Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A multi-functional iodoplumbate-based hybrid crystal: 1-propyl-4-aminopyridinium triiodoplumbate†
Hai-Bao Duan‡
 *,
Shan-Shan Yu‡,
Shao-Xian Liu and
Hui Zhang*
*,
Shan-Shan Yu‡,
Shao-Xian Liu and
Hui Zhang*
School of Environmental Science, Nanjing Xiaozhuang University, Nanjing 211171, P. R. China. E-mail: duanhaibao4660@163.com
First published on 27th April 2017
Abstract
The temperature-dependent photoluminescence, dielectric and impedance spectra, as well as crystal structures have been investigated for the iodoplumbate-based hybrid crystal, [C3-Apy][PbI3] (1) (C3-Apy+ = 1-propyl-4-aminopyridinium). Compound 1 in the room temperature (RT) contains [PbI3]∞ chains formed by face-sharing distorted PbI6 octahedral. In the low-temperature (LT) phase, the disordered propyl groups in HT phase are partly ordered. 1 shows novel dielectric relaxation and thermochromic luminescent properties.
The design of multi-functional molecular materials, especially those technologically useful properties are combined in a conventional inorganic solid with a continuous lattice, has recently attracted numerous of research attentions, because the combination of two cooperative properties in a material might result in new phenomena and novel applications.1–3 The versatility of molecular chemistry makes possible the design of new molecule-based materials that combine two (or more) physical properties. Thus, a possible approach to obtaining such materials is the so-called hybrid approach in which two-network solids are constructed through the self-assembly of two different molecular fragments where each molecular fragment furnishes distinct physical property. The haloplumbate-based hybrids have tunable structures from the discrete mononuclear or polynuclear species to infinite variety with higher dimensionality4–8 and a wide range of novel physical properties from optics9–12 to electronics.13–15 Some organic–inorganic hybrid crystal with interesting thermochromic luminescence properties have been reported.16–19
In our previous studies, the octahedral [PbI6]4− building blocks were chosen for self-assembly with the different conformation counterions to give a variety of structure,4,11,20,21 and some of them exhibits multiple emissions or thermochromic luminescence properties.4,20,21 On the other hand, flexible organic counter cations can separate inorganic chain and further enhance their thermal stability. Basically, the dielectric response of the materials is due to the rotational or hopping moving of the polar molecules or ions and orientationally ordered states.22–25 Thus, from the point of crystal engineering, introducing flexible organic counter cations into haloplumbate-based crystal lattices may obtain luminescence-dielectric multi-functional molecular materials. In this paper, we prepared and crystal structure of an iodoplumbate-based hybrid, [C3-Apy][PbI3] (1) where C3-Apy+ = 1-propyl-4-aminopyridinium, which exhibits thermochromic luminescence and dielectric relaxation behavior.
The yellowish needle-shaped hybrid crystals of 1 were achieved by slowly evaporating the mixture of PbI2, KI and [C3-Apy]Br with molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in DMF at ambient temperature over 30 days and the yield is ca. 75% based on the reactant PbI2. The synthesized compound was insoluble in common organic solvents and water. The purity of hybrid was examined using elemental analyses for C, H and N and powder X-ray diffraction technique (Fig. S1†). Hybrid crystal 1 is thermally stable up to ca. 350 °C (Fig. S2†).§
1 in DMF at ambient temperature over 30 days and the yield is ca. 75% based on the reactant PbI2. The synthesized compound was insoluble in common organic solvents and water. The purity of hybrid was examined using elemental analyses for C, H and N and powder X-ray diffraction technique (Fig. S1†). Hybrid crystal 1 is thermally stable up to ca. 350 °C (Fig. S2†).§
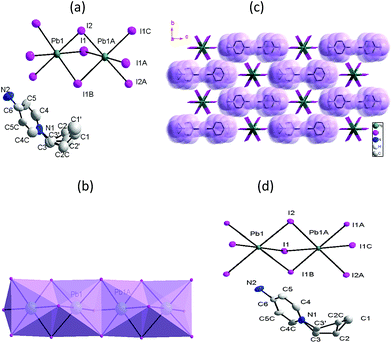
Compound 1 in the room temperature (RT) crystallizes in the orthorhombic space group Pnma, the asymmetric unit contains one crystallographically distinct Pb2+ ions, two different I− anions and half C3-Apy+ cation (Fig. 1a). The cell parameter are analogue to our previous reported [C2-Apy][PbI3] compound,26 however, the packing structure and physical properties of the two compounds are different. Pb atom is coordinated with six I− from two types I− to form the slightly distorted PbI6 octahedron. The Pb(1)–I(1) bond length is 3.204 Å, and Pb(1)–I(2) bond length is 3.256 Å, all within the van der Waals contact limit. Two of I− anions have μ2 connections model linking each Pb2+ ions. Thus, each PbI6 octahedron shares faces with other two PbI6 octahedron forming an infinite one dimension (1D) chain (Fig. 1b). The cations adopt the bent conformation, alkyl chain slightly disrupted close to the pyridyl ring with an almost completely trans-planar conformation. It is noted that the alkyl chains exhibit heavily disordered in RT phase although the equivalent and anisotropic displacement factors in [PbI3]− inorganic moieties and the 4-NH2Py moieties are comparable to those at LT phase. The C3-Apy+ cation interacted with each other through propyl–propyl interactions. Along the a-axis direction, these interaction lead to information of 1D channel, and [PbI3]− is resided in the channel (Fig. 1c). The channel size is about 14.01 × 18.67 Å2. The crystal structure of 1 at 120 K (in LT phase) is quite analogous to that at RT phase. In LT phase, the cell parameters and packing structure is almost not changed. From 120 to 293 K, the a-, b- and c-axes shrinks respectively by 0.89%, 0.96%, and 1.24%, and the cell volume shrinks by 3.21%. However, the displacement parameters in propyl chain are obviously small than those at 293 K. The Pb2+ ions also are all situated in a distorted octahedral coordination environment and the Pb–I distances is similar with RT phase. The most remarkable change is that the structurally disordered propyl group in the HT phase becomes partly ordered at 120 K (Fig. 1d).
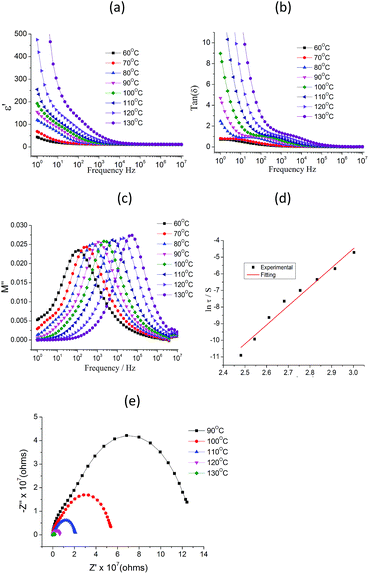
Frequency dependences of ε′ and tan(δ) = ε′′/ε′ are shown in Fig. S3, S4† and 2 for 1 in the temperature range 10–130 °C. At f < 10 Hz, there is a sharp increase in the ε′ data with decreasing frequency that is attributed to space charge polarization associated with sample-electrode interface polarization and the ε′ value of low frequency gradually decrease with temperature decreasing (Fig. 2a). Where thermal motion of dipole units almost be suppressed in the low temperature. At f > 100 Hz, the curves of dielectric loss tan(δ) − f (Fig. 2b) show a wide rounded maximum in the selected temperature, attributed to the polarization arising from the dielectric relaxation of the lattice. However, it is difficult to analyze the frequency dependence of ε′ and tan(δ) because a strong low-frequency dispersion appears when the temperature increased. Here, we use dielectric modulus to analyze the dielectric relaxation processes in 1, which can significantly reduce electrode polarization effect at the low-frequency.27,28 The electric modulus is calculated by eqn (1).29,30
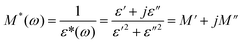 | (1) |
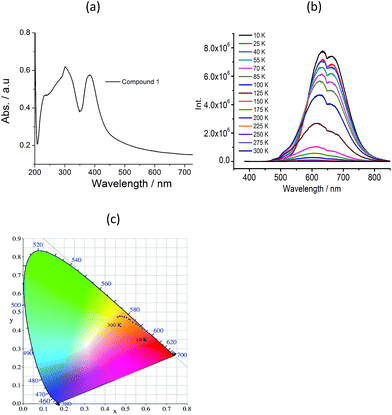
The absorption spectra of 1 in the solid state are shown in Fig. 3a. The shape of broad absorption band is similar with compound [C2-Apy][PbI3]. It contains a broad band below 350 nm and a narrow band centered around 385 nm. However, the emission spectra of two compounds are very different. The room temperature photoluminescence (PL) spectra of 1 shows a weak broad emission band extending from 530 nm to 780 nm on the excitation of λex = 335 nm (Fig. S7†). The observed broad emission can be attributed to band-edge emission and energy transfer from the excition state of the inorganic chain to the excited triplet state of the organic component. Compared to compound [C2-Apy][PbI3], the emission bands below 350 nm nearly not be observed in 1, which may be attributed to very strong π–π interaction within the pyridyl rings in 1. Interestingly, the temperature-dependent photoluminescent investigation indicated that 1 shows thermochromic luminescent properties. As the temperature decreasing, the broad emission band divided into two obvious emission center (center at 635 and 664 nm) (Fig. 3b), and a very weak new emission band centered at 470 nm is observed, which can be attributed to the π* ← π within the pyridyl rings in the cations.16 The emission intensity of the broad band progressively increase as the temperature decreasing, which is because thermal activation of the nonradiative-decay is suppressed. The corresponding luminescence color change from orange to yellow, as shown in the CIE (x, y) chromaticity diagram at different temperature (Fig. 3c).
In summary, we have synthesized and crystal structure characterized a multi-functional haloplumbate-based hybrid crystal (1). The crystal structure of 1 at LT phase is quite analogous to that at RT phase. Each PbI6 octahedron shares faces with other two PbI6 octahedron forming an infinite 1D chain. The alkyl chains of cations exhibit heavily disordered in RT phase and ordered in LT phase. 1 shows an novel dielectric relaxation in the low frequency region, which is related to the propyl groups swing moving. In addition, the temperature-dependent photoluminescent indicated that 1 shows thermochromic luminescent properties. This result offers promising prospects for creating mobility and high carrier conductivity molecular crystal for multi-functional luminescent thermometer. To the best of our knowledge, this is a rare example that a crystalline hybrid semiconductor displays such features.
Acknowledgements
The authors thanks National Nature Science Foundation of China and Natural Science Training Foundation of Nanjing Xiaozhuang University for their financial support (grant No: 21201103, 21301093 and 2016NXY12).Notes and references
- E. Coronado, J. R. Galán-Mascarós, C. J. Gómez-García and V. Laukhin, Nature, 2000, 408, 447 CrossRef CAS PubMed; M. Clemente-Leόn, E. Coronado, M. Lόpez-Jordá, G. M. Espallargas, A. Soriano-Portillo and J. C. Waerenborgh, Chem.–Eur. J., 2010, 16, 2207 CrossRef PubMed.
- J.-M. Rueff, J.-F. Nierengarten, P. Gilliot, A. Demessence, O. Cregut, M. Drillon and P. Rabu, Chem. Mater., 2004, 16, 2933 CrossRef CAS; M. Gonidec, F. Luis, Á. Vílchez, J. Esquena, D. B. Amabilino and J. Veciana, Angew. Chem., Int. Ed., 2010, 49, 1623 CrossRef PubMed.
- M. E. Itkis, X. Chi, A. W. Cordes and R. C. Haddon, Science, 2002, 296, 1443 CrossRef CAS PubMed.
- Y. B. Tong, L. T. Ren, H. B. Duan, J. L. Liu and X. M. Ren, Dalton Trans., 2015, 44, 17850 RSC.
- Z. Tang and A. M. Guloy, J. Am. Chem. Soc., 1999, 121, 452 CrossRef CAS.
- Z. J. Zhang, S. C. Xiang, Y. F. Zhang, A. Q. Wu, L. Z. Cai, G. C. Guo and J. S. Huang, Inorg. Chem., 2006, 45, 1972 CrossRef CAS PubMed.
- J. J. Liu, Y. F. Guan, C. Jiao, M. J. Lin, C. C. Huang and W. X. Dai, Dalton Trans., 2015, 44, 5957 RSC.
- L. L. Mao, H. Tsai, W. Nie, L. Ma, J. Im, C. C. Stoumpos, C. D. Malliakas, F. Hao, M. R. Wasielewski, A. D. Mohite and M. G. Kanatzidis, Chem. Mater., 2016, 28, 7781 CrossRef CAS.
- E. R. Dohner, E. T. Hoke and H. I. Karuadasa, J. Am. Chem. Soc., 2014, 136, 1718 CrossRef CAS PubMed.
- C. Wehrenfenging, M. Liu, H. J. Snaith, M. B. Johnston and L. M. Herz, J. Phys. Chem. Lett., 2014, 5, 1300 CrossRef PubMed.
- J. I. Fujisawa and T. Ishihara, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 70, 113203 CrossRef.
- G. N. Liu, J. R. Shi, X. J. Han, X. Zhang, K. Li, T. Zhang, Q. S. Liu, Z. W. Zhang and C. C. Li, Dalton Trans., 2015, 44, 12561 RSC.
- W. S. Yang, J. H. Noh, N. J. Jeon, Y. C. Kim, S. Ryu, J. Seo and S. I. Seok, Science, 2015, 348, 1234 CrossRef CAS PubMed.
- M. Liu, M. B. Johnston and H. J. Snaith, Nature, 2013, 501, 395 CrossRef CAS PubMed.
- G. Xing, N. Mathews, S. Sun, S. S. Lim, Y. M. Lam, M. Grätzel, S. Mhaisalkar and T. C. Sum, Science, 2013, 18, 344 CrossRef PubMed.
- Z. H. Yan, X. Y. Li, L. W. Liu, S. Q. Yu, X. P. Wang and D. Sun, Inorg. Chem., 2016, 55, 1096 CrossRef CAS PubMed.
- S. Yuan, Y. K. Deng and D. Sun, Chem.–Eur. J., 2014, 20, 10093 CrossRef CAS PubMed.
- H. Y. Zhuo, H. F. Su, Z. Z. Cao, W. Liu, S. A. Wang, L. Feng, G. L. Zhuang, S. C. Lin, M. Kurmoo, C. H. Tung, D. Sun and L. S. Zheng, Chem.–Eur. J., 2016, 22, 17619 CrossRef CAS PubMed.
- Z. Wang, G. L. Zhuang, Y. K. Deng, Z. Y. Feng, Z. Z. Cao, M. Kurmoo, C. H. Tung and D. Sun, Inorg. Chem., 2016, 55, 4757 CrossRef CAS PubMed.
- H. B. Duan, H. R. Zhao, X. M. Ren, H. Zhou, Z. F. Tian and W. Q. Jin, Dalton Trans., 2011, 40, 1672 RSC.
- H. B. Duan, S. S. Yu, Y. B. Tong, H. Zhou and X. M. Ren, Dalton Trans., 2016, 45, 4810 RSC.
- Z. Y. Du, Y. Z. Sun, S. L. Chen, B. Huang, Y. J. Su, T. T. Xu, W. X. Zhan and X. M. Chen, Chem. Commun., 2015, 51, 15642 Search PubMed.
- P. C. Guo, T. Y. Chen, X. M. Ren, W. H. Ning and W. Q. Jin, New J. Chem., 2014, 38, 2254 RSC.
- F. K. He, C. Yuan, K. Li, S. Diao, K. K. Jin, J. J. Wang, J. W. Tong, J. Ma and Q. Fang, RSC Adv., 2013, 3, 23128 RSC.
- S. S. Yu, S. X. Liu and H. B. Duan, RSC Adv., 2015, 44, 20822 CAS.
- H. B. Duan, S. S. Yu, S. X. Liu and H. Zhang, Dalton Trans., 2017, 46, 2220 RSC.
- J. Świergiel and J. Jadizyn, Ind. Eng. Chem. Res., 2011, 50, 11935 CrossRef.
- J. Jadizyn and J. Świergiel, Ind. Eng. Chem. Res., 2012, 51, 807 CrossRef.
- W. Trigui, A. Oueslati, I. Chaabane, G. Corbel and F. Hlel, Appl. Phys. A, 2015, 119, 673 CrossRef CAS.
- M. F. Mostafa and A. S. Atallah, Phys. Lett. A, 1999, 264, 242 CrossRef CAS.
- G. M. Sheldrick, SHELX-97, Program for the refinement of crystal structure, University of Göttingen, Germany, 1997 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: PXRD, TG, emission spectra in solids at room temperature, dielectric permittivity, impedance spectra and CIE coordinates. CCDC 1509582 and 1510132. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7ra03524a |
| ‡ Hai-Bao Duan and Shan-Shan Yu contribute equally. |
| § Crystal data for compound 1 at rome temperature (CCDC 1509582), C16H26I6N4Pb2, M = 1450.2, a = 7.8748(5) Å, b = 10.4050(8) Å, c = 19.3554(17) Å, α = β = γ = 90°, V = 1585.9(5) Å3, Z = 2, Dc = 3.307 g cm−3, F(000) = 1264.0, Rint = 0.1129, R1 = 0.0832, wR2 = 0.0949. Single crystal diffraction data for 1 at room temperature and 120 K (CCDC 1510132) were collected on a Siemens SMART-CCD diffractometer with graphite monochromatic Mo Kα radiation (λ = 0.71073 Å). The structures were solved by direct method and refined on F2 using full matrix least-squares method with SHELXTL.31 Anisotropic thermal parameters were refined for the non-hydrogen atoms. The hydrogen atoms were placed at calculated positions. |
| This journal is © The Royal Society of Chemistry 2017 |