residual indicated that there was still a small amount of roscoelite left in the leaching residual. Ca3Si2O7·H2O, a kind of C–S–H gel,27 produced during the water leaching process probably covered the surface of the roscoelite and thus prevented the effective recovery of vanadium from the roscoelite.
4. Conclusions
(1) The novel NaOH molten roasting and water leaching technology, an eco-friendly process with good selectivity and good availability, was found to be feasible for the recovery of vanadium from refractory stone coal.(2) During the NaOH molten roasting process, the monoclinic crystalline structure of muscovite (K(Al,V)2[Si3AlO10](OH)2) in stone coal was converted into the orthorhombic crystalline structure of Na2SiO3 and the tetragonal crystalline structure of gehlenite (Ca2Al2SiO7), which could promote the liberation and recovery of vanadium.
(3) During the water leaching process, the tetragonal crystalline structure of gehlenite (Ca2Al2SiO7) was converted into the monoclinic crystalline structure of Ca3Si2O7·H2O. Ca3Si2O7·H2O, a kind of C–S–H gel, produced during the water leaching process probably covered the surface of the roscoelite and thus prevented the effective recovery of vanadium from the roscoelite.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 51604197) and the Natural Science Foundation of Hubei Province, China (No. 2016CFB197).References
- W. Li, Y. M. Zhang, T. Liu, J. Huang and Y. Wang, Hydrometallurgy, 2013, 131, 1 CrossRef.
- Y. T. Chen, W. Chen, Q. H. Tang, Z. Guo, Y. H. Yang and F. B. Su, Catal. Lett., 2011, 141, 149 CrossRef CAS.
- T. Hong and F. Q. Xue, Paper presented at the 2009 Asia-Pacific Power and Energy Engineering Conference, 2009, p. 1 Search PubMed.
- X. B. Li, C. Wei, Z. G. Deng, M. T. Li, C. X. Li and G. Fan, Hydrometallurgy, 2011, 105, 359 CrossRef CAS.
- K. Mazurek, Hydrometallurgy, 2013, 134–135, 26 CrossRef CAS.
- K. Yang, X. Zhang, X. Tian, Y. Yang and Y. Chen, Hydrometallurgy, 2010, 103, 7 CrossRef CAS.
- M. D. Okudan, A. Akcil, A. Tuncuk and H. Deveci, Hydrometallurgy, 2015, 152, 76 CrossRef CAS.
- M. T. Li, C. Wei, G. Fan, C. X. Li, Z. G. Deng and X. B. Li, Trans. Nonferrous Met. Soc. China, 2010, 20, s112 CrossRef CAS.
- C. X. Li, C. Wei, Z. G. Deng, M. T. Li, X. B. Li and G. Fan, Trans. Nonferrous Met. Soc. China, 2010, 20, s127 CrossRef CAS.
- Z. J. Ju, C. Y. Wang and F. Yin, Int. J. Miner. Process., 2015, 138, 1 CrossRef CAS.
- X. Zhang, K. Yang, X. Tian and W. Qin, Int. J. Miner. Process., 2011, 100, 184 CrossRef CAS.
- D. He, Q. Feng, G. Zhang, L. Ou and Y. Lu, Miner. Eng., 2007, 20, 1184 CrossRef CAS.
- M. Aarabi-Karasgani, F. Rashchi, N. Mostoufi and E. Vahidi, Hydrometallurgy, 2010, 102, 14 CrossRef CAS.
- H. Y. Li, H. X. Fang, K. Wang, W. Zhou, Z. Yang, X. M. Yan, W. S. Ge, Q. W. Li and B. Xie, Hydrometallurgy, 2015, 156, 124 CrossRef CAS.
- W. C. Song, H. Li, F. X. Zhu, K. Li and Q. Zheng, Trans. Nonferrous Met. Soc. China, 2014, 24, 2687 CrossRef CAS.
- M. Wang, X. Xiang, L. Zhang and L. Xiao, Rare Met., 2008, 27, 112 CrossRef CAS.
- Y. Zhao, Y. Zhang, S. Bao, T. Chen and X. Liu, Ind. Eng. Chem. Res., 2014, 53, 157 CrossRef CAS.
- C. L. Li, X. Y. Zhou, H. Wang, T. K. Zhang, J. Li, X. Ou and X. D. Jiang, J. Cent. South Univ., 2011, 42, 7 CAS.
- G. Zhang and S. Ma, Chin. J. Rare Met., 2007, 31, 813 Search PubMed.
- X. Zeng, F. Wang, H. Zhang, L. Cui, J. Yu and G. Xu, Fuel, 2015, 142, 180 CrossRef CAS.
- Z. L. Cai, Y. M. Zhang, T. Liu and J. Huang, Minerals, 2016, 6, 14 CrossRef.
- Z. L. Cai, Y. M. Zhang, T. Liu and J. Huang, JOM, 2015, 67, 2629 CrossRef CAS.
- Z. H. Wang, S. L. Zheng, S. N. Wang, B. Liu, D. W. Wang, H. Du and Y. Zhang, Trans. Nonferrous Met. Soc. China, 2014, 24, 1273 CrossRef CAS.
- D. S. Chen, L. S. Zhao, Y. H. Liu, T. Qi, J. C. Wang and L. N. Wang, J. Hazard. Mater., 2013, 244–245, 588 CrossRef CAS PubMed.
- E. B. Webb and S. H. Garofalini, Surf. Sci., 1994, 319, 381 CrossRef CAS.
- W. E. Adam, K. Neumann and J. P. Ploumen, Fette, Seifen, Anstrichm., 1979, 81, 445 CrossRef CAS.
- O. Mendoza, C. Giraldo, S. S. Camargo Jr and J. I. Tobón, Cem. Concr. Res., 2015, 74, 88 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |