Open Access Article
Open Access ArticleA turn-on fluorescent chemosensor based on acylhydrazone for sensing of Mg2+ with a low detection limit†
Jing-Han Hu *,
Jian-Bin Li,
You Sun,
Peng-Xiang Pei and
Jing Qi
*,
Jian-Bin Li,
You Sun,
Peng-Xiang Pei and
Jing Qi
College of Chemical and Biological Engineering, Lanzhou Jiaotong University, Lanzhou, Gansu 730070, P. R. China. E-mail: hujinghan62@163.com
First published on 7th June 2017
Abstract
A novel highly selective chemosensor for Mg2+ ions based on the naphthalene group as the fluorophore has been designed and synthesized, which shows a fluorescence turn-on response from colorless to green for Mg2+ ions in DMSO–H2O solutions. L could perform as an “OFF–ON” molecular switch, which was cyclically controlled by Mg2+ and EDTA. Moreover, further study demonstrated that the detection limit for the fluorescence response of L to Mg2+ was down to 9.2 × 10−10 M, which was lower than with other sensors for Mg2+. Test strips based on L were also fabricated, which could act as a convenient and efficient Mg2+ test kit. The sensor L could detect Mg2+ in two different sources of water, distilled and tap water.
Introduction
The magnesium ion (Mg2+) plays vital roles in biological systems.1–3 For plants, magnesium is one of the ingredients for forming chlorophyll and is involved with forming phosphate compounds and phosphate metabolism.4,5 In the cells of the human body, Mg2+ is one of the most abundant cations, and participates in numerous basic biochemical reactions. Magnesium also plays a crucial role in skeletal development and bone remodeling.6,7 Magnesium ion can influence nervous impulses and tension development in muscle, and modulates, amongst others, ion transport in nerves and mitochondria.8–10 If the concentrations of magnesium in the cytosol and subcellular regions are abnormal, there is a possibility of the occurrence of a disease, such as diabetes, hypertension, epilepsy and Alzheimer's disease.11–13 In particular, detection of Mg2+ has been of considerable interest and a great amount of effort has been devoted to the design and synthesis of sensitive and selective sensors for Mg2+ ion.Nonetheless, reports about fluorescent sensors for Mg2+ are still few compared with those on other important ions, such as Zn2+, Al3+, Hg2+ and CN−.14–27 Even so, some methodologies for detecting Mg2+ are often interfered with by Zn2+ and Ca2+. To date, as far as we know, β-diketone, crown ether derivatives, and nanoparticles have been employed for selective detection of Mg2+ ions.28–30 It is worth mentioning that their synthetic processes are intricate. Hence, there is interest in designing a highly selective, sensitive and simple chemosensor that recognizes Mg2+ without the interference of other metal ions.31,32
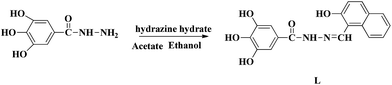
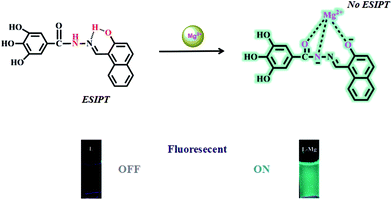
Our research group has a longstanding interest in molecular recognition.33–38 We have successfully developed a simple and efficient optical fluorescent acylhydrazone chemosensor L that can detect Mg2+ in DMSO–H2O solution. To the best of our knowledge, acylhydrazone is easy and inexpensive to prepare. The naphthalene group acts as the fluorophore, while the hydroxyl and imine groups in the sensor molecule L enhance the coordination capacity. As a result, the addition of Mg2+ inhibits the ESIPT process and increases its structural rigidity, which leads to a strong green emission. The mechanism of this process was verified by spectroscopic methods, including 1H NMR, DFT and mass spectrometry. The fluorescent detection limit of the sensor for Mg2+ was 9.2 × 10−10 M, while the corresponding detection limit by the naked eye was determined to be 5.0 × 10−8 M under a UV lamp at 365 nm.
Results and discussion
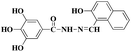
The molecule L was conveniently synthesized via the condensation of 3,4,5-trihydroxybenzhydrazide and 2-hydroxy-1-napthaldehyde. The recognition abilities of L were investigated by adding perchlorate salts (Fe3+, Hg2+, Ag+, Ca2+, Cu2+, Co2+, Ni2+, Cd2+, Pb2+, Zn2+, Cr3+ and Mg2+) into DMSO–H2O solutions (Scheme 1).Receptor L was found to have limited solubility in water, and this compelled us to use this sensor in mixed solvents. The fluorescence emission was examined upon adding various metal ions: Fe3+, Hg2+, Ag+, Ca2+, Cu2+, Co2+, Ni2+, Cd2+, Pb2+, Zn2+, Cr3+ and Mg2+ ions in DMSO/H2O (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
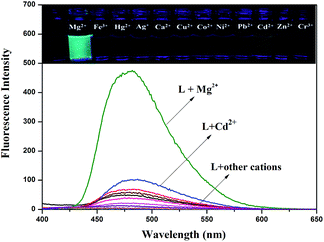
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v, 0.01 M HEPES, pH = 8.5). Only compound L displayed a weak, single fluorescence emission band at 480 nm when excited at 378 nm in DMSO–H2O media. Changes in the spectral pattern were observed only with the addition of 20 equivalents of Mg2+, when sensor L produced a strong fluorescence band at 480 nm and responded with a dramatic color change, from colorless to green. However, upon addition of other metal ions no significant changes in color were observed (Fig. 1). In addition, the fluorescence emission was also examined upon adding MnCl2 and CaCl2 (Fig S3†). The addition of Mn2+ caused no significant spectral change. The absorption spectra of L in the presence of other ions were also tested. The results suggested that sensor L could display an excellent selectivity for Mg2+ over all other ions tested (Fig S4†).
3, v/v, 0.01 M HEPES, pH = 8.5). Only compound L displayed a weak, single fluorescence emission band at 480 nm when excited at 378 nm in DMSO–H2O media. Changes in the spectral pattern were observed only with the addition of 20 equivalents of Mg2+, when sensor L produced a strong fluorescence band at 480 nm and responded with a dramatic color change, from colorless to green. However, upon addition of other metal ions no significant changes in color were observed (Fig. 1). In addition, the fluorescence emission was also examined upon adding MnCl2 and CaCl2 (Fig S3†). The addition of Mn2+ caused no significant spectral change. The absorption spectra of L in the presence of other ions were also tested. The results suggested that sensor L could display an excellent selectivity for Mg2+ over all other ions tested (Fig S4†).
It was well known that fluorescent probes for Mg2+ might encounter interference from other cations, particularly Zn2+ and Cd2+. Thus, competitive behavior was tested to further elucidate whether the coexistence of competing metal cations interfered with the detection of Mg2+. In the solutions of sensor L with Mg2+, when solutions of L were added to solutions of other competing cations, it was clear that the receptor L was highly selective for the detection of Mg2+ in DMSO/H2O (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v, 0.01 M HEPES, pH = 8.5) (Fig. 2).
3, v/v, 0.01 M HEPES, pH = 8.5) (Fig. 2).
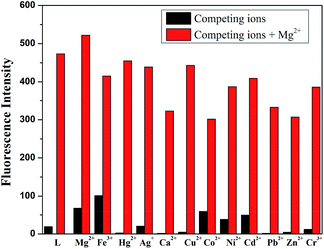 | ||
Fig. 2 Fluorescence spectra response of L (2.0 × 10−5 M) in the presence of various cations in DMSO/H2O (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v, 0.01 M HEPES, pH = 8.5) in response to Mg2+. 3, v/v, 0.01 M HEPES, pH = 8.5) in response to Mg2+. | ||
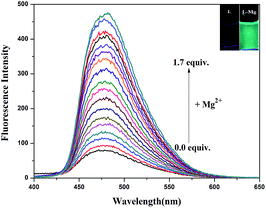
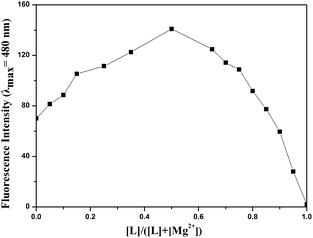
As shown in Fig. 3, the fluorescence titration of Mg2+ was performed using a 20 μM solution of L in DMSO/H2O (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v, 0.01 M HEPES, pH = 8.5). With the addition of Mg2+ (0.2 M), a gradual increase in the fluorescence intensity of chemosensor L at 480 nm was observed as the Mg2+ volume increased from 0 to 1.7 equivalents. The solution containing Mg2+ also showed a change in fluorescence color from colorless to grass green.
3, v/v, 0.01 M HEPES, pH = 8.5). With the addition of Mg2+ (0.2 M), a gradual increase in the fluorescence intensity of chemosensor L at 480 nm was observed as the Mg2+ volume increased from 0 to 1.7 equivalents. The solution containing Mg2+ also showed a change in fluorescence color from colorless to grass green.
The detection limit is one of the most important parameters in ion sensing. For various practical purposes, it is very important to detect analytes at low concentrations. The naked eye detection limit for Mg2+ was determined to be 5.0 × 10−8 M, through use of a UV lamp at 365 nm (Fig. 4). The detection limit for fluorescent spectrum changes was 9.2 × 10−10 M for Mg2+, as calculated on the basis of 3δ/S (where δ is the standard deviation of the blank solution and S is the slope of the calibration curve), which indicates the high detection sensitivity (Fig. 5). The result of the analysis is as follows: linear calibration equation: I = 1.30432C + 53.84714, R2 = 0.997.
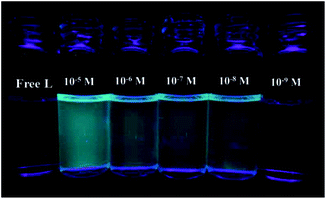 | ||
| Fig. 4 Naked-eye detection limit under UV light at 365 nm. From left to right the concentration of Mg2+: 10−5 M, 5 × 10−6 M, 5 × 10−7 M, 5 × 10−8 M, 5 × 10−9 M. | ||
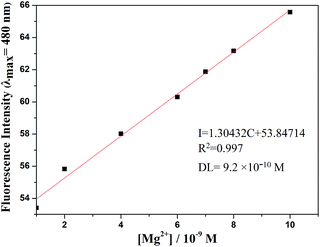 | ||
Fig. 5 Fluorescence detection limit spectra of L (2.0 × 10−5 M) in DMSO/H2O (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v, 0.01 M HEPES, pH = 8.5) solution upon adding of a concentration of Mg2+. 3, v/v, 0.01 M HEPES, pH = 8.5) solution upon adding of a concentration of Mg2+. | ||
We also made comparisons with other reported magnesium selective sensors, and the detection limit of L was much lower.29–31 Some did not explain the detection limits. This indicated that probe L could be used to quantitatively detect Mg2+ at extremely low concentrations and in a relatively wide range (Table 1).
The pH dependence of the probe L system in DMSO/H2O (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v, 0.01 M HEPES, pH = 8.5) was also checked by fluorescence emission spectroscopy. Mg2+ ion was added to buffered solutions of L at different pH values (1–12). The probe L was insensitive to H+ and OH−. However, the results with the L–Mg2+ system in DMSO–H2O media indicated that the reaction of L (2.0 × 10−5 M) with Mg2+ only occurred effectively in the pH range between 8 and 11, where the fluorescence intensity at 480 nm was enhanced more significantly, which indicated that the sensor L and Mg2+ ions and formed a magnesium complex in weak alkaline solutions (Fig. S5†).
3, v/v, 0.01 M HEPES, pH = 8.5) was also checked by fluorescence emission spectroscopy. Mg2+ ion was added to buffered solutions of L at different pH values (1–12). The probe L was insensitive to H+ and OH−. However, the results with the L–Mg2+ system in DMSO–H2O media indicated that the reaction of L (2.0 × 10−5 M) with Mg2+ only occurred effectively in the pH range between 8 and 11, where the fluorescence intensity at 480 nm was enhanced more significantly, which indicated that the sensor L and Mg2+ ions and formed a magnesium complex in weak alkaline solutions (Fig. S5†).
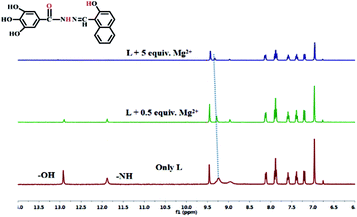
According to 1H NMR spectra (Fig. 6), the addition of Mg2+ made the two proton signals of –OH and –NH at 12.96 (s, 1H) and 11.92 (s, 1H) ppm gradually disappear. Due to coordination of the O atom of the carbonyl group in L, the signal of the two hydroxyl atoms at 9.29 (s, 2H) ppm in the benzene ring showed a significant downfield shift. To further investigate the interaction between the sensor L and Mg2+, IR spectra were collected, and the stretching vibration peak of (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) in L at 1605 cm−1 shifted to 1619 cm−1 when L interacted with Mg2+ (Fig. S6†). These phenomena indicate that in L–Mg2+, the Mg2+ coordinated with the oxygen atoms on the acylhydrazone group.
O) in L at 1605 cm−1 shifted to 1619 cm−1 when L interacted with Mg2+ (Fig. S6†). These phenomena indicate that in L–Mg2+, the Mg2+ coordinated with the oxygen atoms on the acylhydrazone group.
An obtained mass spectrum confirmed that the sensor L ion peak was detected at m/z 339.08, which corresponded to [L + H]+, and the magnesium complex ion peak appeared at m/z 439.09, which indicated that after deprotonation, probe L reacted with Mg2+ and one DMSO to form a stable complex [L − 2H+ + Mg2+ + 1DMSO +1H+]+ (Fig. S7†). A job plot analysis showed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry for the L–Mg2+ complex (Fig. 7). Based on the data in the job plot, 1H NMR spectra and IR spectra, a stable complex between L and Mg2+ was proposed.
1 stoichiometry for the L–Mg2+ complex (Fig. 7). Based on the data in the job plot, 1H NMR spectra and IR spectra, a stable complex between L and Mg2+ was proposed.
The sensing mechanism was estimated based on the above experiments. In solution, L exhibited weak fluorescence owing to ESIPT. The addition of Mg2+ broke the intramolecular hydrogen bond and deprotonated the secondary amine. Hence, excited state intramolecular proton transfer was inhibited. The sensor L showed strong green fluorescence (Scheme 2).
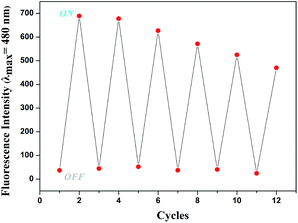
In order to investigate the reversibility of the sensor L, the addition of Mg2+ to a solution of sensor L that had no fluorescence showed increasing fluorescence (That was “ON”). After adding of EDTA to the solution of L–Mg2+ complex, there was no fluorescence (That was “OFF”). In fact, this “OFF–ON–OFF” switching process could be repeated several times with little fluorescent efficiency loss (Fig. 8).
To further confirm the proposed mechanism of sensor L with Mg2+, we performed DFT calculations at the B3LYP/6-311g (2d, p) level of theory. The highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) of L and the L + Mg2+ complex were investigated and are shown in Table 2. The HOMO–LUMO energy band gap of L + Mg2+ complex was 0.12157 au, which was lower than that of L (0.13522 au).
| L | L + Mg2+ | |
|---|---|---|
| a Blue ball is nitrogen, grey ball is carbon, red ball is oxygen, yellow ball is magnesium, and white ball is hydrogen. | ||
| Optimized structure |  |
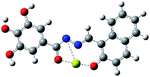 |
| Diagrams of LUMOs | 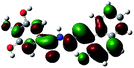 |
 |
| Diagrams of HOMOs |  |
 |
| Energy gaps | 0.13522 au | 0.12157 au |
To investigate the practical application of chemosensor L, test strips were prepared by immersing filter papers into a DMSO/H2O (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
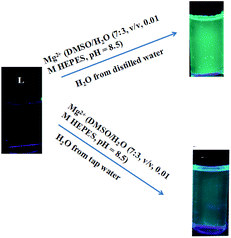
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v, 0.01 M HEPES, pH = 8.5) solution of L (0.1 M) and then drying in air. The test strips containing L were utilized to sense Mg2+ and other metal ions. As shown in Fig. 9, when the test strips were added to solutions of Mg2+ and the other ions, a significant color change was observed only with Mg2+ solution under a 365 nm UV lamp. Moreover, potentially competitive ions exerted no influence on the detection of Mg2+ by the test strips. Therefore, the test strips based on L could quickly and conveniently detect Mg2+ in solutions. The practical application of sensor L for selective sensing of Mg2+ in two different sources of water, distilled and tap water, has also been demonstrated. The results showed that sensor L was a sensitive sensor and could be applied in environmental analysis (Fig. 10).
3, v/v, 0.01 M HEPES, pH = 8.5) solution of L (0.1 M) and then drying in air. The test strips containing L were utilized to sense Mg2+ and other metal ions. As shown in Fig. 9, when the test strips were added to solutions of Mg2+ and the other ions, a significant color change was observed only with Mg2+ solution under a 365 nm UV lamp. Moreover, potentially competitive ions exerted no influence on the detection of Mg2+ by the test strips. Therefore, the test strips based on L could quickly and conveniently detect Mg2+ in solutions. The practical application of sensor L for selective sensing of Mg2+ in two different sources of water, distilled and tap water, has also been demonstrated. The results showed that sensor L was a sensitive sensor and could be applied in environmental analysis (Fig. 10).
Conclusions
In summary, we have developed a sensor that can detect Mg2+ ions in a weakly alkaline medium of DMSO/H2O (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v, 0.01 M HEPES, pH = 8.5) with high selectivity and special sensitivity. Moreover, the fluorescence detection limit was low 9.2 × 10−10 M for Mg2+. In addition, L could be used as a novel NOR logic gate triggered by Mg2+ and EDTA. Test strips based on L were also fabricated, which could serve as a practical fluorescent sensor to detect Mg2+ in field measurements or in test kits. Thus, the probe could have potential applications in both environmental and biological systems for the monitoring of magnesium.
3, v/v, 0.01 M HEPES, pH = 8.5) with high selectivity and special sensitivity. Moreover, the fluorescence detection limit was low 9.2 × 10−10 M for Mg2+. In addition, L could be used as a novel NOR logic gate triggered by Mg2+ and EDTA. Test strips based on L were also fabricated, which could serve as a practical fluorescent sensor to detect Mg2+ in field measurements or in test kits. Thus, the probe could have potential applications in both environmental and biological systems for the monitoring of magnesium.
Acknowledgements
This study was supported by the National Nature Science Foundation of China (No. 21467012).Notes and references
- G. Men, C. Chen, S. Zhang, C. Liang, Y. Wang, M. Deng, H. Shang, B. Yang and S. Jiang, Dolton Trans., 2015, 44, 2755 RSC.
- T. Dudev, K. Mazmanian and C. Lim, Phys. Chem. Chem. Phys., 2016, 18, 16986 RSC.
- H. S. Yin, B. C. Li, Y. L. Zhou, H. Y. Wang, M. H. Wang and S. Y. Ai, Biosens. Bioelectron., 2017, 96, 106 CrossRef CAS PubMed.
- B. O'Rourke, P. H. Backx and E. Marban, Science, 1992, 257, 245 Search PubMed.
- J. Smith, J. Am. Chem. Soc., 1927, 69, 1492 CrossRef.
- L. Wang, W. Qin, X. Tang, W. Dou and W. Liu, J. Phys. Chem. A, 2011, 115, 1609 CrossRef CAS PubMed.
- H. O. Trowbridge and J. L. Seltzer, J. Periodontal Res., 1967, 2, 147 CrossRef CAS PubMed.
- H. C. Politi and R. R. Preston, Neuroreport, 2003, 14, 659 CrossRef PubMed.
- Y. Ma, H. Liu, S. Liu and R. Yang, Analyst, 2012, 137, 2313 RSC.
- C. Berti, V. Zsolnay, T. R. Shannon, M. Fill and D. Gillespie, J. Mol. Cell. Cardiol., 2017, 103, 31 CrossRef CAS PubMed.
- J. Orrego-Hernández, N. Nunez-Dallos and J. Portilla, Talanta, 2016, 152, 432–437 CrossRef PubMed.
- L. Y. Shen, Y. H. Zhao, L. Mu, X. Zeng, R. Carl and G. Wei, Sens. Actuators, B, 2016, 226, 279 CrossRef.
- M. Jamshidi, O. Rezaei, A. R. Belverdi, S. Malekian and A. R. Belverdi, J. Mol. Struct., 2016, 1123, 111 CrossRef CAS.
- D. Ray and P. K. Bharadwaj, Inorg. Chem., 2008, 47, 2252 CrossRef CAS PubMed.
- C. Y. Lu, Y. W. Liu, P. J. Huang, C. F. Wan and A. T. Wu, Analyst, 2013, 138, 2527 RSC.
- S. Devaraj, Y. Tsui, C. Chiang and Y. Yen, Spectrochim. Acta, Part A, 2012, 96, 594 CrossRef CAS PubMed.
- R. Alam, T. Mistri, A. Katarkar, K. Chaudhuri, S. K. Mandal, A. R. K. Bukhsh, K. K. Das and M. Ali, Analyst, 2014, 139, 4022 RSC.
- V. K. Gupta, A. K. Singh and L. K. Kumawat, Sens. Actuators, B, 2014, 204, 507 CrossRef CAS.
- D. Sarkar, A. K. Pramanik and T. K. Mondal, J. Lumin., 2014, 146, 480 CrossRef CAS.
- B. B. Shi, P. Zhang, T. Wei, H. Yao, Q. Lin and Y. Zhang, Chem. Commun., 2013, 49, 7812 RSC.
- S. Wang, X. Fei, J. Guo, Q. Yang, Y. Li and Y. Song, Talanta, 2016, 148, 229 CrossRef CAS PubMed.
- Y. Sun, J. H. Hu, J. Qi and J. B. Li, Spectrochim. Acta, Part A, 2016, 167, 101 CrossRef CAS PubMed.
- D. S. Kopchuk, A. M. Prokhorov, P. A. Slepukhin and D. N. Kozhevnikov, Tetrahedron Lett., 2012, 53, 6265 CrossRef CAS.
- Q. Lin, Y. P. Fu, P. Chen, T. B. Wei and Y. M. Zhang, Dyes Pigm., 2013, 96, 1 CrossRef CAS.
- (a) J. Liu, Q. Lin, Y. M. Zhang and T. B. Wei, Sens. Actuators, B, 2014, 196, 619 CrossRef CAS; (b) G. Chen, Z. Guo, G. Zeng and L. Tang, Analyst, 2015, 140, 5400 RSC.
- J. H. Hu, J. Qi, Y. Sun and P. X. Pei, Phosphorus, Sulfur Silicon Relat. Elem., 2017, 192, 565 CrossRef CAS.
- P. Zhang, B. B. Shi, X. M. You, Y. M. Zhang, Q. Lin, H. Yao and T. B. Wei, Tetrahedron, 2014, 70, 1889 CrossRef CAS.
- H. M. Kim, P. R. Yang, M. S. Seo, J. S. Yi, J. H. Hong, S. J. Jeon, Y. G. Ko, K. J. Lee and B. R. Cho, J. Org. Chem., 2007, 72, 2088 CrossRef CAS PubMed.
- Y. Liu, M. Han, H. Zhang, L. Yang and W. Jiang, Org. Lett., 2008, 10, 2973 Search PubMed.
- E. J. Park, M. Brasuel, C. Behrend and M. A. Philbert, Anal. Chem., 2003, 75, 3784 CrossRef CAS PubMed.
- P. S. Hariharan and S. P. Anthony, RSC Adv., 2014, 4, 41565 RSC.
- H. Sharma, N. Kaur, A. Singh, A. Kuwar and N. Singh, J. Mater. Chem. C, 2016, 4, 5154 RSC.
- J. H. Hu, J. B. Li, J. Qi and Y. Sun, Sens. Actuators, B, 2015, 208, 581 CrossRef CAS.
- J. H. Hu, J. B. Li, J. Qi and Y. Sun, New J. Chem., 2015, 36, 4041 RSC.
- J. B. Li, J. H. Hu, J. J. Chen and J. Qi, Spectrochim. Acta, Part A, 2014, 133, 773 CrossRef CAS PubMed.
- J. H. Hu, N. P. Yan and J. J. Chen, J. Chem. Res., 2012, 36, 619 CrossRef CAS.
- J. H. Hu, N. P. Yan, J. J. Chen and J. B. Li, Chem. Res. Chin. Univ., 2013, 34, 1368 CAS.
- J. Qi, J. H. Hu, Y. Sun and J. B. Li, Curr. Anal. Chem., 2016, 12, 119 CrossRef CAS.
- G. Wang, J. Qin, L. Fan, C. Li and Z. Yang, J. Photochem. Photobiol., A, 2016, 314, 29 CrossRef CAS.
- J. Qin, Z. Yang and G. Wang, Inorg. Chim. Acta, 2015, 435, 194 CrossRef CAS.
- L. Jin, Z. Guo, Z. Sun, A. Li, Q. Jin and M. Wei, Sens. Actuators, B, 2012, 161, 714 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Complete experimental procedures and some of the spectroscopic. See DOI: 10.1039/c7ra04462c |
| This journal is © The Royal Society of Chemistry 2017 |