Open Access Article
Open Access ArticleInfluence of anion hydration status on selective properties of a commercial anion exchange membrane electrochemically impregnated with polyaniline deposits
A. Montes-Rojas *a,
J. A. Q. Renteríaa,
N. B. J. Cháveza,
J. G. Ávila-Rodrígueza and
B. Yañez Soto
*a,
J. A. Q. Renteríaa,
N. B. J. Cháveza,
J. G. Ávila-Rodrígueza and
B. Yañez Soto b
b
aLaboratorio de Electroquímica, Facultad de Ciencias Químicas, Universidad Autónoma de San Luis Potosí, Av. Dr. Manuel Nava, Zona Universitaria, San Luis Potosí, S.L.P. CP. 78210, México. E-mail: antonio.montes@uaslp.mx
bInstituto de Física, Universidad Autónoma de San Luis Potosí, Av. Manuel Nava No. 6, Zona Universitaria, San Luis Potosí, S.L.P. CP. 78290, México
First published on 10th May 2017
Abstract
There is currently great interest in the use of polyaniline (PAni) to impart particular properties to anion exchange membranes, employed in several fields. This special polymeric material can be obtained both chemically and electrochemically; however, the latter affords better control of morphology, which could be used to promote some properties of PAni-based membranes, such as their selectivity or hydrophobic/hydrophilic balance. In this work, commercially available membranes were electrochemically modified with PAni to improve their hydrophobic/hydrophilic balance, and this was achieved by cyclic voltammetry in acidic solutions. The performance of the modified membranes in the electrodialysis process of a nitrate–chloride mixture in neutral solutions was then evaluated. The impregnation imparts specific selective properties to the commercial membrane that lead to a greater retention of chloride ions. This property derives from the higher hydrophobicity of the PAni deposited on the membrane, which leads to a more effective exclusion of the more hydrated chloride anions.
1. Introduction
The selectivity of an ion exchange membrane (IEM) is influenced by the interactions between the species passing through it and each of the components of the membrane.1–4 These interactions are controlled by different factors, such as the hydrophobic/hydrophilic balance of the membrane, or the intensity of the electrostatic interactions.5,6 For example, the selectivity of the membrane increases when the concentration of fixed charged groups in the membrane is high; however, an excess of charge results in poor mechanical properties. Furthermore, an increase in the number of fixed charges produces accumulation of solvating water, which in turn results in a higher electrical resistance of the membranes, increasing the costs of the processes in which they are employed. The nature of the species passing through the membrane also plays an important role: it has been shown that the ion exchange membranes exhibit greater selectivity towards higher-charge and more hydrated ions.6Several studies reported in the literature describe attempts to improve the selectivity of a membrane by exploiting the effects discussed above.7–13 For example, polyaniline (PAni) has been used to impart specific properties to the membranes because – besides its flexibility and unique electrical conductivity – this material has the special capacity to generate charges in its bulk under certain conditions (such as chemical or electrochemical doping).11–17 Furthermore, its different chemical forms exhibit not only specific structural characteristics, but also particular hydrophobic/hydrophilic properties. For example, the nonprotonated form of PAni, named leucoemeraldine base, is more hydrophobic due to the polar C–N, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, and N–H bonds in its structure; moreover, due to its ability to form hydrogen bonds, leucoemeraldine base presents the most compact and rough structure among the different PAni forms. On the other hand, protonated emeraldine (doped) is the oxidized form of PAni and has a lower hydrophobicity, due positive charges present in its structure; moreover, the repulsive interactions associated to these charges produce a more homogeneous and porous material. The last form of PAni, called pernigraniline, is the most oxidized one and contains single C–N and double C
N, and N–H bonds in its structure; moreover, due to its ability to form hydrogen bonds, leucoemeraldine base presents the most compact and rough structure among the different PAni forms. On the other hand, protonated emeraldine (doped) is the oxidized form of PAni and has a lower hydrophobicity, due positive charges present in its structure; moreover, the repulsive interactions associated to these charges produce a more homogeneous and porous material. The last form of PAni, called pernigraniline, is the most oxidized one and contains single C–N and double C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds, which cause it to be highly hydrophobic.14,18–20
N bonds, which cause it to be highly hydrophobic.14,18–20
According to the literature, PAni could be obtained in any of these chemical forms by varying the potential applied to it, using either a voltammetric potential scan or a potential pulse. For example, at potential values lower than +0.2 V vs. SCE, PAni is found in the leucoemeraldine form, whereas pernigraniline is formed at potentials higher than +0.55 V vs. SCE. Between these two limits, PAni is found in the protonated emeraldine form. These data suggest that a specific chemical form of PAni can be selected by adjusting the imposed potential.19
These characteristics of PAni have generated expectations that the selectivity properties of membranes can be modified. Despite this, however, efforts have concentrated mainly on their use as cation-exchange membranes,11–13,21–24 while there is very little information on anion exchange membrane modifications using different chemical forms of polyaniline.21
Cation-exchange membranes modified with PAni have been used to establish the influence of ion hydration and the hydrophobic balance of the membrane on selectivity. For example, van der Bruggen et al.22 and Farrokhzad et al.23 established that small, hydrated, cations can penetrate and diffuse through a hydrophilic surface and the modified membrane consequently has a high cation-removing capability.
A similar result was obtained by Sata when using anion exchange membranes chemically modified with polypyrrole.24 The author suggests that the affinities of less-hydrated anions for these membranes increase with decreasing membrane hydrophilicity, irrespective of the charge.
This work investigates the selectivity of commercially available anion exchange membranes modified with PAni; in particular, we investigate the effect of applying a specific potential in order to promote the formation of a chemical form of PAni, which impart a different hydrophobic/hydrophilic character to the membrane. The properties of membranes modified with PAni were fully characterized, in order to rationalize their selectivity in the electrodialysis (ED) of a nitrate–chloride mixture with neutral pH. The selected ionic species in the mixture have similar size and charge but a significantly different hydration number.
2. Experimental
2.1 Materials and methods
| Property | NO3− | Cl− |
|---|---|---|
| Molecular weight (g mol−1) | 62.01 | 35.50 |
| Ionic radius (nm) | 0.3 | 0.3 |
| Charge | −1 | −1 |
| Hydration number | 4 | 6 |
| Gibbs hydration energy (kJ mol−1) | −270 | −317 |
The data in the table show that the two anions have similar characteristics such as charge, ionic radius, and conductivity at infinite dilution, whereas other properties such as molar mass and hydration number differ. In particular, the table shows that the chloride ion is more hydrated, with six water molecules in its hydration shell, compared to only four molecules for the nitrate ion.
| Parameter | Value |
|---|---|
| Electrical resistance (Ω cm2) | 0.4–1.5 |
| Transport number of NO3− in NaNO3 0.1 M | 0.93 |
| Transport number of Cl− in NaCl 0.1 M | 0.92 |
| Thickness (μm) | 130–180 |
| Ion exchange capacity (meq g−1) | 2.0–3.5 |
Circular cuts of the membrane with an area of 4.9 cm2 were used for the different experiments carried out in this work. In addition, a pretreatment was used to remove any possible contaminants introduced during the transportation of the membrane to the laboratory. The pretreatment consisted in placing the membrane in a 1 M nitric acid bath for 24 h with moderate agitation, followed by rinsing several times with deionized water in order to eliminate any trace of HNO3. Finally, the membranes where kept in H2SO4 1 M for 24 h before the modification treatment.28–30
2.2 Modification of the commercial membrane with polyaniline
The amount of PAni deposited on the membrane was controlled by cyclic voltammetry (CV) in acidic solution following the methodology described below.All electrodes were connected to an Autolab PGSTAT 302 N potentiostat/galvanostat, which was used to modify the membrane by carrying out potential scans from −100 to +1000 mV for 40 cycles at a scan rate of 100 mV s−1. It should be noted that the initial and final potential during this process was −100 mV. Following the formation of the PAni deposits, the membrane was separated from the carbon paste electrode and washed using chloroform and an ultrasonic bath until every trace of paste was eliminated. After this procedure, the membrane was rinsed several times with deionized water and finally stored in a 1 M NaCl solution.29,30 This procedure was repeated as many times as needed to produce sufficiently modified membranes, suitable for the subsequent experiments.
Each experiment was performed in triplicate on a newly modified membrane, obtained through the sequence of steps described above.
In the case of electrodialysis experiments only the recently modified membranes were used.
2.3 Characterization of the modified membranes
The modified membranes were characterized by analyzing their counterion transport number (ta), ion exchange capacity (IEC), water content (% H2O), contact angle as well as their I–E curves and infrared spectra. The methodology used to obtain each set of data is described in the following sections.The corresponding experimental setup involved placing the membrane (of exposed area 0.7853 cm2) between two symmetric glass beakers with a tube of 1 cm length and 1 cm internal diameter. Two Ag|AgCl|NaCl (3 M) reference electrodes were used to measure the membrane potential. Each electrode was placed inside a glass extension filled with NaCl 3 M. The top of the glass extension was blocked with an agar plug (3% w/w), which prevented loss of NaCl solution and established the ionic contact. The membrane potential was measured every 10 s for 15 min with a UNI-T UT70C multimeter, which was connected to a computer by a R232 port and was controlled through the UT70C software. The experiments were performed under constant agitation and the initial and final temperatures were recorded in each experiment.
For the determination of the transport number at a concentration of 0.01 M, we employed a set of four solutions of different concentrations (namely, two higher and two lower concentrations); ta was determined from the measured membrane potential, Em, according to the following equation:
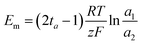 | (1) |
When working with the modified membranes, special care was taken to ensure that the modified side of the membrane was in contact with the solution of higher concentration.
The membranes examined in this study were equilibrated in a normalized NaOH solution (0.1 N, Hycel) for 24 h. After this step, the membrane was rinsed with deionized water and equilibrated for 24 h in 50 mL of a 0.1 M solution of NaNO3. The increase in basicity of the NaNO3 solution was monitored by acid-base titrations using a normalized HCl solution (0.1 N, CTR Scientific) and a Metrohm 827 pH Lab pH meter. Finally, the IEC was calculated as:
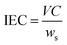 | (2) |
The methodology employed for determining the water content consists in evaporating the water molecules inside the membrane by drying at constant temperature. For this purpose, the membrane was kept in deionized water for 24 h, after which it was superficially dried with a paper towel and weighed (W0). Then, the membrane was dried at 55 °C for 2 h or until a constant weight (Wf) was reached. The water content in the membrane was then calculated using the following expression:
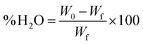 | (3) |
The I–E curves were obtained by performing linear current scans with a scan rate of 1 × 10−5 A s−1. An Autolab PGSTAT 302 N potentiostat/galvanostat was employed to control the applied current and measure the potentials. The working solution was renewed after every experiment and all experiments were performed in triplicate.
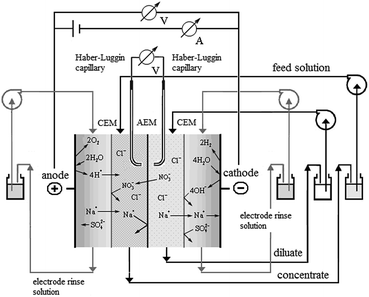
2.4 Electrodialysis
The use of the modified membranes for the separation of nitrates in a nitrate–chloride mixture was assessed by electrodialysis under a constant electric current density, as detailed in the following sections.According to the design of the ED stack, three separated circuits for the diluate, concentrate, and electrode rinsing solutions were obtained. Each solution was 50 mL in volume, and was recirculated by a separate peristaltic pump (Mityflex). The initial concentrations in the diluate and concentrate compartments were 0.1 M NaCl + 0.1 M NaNO3. The membrane stack was connected to a homemade DC power supply operated under constant current during a 2 h experiment.
In addition, two multimeters (UNI-T UT70C) and a pH meter (Oaklon) were used to complement the experimental setup (Fig. 1).
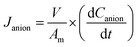 | (4) |
The ion selectivity of each membrane was calculated at the end of the treatment using eqn (5):24
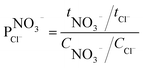 | (5) |
3. Results and discussion
3.1 Optimization of the number of cyclic voltammetry cycles for the electrosynthesis of polyaniline
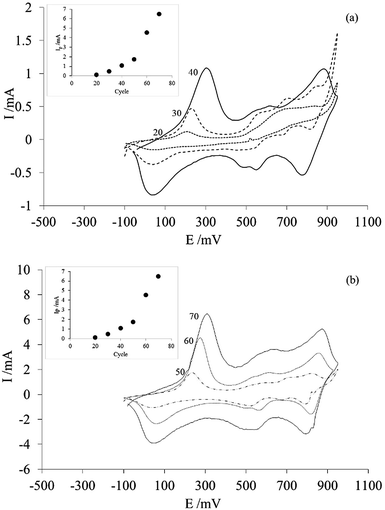
Cyclic voltammetry (CV) is a versatile technique that has proven its relevance in the electrosynthesis of conductive polymers.34 Among the main characteristics of CV, is the possibility to control both the morphology of the conductive polymer and the amount deposited onto a surface by means of the number of potential scanning cycles, at a certain potential scan rate and between the appropriate potential limits.To optimize our procedure, we determined the appropriate number of potential scanning cycles needed to modify the commercial membrane used in this work. To achieve this, different membranes were modified with PAni deposits, which were obtained using 20–70 sweep cycles. Based on previous studies, the potential range was fixed between −100 and 1000 mV and the sweep velocity was fixed at 100 mV s−1.29,30
Fig. 2 shows the voltammograms of each of the PAni deposits obtained by varying the number of cycles in a 0.5 M sulfuric acid solution.
From the voltammograms, it can be seen that the order of magnitude of the current for each of the responses obtained is directly proportional to the number of sweep cycles. This is clearer in the inset with the peak current (Ip) of the anodic peak located between −100 mV and 500 mV. Assuming that the area under the curve corresponds to the charge and that the charge is proportional to the amount of material deposited, the amount of PAni deposited on the membrane increases with increasing number of sweep cycles. In addition, another characteristic of these curves is that the obtained voltammograms (Fig. 1) correspond to the typical response of PAni only if the number of sweep cycles is greater than 40.20
This confirms that the number of potential scanning cycles determines the amount of PAni deposited onto the commercial membrane; thus, membrane modification can be controlled using cyclic voltammetry.
Finally, considering this, we decided to use the modified membrane, prepared using 40 scanning cycles, to ascertain how small amounts of PAni could affect membrane selectivity during electrodialysis.
3.2 Electrochemical modification of the membranes via PAni deposition
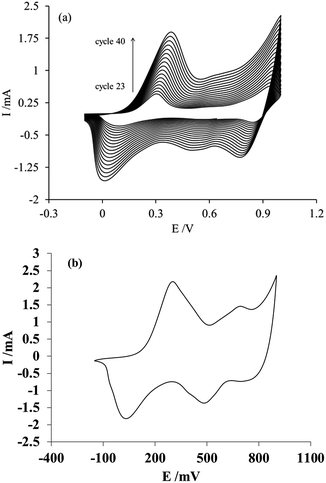
Fig. 3 shows the last 17 scan cycles recorded during the electropolymerization process of PAni on the membrane (Fig. 2a), along with the voltammetric response of the electrode/modified membrane assembly obtained in an acidic solution free of aniline monomer (Fig. 3b). The main feature of the I–E curves in Fig. 2a is that the current recorded during the electropolymerization of PAni increases with each scan cycle, which is a behavior typical of the growth of an electroactive film on the electrode (paste/membrane assembly). Additionally, all voltammograms exhibit the characteristic features (number and shape of peaks) of PAni.20,34 These same features are also present in the voltammetric response obtained after moving the post-modification electrode assembly to a solution free of aniline monomer (Fig. 3b). The characteristics of both responses confirm the progressive growth of polyaniline on the paste/membrane assembly.3.3 Electrochemical characterization of the membrane modification
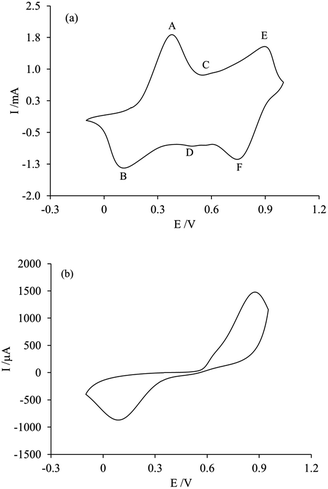
The electrochemical modification of the membrane by PAni deposition via cyclic voltammetry implies a better control of the characteristics of the polymeric material; however, due to the modification procedure, which involves attaching the membrane to the surface of the carbon paste, it is necessary to determine if both sides of the membrane were successfully modified with PAni. For this purpose, the voltammetric responses of both sides of the membrane were obtained in a 1 M H2SO4 electrolytic solution free of aniline monomer.Fig. 4 shows the voltammetric responses of the membrane side in contact with the carbon paste (Fig. 3a) and with the solution (Fig. 3b), obtained after attaching the membrane over a renewed surface of carbon paste electrode, during the modification procedure.
As can be seen in the figure, only one side of the membrane shows the characteristic response of PAni (Fig. 4a), whereas the other side shows the response of an unmodified membrane, which will be discussed later. This indicates that the membrane modification only occurs on the side of the membrane in contact with the carbon paste electrode during the electropolymerization process. The voltammetric response presents the typical features of PAni,20 which consist of three peaks (A, C, E) on the anodic side and their cathodic counterparts (B, D, F) representing the redox reactions occurring in the polymer. The A/B pair of peaks, between 0 and +300 mV, correspond to the PAni transformation from its reduced form leucoemeraldine to the partially oxidized state emeraldine. The peaks E/F, located approximately at +800 and +950 mV, are related to the transition from leucoemeraldine to pernigraniline. While the peaks C/D at +500 and +600 mV correspond to the redox reaction of p-benzoquinone, they are generally less intense than the previous ones, and their intensity provides insight into the over-oxidation of the polymer.35,36 As will be discussed later, an applied potential could be used to favor the occurrence of a specific chemical form of PAni, in order to impart particular separation characteristics to the membrane.19,20
Since the voltammetric response of PAni (shown in Fig. 4a) was obtained after moving the paste/membrane/PAni assembly to an acidic solution free of aniline monomer, it can be inferred that the deposited PAni is located at the interface between the carbon paste and the membrane.
In practice, since the membrane is not an electronic conductor (whereas a conductor would be required for the electropolymerization of aniline to proceed) the polymerization process begins at the carbon paste surface not blocked by the membrane, following which the deposit grows towards the solution, reaching the channels of the membrane nearest to the carbon paste and impregnating its walls. However, the number of potential scans performed is not sufficient for the polymerization process to reach the other side of the membrane, which explains why the deposits are located only at the side of the membrane in contact with the carbon paste electrode.
One possible way to assess how the impregnation with PAni affects the membrane involves determining the capacitance of the electrochemical double layer (Cdl) of the system formed by the paste/PAni/membrane electrode and the electrolyte, using cyclic voltammetry.37
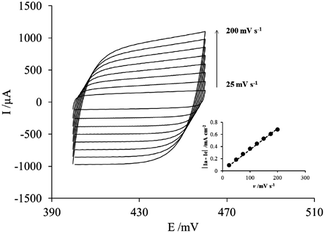
Fig. 5 shows the voltammetric response between 420 and 440 mV (Fig. 4a) and the plot of |Ia − Ic| (where Ia and Ic are the anodic current and cathodic current respectively) as a function of the scan rate (v) used to determine Cdl at E = 425 mV.
All curves in Fig. 5 exhibit a rectangular shape regardless of the scan rate used, without current peaks associated to faradaic processes. The absence of faradaic processes implies that the current flowing through the electrode/PAni/membrane system is used exclusively for the charge and discharge of the electrochemical double layer; this behavior is characteristic of a parallel plate capacitor.38,39 However, this characteristic is observed only at low scan rates, whereas at scan rates higher than 100 mV s−1 the rectangular shape tends to tilt slightly to the right. While in the case of the carbon paste the processes taking place correspond only to the arrangement of the double layer, in the case of the paste/PAni/membrane system the relevant processes involve the insertion–deinsertion of the doping anions in the PAni deposit from or to the electrolyte. In other words, at low scan rates the ions diffusing from the electrolyte can access all sites (pores) available in the PAni deposit; therefore, the observed behavior resembles that of an ideal capacitor. On the other hand, at high scan rates, the insertion process is incomplete. The electrode thus behaves as an ideal parallel plate capacitor at low scan rates, through the whole potential range explored; however, if the scan rate is higher than 100 mV s−1, the electrode assembly deviates from this ideal behavior.
The capacitance of the electrochemical double layer determined from the slope of the curve in inset of Fig. 5 was 1.736 μF cm−2 for the paste/PAni/membrane/electrolyte assembly, whereas the value measured for the paste/electrolyte system was only 2.5 nF cm−2. This result can be explained considering that the capacitance of the electrochemical double layer is given by the equation:
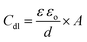 | (6) |
3.4 Reproducibility and homogeneity of the PAni deposits
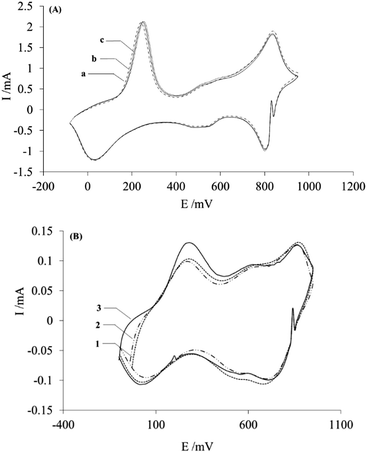
Another aspect investigated in this work was the reproducibility and the homogeneity of the modification procedure. For this purpose, the voltammetric responses of three modified membranes are shown in Fig. 6A.As the figure shows, the voltammetric responses of the modified membranes show essentially the same form and intensity: this indirectly proves that the deposits on each membrane contain the same amount of PAni material, which indicates that the modification procedure is reproducible.
In addition, the homogeneity of the membranes was also investigated. For this purpose, a modified membrane was cut into pieces, and each piece was attached, from the modified side, to the carbon paste electrode, in order to obtain the corresponding voltammetric response in sulfuric acid without monomer, shown in Fig. 6B.
The obtained I–E curves display similar shape and intensity, which again reflects the presence of similar amounts of deposited PAni in different regions of the membrane, thus proving that PAni is homogeneously distributed over the membrane surface. Moreover, the curves obtained, Fig. 5B, show lower current values compared with Fig. 6A, which is attributed to the use of a smaller membrane area in this experiment, and therefore a lower quantity of PAni.
3.5 Characterization by Fourier transform infrared spectroscopy of the modified membranes after the application of a potential pulse
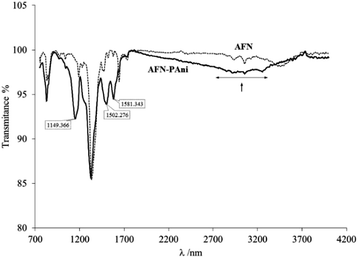
The application of a potential pulse to the PAni deposits produces a change in their chemical forms, as reported before by several authors.40–42 A potential value was thus applied for 20 min to the PAni deposits on the membrane, in order to promote a chemical form in the modification. The potential value selected was −100 mV, to promote the formation of the partially oxidized emeraldine structure. In order to confirm the presence of this form of PAni, analyses of the Fourier Transform Infrared (FTIR) spectra of the PAni deposited on the membranes can provide information on this matter. Fig. 7 depicts the FTIR spectra of unmodified (AFN) and modified (AFN-PAni) membranes.The spectrum of the modified membrane (AFN-PAni) exhibits a broad band between 2200 and 3700 cm−1, which is associated with N–H bond stretching and is characteristic of PAni deposits.23 Additionally, the FTIR spectrum shows other characteristics peaks associated with PAni deposited on ion exchange membranes. For example, the band at 1149 cm−1 is related to in-plane C–H bending of an aromatic ring, while the band at 1581 cm−1 is attributed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching vibration of the quinoid unit, and the band at 1502 cm−1 is due to the C
N stretching vibration of the quinoid unit, and the band at 1502 cm−1 is due to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration of the benzenoid unit.23,43,44 In fact, these peaks confirm that the emeraldine structure of PAni is the predominant form on the membrane surface, as mentioned above.
C stretching vibration of the benzenoid unit.23,43,44 In fact, these peaks confirm that the emeraldine structure of PAni is the predominant form on the membrane surface, as mentioned above.
3.6 Characterization of the surface of the modified membrane
Fig. 8 shows the surface morphologies obtained by Scanning Electronic Microscopy (SEM) of a membrane before (Fig. 8a) and after (Fig. 8b) electrochemical modification with PAni. Membrane tissue reinforcement and morphological changes to the surface after modification with PAni are evident in these images. Indeed, before modification (Fig. 8a), the surface membrane is completely smooth, while after modification with PAni (Fig. 8b) the modified side of the membrane is rougher due to the homogeneous distribution of small clusters (see arrows).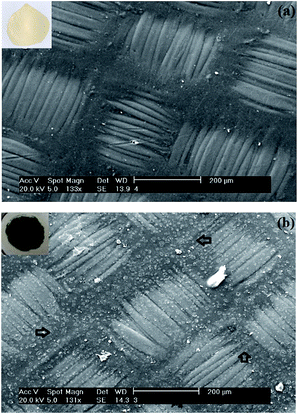 | ||
| Fig. 8 SEM micrographs of the membrane surface before (a) and after (b) electrochemical modification with PAni in acidic solution. | ||
This rough character of the modified-membrane surface affects the ionic transport properties of the membrane, as can be deduced by analysis of the I–E curves shown in Fig. 9.
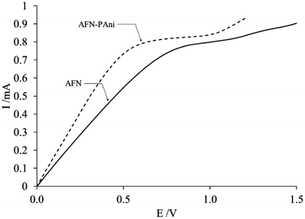 | ||
| Fig. 9 I–E curves of the modified (AFN-PAni) and unmodified (AFN) membranes in a 10−2 M solution of NaNO3. | ||
The I–E curves in Fig. 9 exhibit the typical shapes of homogeneous ion exchange membranes, with three well-defined zones,45–47 implying that modification with PAni does not affect the overall structure of the commercial membrane. The first zones of these polarization curves, which begin at the origin and end at the potential value (E(Ilim)), where the current becomes constant (Ilim), are referred to as “ohmic regions” because of the linear relationship between current and voltage displayed in these zones. These zones are of particular interest because, according to some authors,45,47 the corresponding potential and current values provide insight into the transport of counterions through the membrane, with no diffusion limitations. Since the linear segments of both I–E curves in Fig. 8 end at the same current value, but different values of E(Ilim), the only possible source of this observed difference counterion transport is the membrane modification. The parameters that quantify this effect are listed in Table 3.
| Membrane | Rt/kΩ | Ilim/mA | E(Ilim)/V | Rm/Ω cm2 |
|---|---|---|---|---|
| AFN | 0.93 | 0.791 | 0.83 | 0.767 |
| AFN-PAni | 0.60 | 0.826 | 0.50 | 0.687 |
According to the data in the table, the Rt and E(Ilim) values of the modified membrane are less than those of the unmodified membrane. This behavior can be understood if we consider that Rt is composed of different components:
| Rt = Rm + Rsol + Rdif + Rdl | (7) |
With respect to the limiting potentials of the two studied membranes, E(Ilim) for the modified membrane was observed to be less than that of the unmodified membrane, which is in agreement with previous reports,48 and implies that the limiting current is reached more easily for the modified membrane that for the unmodified analog. On the other hand, the current limit is slightly higher for modified membrane than for the unmodified membrane, which implies that a greater amount of counterion exists in the diffusion layer next to the surface of the modified membrane. Both parameters are related to the surface roughness of this membrane. In agreement with different authors,49 in the case of the modified membrane, its surface roughness (Fig. 6) promotes depletion of the counterion in the diffusion layer near the membrane more easily than the smooth surface of the unmodified membrane. Additionally, this roughness also promotes a mixture of counterion concentration gradients, in this case of the nitrate ions, in the diffusion layer and therefore an additional supply of nitrate ions, which leads to an increase in the value of the limit current associated to the modified membrane.
Despite these results, it is possible that the changes in the values of the parameters associated with the modified membrane are caused by the membrane itself, since, as mentioned above, it exhibits moderate electroactivity (Fig. 3b). In order to evaluate this possibility, a potential pulse of −100 mV (AFN red) was applied to unmodified membranes, and values of Rm and t(NO3−) were determined for these samples (Table 4).
| Membrane | Rm/Ω cm2 | t(NO3−) |
|---|---|---|
| AFN | 0.767 ± 0.069 | 0.947 ± 0.012 |
| AFN red | 0.838 ± 0.054 | 0.949 ± 0.004 |
Taking as a reference the Rm value of a pristine membrane (0.767 Ω cm2), the application of a potential pulse causes an increase in Rm, which means that the pristine membrane opposes less resistance to the passage of ions; however, the value of t(NO3−) obtained after the pulse is the same regardless of the value of the potential applied. These results demonstrate that the electroactive component of the membrane does not affect its selectivity properties.
Moreover, because the Rm value for the membrane with potential pulse, shown in Table 4, is higher than those of the modified membranes (see Table 3), the effect of the PAni modification on Rm is consistent with the previous discussion, that is, the lower Rm of the membrane modified by a negative potential pulse is due to the greater conductivity of the emeraldine form of PAni deposit.
Based on the previous observations, it is possible that the electroactive properties of the membrane affect other aspects of the modified membranes, for example the polymerization process of PAni or different parameters relevant for its characterization and use in electrodialysis. This prompted us to investigate the origin of the electrochemical activity of the membrane, along with possible ways to minimize it.
The voltammetric response of the membrane in Fig. 3b shows a pair of current peaks separated by 0.5 V, which denotes an irreversible process. This irreversibility is also indicated by the lack of symmetry in the peaks, where the anodic peak reaches a higher current value, whereas the value reached by the cathodic peak is very small. It is important to note that the peaks observed are related to the fabrication process of the membrane. Copolymers such as 4-vinylpyridine and 2-methyl-5-vinylpyridine are used in the fabrication of the AFN membrane.50 According to some authors,51 these copolymers show a moderate electroactivity associated to the pyridine group, which is indicated by a marked irreversibility in their voltammetric responses. Due to the latter effect, the peaks observed in the voltammetric response of the membrane are most likely caused by the electroactivity of the copolymer residues.
In order to reduce the impact of the membrane electroactivity on the process of electropolymerization and on the other properties of the modified membranes, the unmodified membrane was scanned in the range where the peaks were detected, for example from −100 to +1200 mV, for several cycles. The intensity of the peaks decreased with each successive scan, until they disappeared. This trend suggests that the peaks, and their possible effect on the properties of the modified membrane, will disappear during the electropolymerization process of aniline.
3.7 Electrodialysis
Based on the results shown elsewhere,52 a current of 40 mA and a flow rate of 4.4 mL min−1 were selected. These parameters were used when removing aliquots from both solutions in order to assess the efficiency of the process.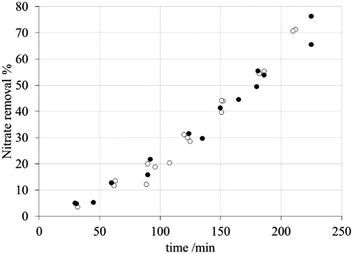 | ||
| Fig. 10 Nitrate removal percentage during electrodialysis with the modified membranes: (●) AFN and (○) AFN-PAni. | ||
Nitrate anion transport numbers, t(NO3−), and anion fluxes through the membrane, listed in Table 5, show a certain degree of correlation with the corresponding nitrate removal capacity.
| Membrane | t(NO3−) | JNO3−/107 mol cm−2 s−1 |
|---|---|---|
| AFN | 0.915 ± 0.012 | 147 |
| AFN-PAni | 0.947 ± 0.012 | 146 |
Firstly, according to these data, the selectivity of the modified membrane is slightly greater than that of the unmodified membrane; however, this difference is not the decisive factor since it is not reflected in the removal curves shown in Fig. 10. Secondly, the nitrate fluxes obtained for both membranes are practically identical, implying that PAni modification does not affect the transport of this ion.
Accordingly, there appears to be another factor that impairs the selectivity of the modified membrane, which is not based purely on electrostatic effects.
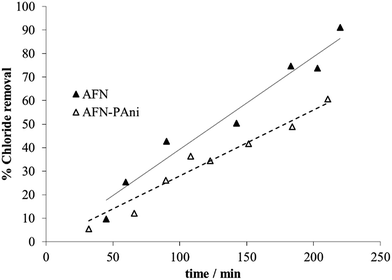 | ||
| Fig. 11 Chloride removal percentage during electrodialysis performed with PAni-modified membrane, compared to the unmodified (AFN) membrane. | ||
The experimental data shown in Fig. 11 clearly indicate that higher chloride removal capacities are achieved using the unmodified membrane, compared to the AFN-PAni-modified membranes, during treatment. In other words, a higher retention of chloride is achieved by the modified membrane, and the difference with respect to the unmodified membrane further increases with increasing treatment time. The modification with a PAni deposit thus increases the chloride exclusion by the membrane, which already presents a slightly rather low intrinsic selectivity towards this ion (see Table 2). For example, at the end of the treatment (180 min) the increases in chloride retention by the modified membrane compared to the unmodified one reach almost 22%.
The chloride separation capacity exhibited by the modified membrane is harder to interpret than their nitrate separation capacity, due to the different parameters, shown in Table 6. These parameters, can be used to understand the behaviour of the modified membrane. For example, the corresponding chloride transport numbers highlight a clear difference in the selectivity of the modified membranes. In particular, the AFN-PAni-modified membrane has a slight low selectivity than the unmodified membrane (low transport number), which explains the higher chloride removal obtained with the latter.
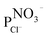 , chloride fluxes, JCl−, water content (% H2O) and water contact angle for the unmodified (AFN) and modified (AFN-PAni) membranes
, chloride fluxes, JCl−, water content (% H2O) and water contact angle for the unmodified (AFN) and modified (AFN-PAni) membranes
This result can be understood by considering the IEC values, which show that, due to its higher IEC, the unmodified membrane exhibits a higher relative selectivity (higher transport number) compared to that of the modified membrane. In this case, the observed decrease in IEC may be due to the blocking of fixed charges by the deposited polymer; however, the difference between the IEC values of the two membranes is not statistically significant and does not decisively explain the different selectivity properties of the two studied membranes.
Several similar examples are found in literature that can help to understand these results, with the exception that cation-exchange membranes modified with PAni were used with cations of different degrees of hydration.22–24 For example, Farrokhzad et al. consider that the PAni deposited on the membrane confers a greater hydrophobicity to its surface, and that because of this, the more-hydrated ions are more strongly repelled.
This behavior is due to the chemical nature of the PAni deposit; that is, the PAni deposit creates a more hydrophobic surface compared to that of the unmodified membrane, owing to the nonpolar N–H and C–N bonds present in the reduced form of polyaniline. In this form, if a hydrated ion, in our case an anion, intends to penetrate the membrane, the energy barrier associated with the partial dehydration of the ion needs to be overcome. The parameter relating to this barrier is the hydration energy of the anion. Hence, an anion with a high hydration energy will have more difficulty in overcoming this barrier and therefore will be more easily rejected by a hydrophobic surface. In our case, the chloride ion has a higher energy of hydration than the nitrate, see Table 1, so the chloride ion will be more strongly rejected by the modified membrane.
This is confirmed by the membrane selectivities (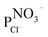 ) and the chloride fluxes (JCl−) listed in Table 6.
) and the chloride fluxes (JCl−) listed in Table 6.
As shown in Table 6, the selectivity of NO3− over Cl− is lower for the modified membrane compared to that of the unmodified counterpart. In addition, the chloride ion flux is higher for the unmodified membrane than for modified membrane, which implies that the chloride ions (more hydrated) are more strongly rejected by the modified membrane (higher hydrophobicity) that by the unmodified membrane. In addition, these data show that the nitrate ions are not only more weakly rejected by the modified membrane due to the decrease of the IEC in the membrane, but also because of weak interactions between the hydrophobic surface and the weakly hydrated ions. Therefore, the affinities of anions with different degrees of hydration, with the anion-exchange membranes in this study are thought to increase with decreasing membrane hydrophilicity.
This hypothesis is further supported by the measured water content (% H2O) of the studied anion-exchange membranes and their contact angles, as shown in Table 6.
Both membranes have a water content (% H2O) in the range of 40–45%; nevertheless, the AFN-PAni-modified membrane has a slightly lower water content (43.9%) compared to the unmodified membrane, which contains around 45% water. In addition, the high value of the contact angle obtained for the modified membrane (91.3°), also listed in Table 6, implies a water repelling surface.
This is not surprising behavior since the PAni in the membrane is in the emeraldine form and in this form PAni possesses functional groups of differing polarity that give rise to a highly hydrophobic surface.
This hydrophobicity of the membrane is expressed through low water content and high contact angles.
4. Conclusions
We investigated the selectivity of an anion exchange membrane that had been electrochemically modified with PAni deposits through the application of a negative potential pulse. The information obtained by voltammetry and infrared spectroscopy established that the PAni in the membrane is mainly in its emeraldine form. This modification not only imparted greater hydrophobic character to the membrane, but also greater roughness to its surface, resulting in different selectivities, depending on the anion being studied. For example, the modified membrane exhibited almost identical selectivity towards the nitrate ion when compared to the unmodified membrane, which is attributed to decreases in repulsions between the PAni and the anion, because the anion is weakly hydrated.On the contrary, when the anion is more hydrated, as is the case with chloride, the anion is more strongly rejected by the more hydrophobic membrane and it is more highly retained.
Finally, the high hydrophobic character of the modified membrane was confirmed by high contact angles and low water content.
Acknowledgements
This work was supported by CONACYT (CB 2008-01, 105875), SEP (UASLP-CA-31) and UASLP (C15-FAI-71.71). FTIR test performed by María Estela Nuñez Pastrana is gratefully acknowledged.References
- J. Ran, L. Wu, Y. He, Z. Yang, Y. Wang, C. Jiang, L. Ge, E. Bakangura and T. Xu, J. Membr. Sci., 2017, 522, 267 CrossRef CAS.
- G. Pourcelly, Desalination, 2000, 147, 359 CrossRef.
- T. Xu, J. Membr. Sci., 2005, 263, 1 CrossRef CAS.
- H. Strathmann, in Ion-Exchange Membrane Separation Processes, Elsevier, Amsterdam, ch. 3, 2004 Search PubMed.
- H. Strathmann, Electrodialysis, Desalination, 2010, 264, 268 CrossRef CAS.
- M. Murder, in Basic Principles of Membrane Technology, Kluwer Academic Publishers, London, 2nd edn, 1996, p. 267–271 Search PubMed.
- T. Sata, J. Membr. Sci., 2000, 167, 1 CrossRef CAS.
- I. Banerjee, R. C. Pangule and R. S. Kane, Adv. Mater., 2011, 23, 690 CrossRef CAS PubMed.
- F. Guesmi, C. Hannachi and B. Hamrouni, Ionics, 2012, 18, 711 CrossRef CAS.
- N. L. Le and S. P. Nunes, SMT: Surface Mount Technology, 2016, 7, 1 CAS.
- P. Sivaraman, J. G. Chavan, A. P. Thakur, V. R. Hande and A. B. Samui, Electrochim. Acta, 2007, 52, 5046 CrossRef CAS.
- S. Tan, A. Laforgue and D. Belanger, Langmuir, 2003, 19, 744 CrossRef CAS.
- M. Kumar, M. A. Khan, Z. A. A. AlOthman and R. Siddiqui, Desalination, 2013, 325, 95 CrossRef CAS.
- F. Ahmed, B. S. Lalia, V. Kochkodan, N. Hilal and R. Hashaikeh, Desalination, 2016, 391, 1 CrossRef CAS.
- N. P. Berezina, N. P. Gnusin, O. A. Demina and L. A. Annikova, Russ. J. Electrochem., 2009, 45, 1226 CrossRef CAS.
- N. P. Berezina, A. A.-R. Kubaisi, N. M. Alpatova, V. N. Andreev and E. I. Griga, Russ. J. Electrochem., 2004, 40, 286 CrossRef CAS.
- N. P. Berezina, S. A. Shkirskaya, M. V. Kolechko, O. V. Popova, I. N. Senchikhin and V. I. Roldugin, Russ. J. Electrochem., 2011, 47, 995 CrossRef CAS.
- E. T. Kang, K. G. Neoh and K. I. Tan, Prog. Polym. Sci., 1988, 23, 277 CrossRef.
- M. K. M. Molapo, P. M. Ndangili, R. F. Ajayi, G. Mbambisa, S. M. Mailu, N. Njomo, M. Masikini, P. Baker and E. I. Iwuoha, Int. J. Electrochem. Sci., 2012, 7, 11859 Search PubMed.
- E. M. Genies and C. Tsintavis, J. Electroanal. Chem., 1986, 200, 127 CrossRef CAS.
- B. G. Choi, H. S. Park, H. S. Im, Y. J. Kim and W. H. Hong, J. Membr. Sci., 2008, 324, 102 CrossRef CAS.
- B. van der Bruggen, A. Koninckx and C. Vandecasteele, Water Res., 2004, 38, 1347 CrossRef CAS PubMed.
- H. Farrokhzad, M. R. Moghbeli, T. Van Gerven and B. Van der Bruggen, React. Funct. Polym., 2015, 86, 161 CrossRef CAS.
- T. Sata, T. Yamaguchi and K. Matsusaki, J. Phys. Chem., 1995, 99, 12875 CrossRef CAS.
- P.-A. Bergstroem, J. Lindgren and O. Kristiansson, J. Phys. Chem., 1991, 95, 8575 CrossRef CAS.
- http://www.astom-corp.jp/en/product/10.html.
- X. Tongwen and Y. Weihua, J. Membr. Sci., 2001, 190, 159 CrossRef.
- A. Montes Rojas, Y. Olivares Maldonado and L. M. Torres Rodríguez, J. Membr. Sci., 2007, 300, 2 CrossRef.
- J. G. Ávila Rodríguez, Master’s Thesis, Universidad Autónoma de, San Luis Potosi, 2012.
- J. A. Quezada Renteria, Master’s Thesis, Universidad Autónoma de, San Luis Potosi, 2014.
- R. Lteif, L. Dammak, C. Larchet and B. Auclair, Eur. Polym. J., 2001, 37, 627 CrossRef CAS.
- F. Karas, J. Hnát, M. Paidar, J. Schauer and K. Bouzek, Int. J. Hydrogen Energ., 2014, 39, 5054 CrossRef CAS.
- A. Jikihara, R. Ohashi, Y. Kakihana, M. Higa and K. Kobayashi, Membranes, 2013, 3, 1 CrossRef CAS PubMed.
- J. Heinze, B. A. Frontana-Uribe and S. Ludwigs, Chem. Rev., 2010, 110, 4724 CrossRef CAS PubMed.
- N. G. R. Mathebe, A. Morrin and E. I. Owuoha, Talanta, 2004, 64, 115 CrossRef CAS PubMed.
- K. R. Prasad and N. Munichandraiah, Synth. Met., 2001, 123, 459 CrossRef.
- E. Gileadi, Physical Electrochemistry, Fundamentals, Techniques and Applications, Wiley-VCH, Germany, 2011, p. 125 Search PubMed.
- P. Staiti and F. Lufrano, J. Electrochem. Soc., 2005, 152, A617 CrossRef CAS.
- B. S. Singu, P. Srinivasan and S. Pabba, J. Electrochem. Soc., 2012, 159, A6 CrossRef.
- L. D. Arsov, W. Plieth and G. Koûmehl, J. Solid State Electrochem., 1998, 2, 355 CrossRef CAS.
- S. Pruneanu, E. Veress, I. Marian and L. Oniciu, J. Mater. Sci., 1999, 34, 2733 CrossRef CAS.
- R. Yan and B. Jin, J. Electroanal. Chem., 2015, 743, 60 CrossRef CAS.
- Z. Ping, G. E. Nauer, H. Neugebauer, J. Theiner and A. Neckel, J. Chem. Soc., Faraday Trans., 1997, 93, 121 RSC.
- E. M. Genies and M. Lapkowski, J. Electroanal. Chem., 1987, 220, 67 CrossRef CAS.
- I. Rubinstein and B. Zaltzman, Phys. Rev. E: Stat. Phys., Plasmas, Fluids, Relat. Interdiscip. Top., 2000, 62, 2238 CrossRef CAS.
- G. Chamoulaud and D. Bélanger, J. Colloid Interface Sci., 2005, 281, 179 CrossRef CAS PubMed.
- J. J. Krol, M. Wessling and H. Strathmann, J. Membr. Sci., 1999, 162, 145 CrossRef CAS.
- N. D. Pis’menskaya, V. V. Nikonenko, N. A. Mel’nika, G. Pourcellib and G. Larchetc, Russ. J. Electrochem., 2012, 48, 610 CrossRef.
- M. K. Urtenov, A. M. Uzdenova, A. V. Kovalenko, V. V. Nikonenko, N. D. Pismenskaya, V. I. Vasil'eva, P. Sistat and G. Pourcelly, J. Membr. Sci., 2013, 447, 190 CrossRef CAS.
- X. T. Le, Electrochim. Acta, 2013, 108, 232 CrossRef CAS.
- J. L. W. Ling, A. Khan, B. Saad and S. Ab Ghani, Talanta, 2012, 88, 477 CrossRef CAS PubMed.
- N. B. Jiménez, Master’s Thesis, Universidad Autónoma de, San Luis Potosi, 2016.
| This journal is © The Royal Society of Chemistry 2017 |