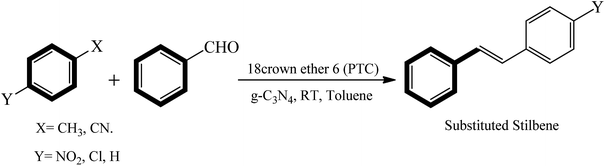
Open Access Article
Open Access ArticleHighly active g-C3N4 as a solid base catalyst for knoevenagel condensation reaction under phase transfer conditions†
Priti Sharma * and
Yoel Sasson
* and
Yoel Sasson
Casali Center of Applied Chemistry, Institute of Chemistry, The Hebrew University of Jerusalem, Jerusalem 91904, Israel. E-mail: priti.sharma@mail.huji.ac.il
First published on 12th May 2017
Abstract
In a promising approach, heterogeneous g-C3N4 as a solid base catalyst exhibits appreciable activity in Knoevenagel condensations at room temperature for the synthesis of substituted stilbene in presence of a crown-ether phase transfer catalyst. High yield of the product substituted stilbene were isolated in a very short reaction time period (∼30 min). The solid base g-C3N4 catalyst was proven to be recyclable for several runs. Various aromatic substrates were screened using heterogeneous base g-C3N4 catalyst, exhibiting appreciable corresponding product yield (∼99%) at room temperature.
Introduction
The base catalyzed organic reactions are of great importance in the chemical process industry. Typical examples are aldol condensations, Knoevenagel condensations and transesterification reactions.1–4 However, the above mentioned name reactions typically require stoichiometric amounts of base along with several reaction conditions such as high reaction temperature, long reaction time and lack of recyclability.5 Over the decades researchers have sought solid base catalysts such as metal oxides6–8 organic materials9 or alkali doped mesoporous,10 zeolites11 or other solid supported materials.12,13 Nevertheless such materials are often instable, and suffer from leaching and deactivation partial decomposition under harsh reaction condition and low turnover numbers are obtained.14 Recently organocatalysis has emerged as a promising technology for application in green catalysis applications.15–17 In the same context, g-C3N4, metal free organocatalyst semiconductors was recently reported as an efficient and environmentally benign photo catalyst with working capacity under visible light.18–22 However, the surface basic nature of g-C3N4 is relatively less explored in organocatalysis application.23 The fabrication of g-C3N4 is based on direct thermal condensation of high nitrogen content precursor example, urea, cyanamide or dicyandiamide.24 Consequently, g-C3N4 opens the doors of Lewis-base characters for coordination chemistry and base-catalyzed organic reactions.25 In a notable work Vinu et al. claims synthesis of g-C3N4 carbon nitride mesoporous nanoparticles with high nitrogen content, exhibited appreciable catalytic transesterification of β-keto esters with different alcohols at temperature of 110 °C.26 In another work Bitter et al. reported basic nitrogen containing carbon nanotubes synthesis (various amount of pyridine nitrogen incorporation) and its catalytic activity in Knoevenagel condensations at 80 °C.27 Recently, Park and coworkers demonstrated mesoporous carbon nitride (MCN) as base catalyst for Knoevenagel condensation of aromatic aldehydes with ethylcyanoacetate by using microwave irradiation.28In addition W. Fan et al. described the controlled synthesis of g-C3N4 with high nitrogen content (modified) and claims higher product yield (40 °C).29 Further, in a remarkable series of work Markus Antonietti exhibited the lewis base character of mpg–C3N4 in the various applications viz.; aliphatic C–H bond oxidation,30 aerobic oxidative coupling of amines,31 selective oxidation of alcohols using visible light.32 Recently Markus Antonietti, extend the application of deprotonated (treated with aq. tBuOK, KOH or K2CO3) mesoporous graphitic carbon nitride (mpg–C3N4) catalysts screened for Knoevenagel and transesterification reactions at 110 °C.33 However, the reported procedure for knoevenagel condensation reaction faces the drawback of high temperature, long reaction time period and most of these catalysts are not completely recyclable. In addition the above fabrication methods are not efficient and not suitable for large scale applications. Stilbenes and its derivatives are important intermediates in the agrochemical34–36 polymer,37,38 food39 pharmaceutical industries40–42 Moreover stilbene synthesis reported procedures suffer from long reaction time, high metal content, high base usage, non recyclable and accidental prone at higher temperature.43,44–46 On the other hand phase transfer catalysis (PTC) offer, efficient, mild and environment friendly reaction conditions, with the replacement of hazardous and sensitive bases or other chemicals.47–52
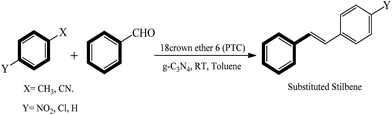
In the present research study we combined the solid base g-C3N4 with a phase transfer agent to catalyze the Knovenagel condensation under ambient conditions, to the best of our knowledge this combination is novel and was not reported previously. We have examined various governing factors such as, various phase transfer catalysts (PTC), solvent, temperature effect and different substrates. The optimized procedure yielded over 90% yield of several stilbene derivatives (Scheme 1).
Results and discussion
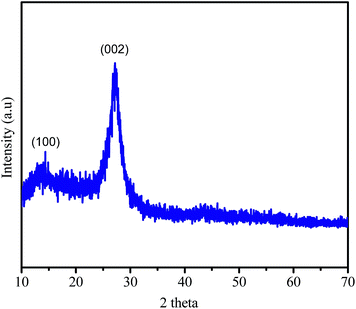
Solid basic g-C3N4 catalyst is formulated via using urea and following the known reported procedure by Wei Chen et al.53 The obtained solid in pale yellow color g-C3N4 was thoroughly analyzed by physicochemical characterization techniques (Scheme 1) (Fig. S1–S8†).The typical X-ray diffraction (XRD) pattern of g-C3N4 material is depicted in Fig. 1. The formation of g-C3N4 is clearly observed in the XRD pattern where after calcination at 550 °C for 3 h, intense typical peaks at 13.20° and 27.20° appeared (Fig. 1). These peaks are in good agreement with reported values which attributes to (100) and (002) diffraction plane, respectively (Fig. 1).54 The intense XRD peak of solid basic g-C3N4 at 27.2° corresponds to the (002) plane and is characteristic for stacking of the conjugated aromatic system (Fig. 1).55
Fabrication of g-C3N4 from urea is well characterized also by FTIR spectroscopy and visualized in Fig S2† (a) urea, (b) g-C3N4. The characteristics absorption bands of urea (1456, 1578, 3435, 3341 cm−1) were disappeared after transformation into solid basic g-C3N4 via controlled calcinations (urea at 550 °C for 3 h) (Fig. S2a and b†). Additionally, the characteristic breathing mode of the triazine repeating units at 810 cm−1, related to the s-triazine ring absorption band vibrations; which are well observed in synthesized g-C3N4 pale yellow material (Fig. S2b†). The strong intense bands at 1625, 1550, 1410 and 1250 cm−1 were in good agreement to the reported values of typical stretching vibration modes of triazine derived repeating units (Fig. S2b†).56
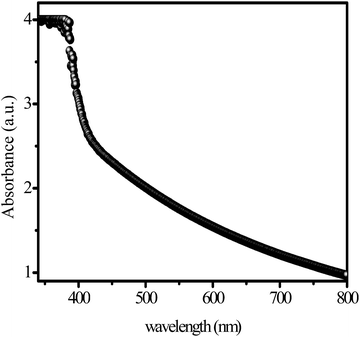
The optical properties of synthesized material, solid basic g-C3N4 were analyzed by UV-vis spectroscopy. Synthesized solid basic g-C3N4 show maximum absorption below 550 nm (visible range), which attributes valence band (VB) to the conduction band (CB) transition (Fig. 2).57
A detail XPS study was carried out for molecular arrangement with chemical properties with systematic formation analysis of heterogeneous basic g-C3N4 catalyst, (Fig. S3A–C†). In the displayed C1s spectrum shows two characteristic peaks corresponding the (–C–N–) and (–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–) at 284.8 and 288 eV; respectively in the fabricated g-C3N4 material (Fig. S3C†). High resolution N1s spectrum of g-C3N4 the N1s spectrum clearly deconvoluted into three intense peaks at 399.9, 401.3 and 405.6 eV, respectively (Fig. S3B†). The peak at 399.9 eV is assigned to the presence of pyridine-N species [sp2-hybridized nitrogen (C
C–) at 284.8 and 288 eV; respectively in the fabricated g-C3N4 material (Fig. S3C†). High resolution N1s spectrum of g-C3N4 the N1s spectrum clearly deconvoluted into three intense peaks at 399.9, 401.3 and 405.6 eV, respectively (Fig. S3B†). The peak at 399.9 eV is assigned to the presence of pyridine-N species [sp2-hybridized nitrogen (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–C)], where each nitrogen (N) atom is bonded to two carbon atoms within aromatic ring located at edges of the graphite planes of g-C3N4 material (Fig. S3B†). The peak at 401.3 eV corresponds to nitrogen atoms trigonally bonded with C carbon (sp2 or sp3) atoms of amino functional groups (C–N–H). Whereas, the peak at 405.6 eV describes the terminal amino groups (–NH2), which is considered to be strong reason for the enhancing basic nature to the solid g-C3N4 material (Fig. S3B†).
N–C)], where each nitrogen (N) atom is bonded to two carbon atoms within aromatic ring located at edges of the graphite planes of g-C3N4 material (Fig. S3B†). The peak at 401.3 eV corresponds to nitrogen atoms trigonally bonded with C carbon (sp2 or sp3) atoms of amino functional groups (C–N–H). Whereas, the peak at 405.6 eV describes the terminal amino groups (–NH2), which is considered to be strong reason for the enhancing basic nature to the solid g-C3N4 material (Fig. S3B†).
The morphologies of g-C3N4 material visualized by SEM spectroscopy with different magnification at 5 μm, 2 μm and 1 μm respectively (Fig. 3A–C). The monomer unit of g-C3N4 appeared to aggregation of uniform sheet structure, each particle agglomeration of many small layers in g-C3N4.58 Moreover, the EDX pattern of g-C3N4 indicates the presence of elementary carbon and nitrogen in the synthesized solid basic g-C3N4 material (Fig. S5†). The morphological representative TEM images of g-C3N4 material at 1 μm, 100 nm and 10 nm scale show crumbled sheets, multilayer with a graphite like structure (Fig. 4A–C).57,59 Furthermore, the SAED pattern shows a broad ring with lower intensity, supports amorphous nature of the synthesized g-C3N4 heterogeneous basic materials (Fig. S6 and S7†).
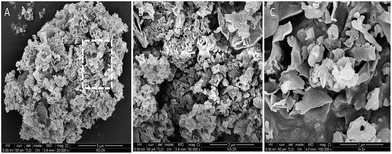 | ||
| Fig. 3 SEM images of g-C3N4 at different magnifications: (A) at 5 μm g-C3N4, (B) at 2 μm g-C3N4, (C) at 1 μm g-C3N4. | ||
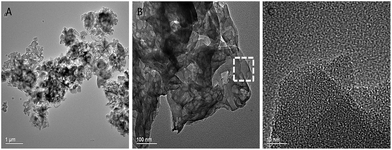 | ||
| Fig. 4 TEM images of g-C3N4 at different magnifications: (A) at 1 μm g-C3N4, (B) at 100 nm g-C3N4, (C) at 10 nm g-C3N4. | ||
The surface textural properties of the synthesized samples are determined with the help of the BET multilayer adsorption and desorption analysis. The calculated BET surface area is 15.00 m2 g−1, total pore volume (Vp) 0.013 cm3 g−1, and mean pore size (radius) Dv(r) of g-C3N4 is found to be 15.3 nm, respectively (Fig. S4†).56
A classical Knoevenagel condensation reaction, proceeds with stoichiometric amount of base at higher temperature with standard substrates.60,61 Here we tried to develop a protocol under mild reaction condition (precisely at room temperature and short reaction time period) using phase transfer catalyst (PTC) with heterogeneous g-C3N4 base in nature catalyst to activate the substrate at room temperature and with minimum time period to afford the appreciable corresponding yield selectively.
In the present study, we exclusively used g-C3N4 basic in nature as the heterogeneous catalyst with phase transfer catalyst for the Knoevenagel condensation reaction (Schemes 2 and 3). For the efficiency measurement of g-C3N4 with phase transfer catalyst a model reaction was chosen between 4-nitrotoluene (1 mmol), benzaldehyde (1 mmol), at room temperature (25 °C) with 18Crown ether 6 (0.1 mmol) (PTC), solvent toluene. (5 mL), g-C3N4 catalyst (30 mg) (Schemes 2 and 3). Within a short reaction time period (30 min) the product formation of 4-nitro trans-stilbene is confirmed with GC in excellent yield (99%). Interestingly, Knoevenagel condensation reaction progresses smoothly in presence of basic g-C3N4 heterogeneous catalyst with PTC in a quite short reaction time period (30 min) at room temperature (25 °C) to afford the corresponding product yield.
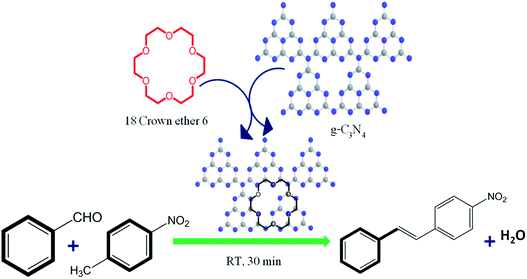 | ||
| Scheme 2 g-C3N4 catalyzed Knoevenagel condensation with 18Crown ether using benzaldehyde and 4-nitrotoluene. | ||
The Knoevenagel reaction has been studied for solvent impact using different solvents namely the highly polar DMSO, DMF and non-polar toluene. Reaction was carried out in highly polar solvent with DMSO and DMF as solvent under optimized reaction condition [4-nitrotoluene (1 mmol), benzaldehyde (1 mmol), room temperature (25 °C), 18Crown ether 6 (0.1 mmol) (PTC), solvent (5 mL), catalyst g-C3N4 (30 mg)] resulting in 10% and 15% yield of corresponding product respectively in 5 h of reaction time period. Surprisingly with non polar solvent toluene with g-C3N4 basic heterogeneous catalyst reaction progress selectively to yield 4-nitrostilbene product with 99% yield under same optimized reaction conditions within 30 min of reaction time period. This might be due to nonpolar solvent toluene might have easily provided the accessibility of 18Crown ether 6 (PTC) to g-C3N4 due to nonpolar nature. Therefore, toluene as solvent is chosen for the rest of the reaction optimization procedures. However without 18Crown ether 6 (PTC) no product formation was detected under optimized reaction condition with toluene solvent even after 5 h.
In the present study, for the establishment the role of phase transfer catalyst we proceeded with model reaction between 4-nitrotoluene (1 mmol), benzaldehyde (1 mmol), at room temperature (25 °C) with PTC (0.1 mmol), solvent toluene (5 mL) and g-C3N4 catalyst (30 mg). We screened the several phase transfer catalysts under optimized reaction conditions, viz.; TBAB (tetrabutyl ammonium bromide), THAB (tetrahexyl ammonium bromide), THAB (tetrabutyl ammonium iodide), THAB (tetrahexyl ammonium chloride), THAB (tetraethyl ammonium chloride) and 18Crown ether 6. The screened results were summarized in Table 1. From Table 1 result describe itself clearly among all the screened phase transfer catalysts only 18Crown ether 6 (PTC) is surprisingly working at room temperature (25 °C) and affords 99% yield of corresponding product within a short reaction time period (1 h) (Table 1 entry 6). The appreciable reactivity of 18Crown ether 6 (PTC) is might be due to the cyclic structure owning highly flexible, good binding affinity, and selective, which is more appropriate to access the g-C3N4 active site, rather than long aliphatic chain of other phase transfer catalysts (Table 1 entries 1–6). Further, Glym62,63 (open-chain analogues of crown ethers) viz.; diglyme [bis(2 methoxyethyl)ether], triglyme [1,2-bis(2 methoxyethoxy)ethane were tested as PTC for the Knoevenagel reaction under optimized reaction conditions with standard reactants. The obtained results exhibited ∼10% conversion of substituted E-stilbene. From Table 1 clears the open chain crown ethers are not much efficient to initiate the reaction in comparison to closed chain crown ether as PTC (Table 1 entries 7, 8).
| Entry | PTC | Time (h) | Yield (%) |
|---|---|---|---|
| a Reaction conditions: 4-nitrotoluene (1 mmol), benzaldehyde (1 mmol), room temperature (25 °C), PTC (0.1 mmol), solvent toluene (5 mL) and g-C3N4 catalyst (30 mg). # glyme (3 mL). | |||
| 1 | Tetrabutyl ammonium bromide (TBAB) | 12 | 10 |
| 2 | Tetrahexyl ammonium bromide (THAB) | 12 | ∼2 |
| 3 | Tetrabutyl ammonium iodide (THAB) | 12 | ∼5 |
| 4 | Tetrahexyl ammonium chloride (THAB) | 12 | ∼2 |
| 5 | Tetraethyl ammonium chloride (THAB) | 12 | ∼1 |
| 6 | 18Crown ether 6 | 30 min | 99% |
| 7# | Diglyme [bis(2-methoxyethyl)ether] | 12 | 10 |
| 8# | Triglyme [1,2-bis(2-methoxyethoxy)ethane] | 12 | 10 |
To designate the basic role of g-C3N4 heterogeneous catalyst in a Knoevenagel condensation reaction under optimized reaction via model reaction using 4-nitrotoluene (1 mmol) and benzaldehyde (1 mmol), at room temperature (25 °C) with PTC (0.1 mmol), solvent toluene (5 mL) and g-C3N4 (30 mg) catalyst is performed. For the reaction progress crosscheck, samples were withdrawn periodically (15 min) from the reaction mixture and analyzed by GC. In a controlled experiment without g-C3N4 catalyst reaction is performed under optimized reaction condition (Table 2, entry 1). The result of controlled experiment shows no progress of reaction in absence of g-C3N4 catalyst. Further, another set of controlled experiments were performed at higher temperature (at 50 °C, 60 °C, 70 °C) without the use of g-C3N4 using model reaction substrates. The result do not shows any appreciable progress of reaction (10%). The result signifies that only higher temperature is not sufficient to initiate reaction, base usage also plays a crucial role in Knoevenagel condensation reaction (Table 2, entry 2).
| Entry | Reaction parameter | Time (h) | Yield (%) |
|---|---|---|---|
| a Reaction conditions: 4-nitrotoluene (1 mmol) and benzaldehyde (1 mmol), at with PTC (0.1 mmol), solvent toluene (5 mL), base & g-C3N4 catalyst (30 mg). | |||
| 1 | 18Crown ether 6 (PTC) | 12 | 5 |
| 2 | 18Crown ether 6 (PTC) + heat (50 °C, 60 °C, 70 °C) | 12 | 5, 10, 10 |
| 3 | 18Crown ether 6 (PTC) + KOH | 1 | 99 |
| 4 | 18Crown ether 6 (PTC) + K2CO3 | 1 | 98 |
| 5 | g-C3N4 | 12 | ∼1 |
| 6 | g-C3N4 + heat | 12 | 88 |
| 7 | 18Crown ether 6 (PTC) + g-C3N4 | 30 (min) | 99 |
| 8 | (Blank reaction) | 12 | 00 |
A new set of reaction with inorganic base (KOH, K2CO3) were screened under optimized reaction condition 4-nitrotoluene (1 mmol) and benzaldehyde (1 mmol), at room temperature (25 °C) with 18Crown ether 6 (PTC) (0.1 mmol) and solvent toluene (5 mL). M. Chidambaram et al. reported64 inorganic base KOH and K2CO3 reactivity with 18Crown ether 6 (PTC), shows 98% yield at room temperature, which we also reproduced with 99% conversion of corresponding product (Table 2, entry 3, 4). But unfortunately this procedure (use of KOH and K2CO3) does not provide the recyclability of base which is essential criteria for environment friendly and industrial process for continuous production.
From all the conducted experiments it is clear that a combination of heterogeneous basic catalyst g-C3N4, with 18Crown ether 6 (PTC) at room temperature (25 °C) is necessary to improve towards more economic Knoevenagel condensation rather than classical condensation reaction (Table 2). The performed reaction establishes a peculiar role of phase transfer catalyst for Knoevenagel condensation reaction at room temperature (Tables 1 and 2).
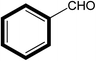
After optimizing the reaction protocol for Knoevenagel condensation model reaction and interesting exclusive results within a short reaction time period, we proceeded to screen the g-C3N4 heterogeneous basic catalyst with 18Crown ether 6 (PTC) combinations with different substrates. Various reactants were analyzed and results are summarized in Table 3. In the referred Knoevenagel condensation reaction under optimized conditions monosubstituted benzaldehyde reacted with 4-nitrotoluene, benzylcynide and 4-nitrobenzylcynide with 99% yield each respectively in a 30 min reaction time period (Table 3, entries 1, 2, 3). At the same time when disubstituted benzaldehyde reacts with monosubstituted another substrate viz.; benzylcynide the productivity of reaction is not affected and shows 85% of corresponding yield (Table 3, entry 5) of corresponding product (Table 3, entry 5, 6, 8). Since –NO2 substituted electron rich substrates are considered to be strong candidate for enhancement of reaction progress (Table 3, entry 1–8). Further, disubstituted benzaldehyde reacted with disubstituted another substrate and shows excellent yield of (18Crown ether 6) exhibit excellent reactivity and not much affected by monosubstituted and disubstituted substrates (Table 3). It is clearly visualized from the Table 3 the synthesized g-C3N4 with phase transfer catalyst PTC (18Crown ether 6) efficiently affords moderate to excellent yields of the corresponding products for Knoevenagel condensation reaction.
| Entry | Substrate 1 | Substrate 2 | Product | Time (min) | Yield (%) |
|---|---|---|---|---|---|
| a Reaction conditions: substrate 1 (1 mmol) and substrate 2 (1 mmol), at room temperature (25 °C), 18Crown ether 6 (PTC) (0.1 mmol), solvent toluene (5 mL) and g-C3N4 basic catalyst (30 mg). | |||||
| 1 | 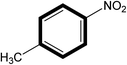 |
 |
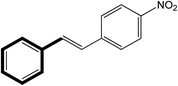 |
30 | 99 |
| 2 | 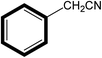 |
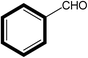 |
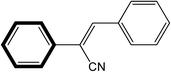 |
30 | 99 |
| 3 | 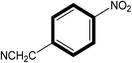 |
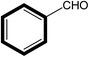 |
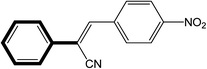 |
30 | 99 |
| 4 | 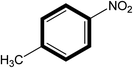 |
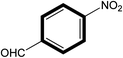 |
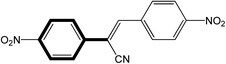 |
30 | 99 |
| 5 | 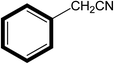 |
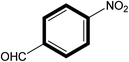 |
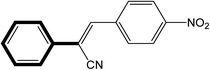 |
30 | 85 |
| 6 | 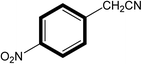 |
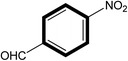 |
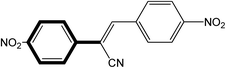 |
30 | 88 |
| 7 | 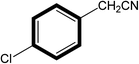 |
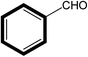 |
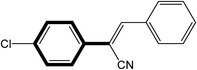 |
30 | 80 |
| 8 | 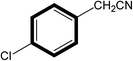 |
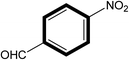 |
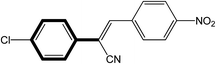 |
30 | 85 |
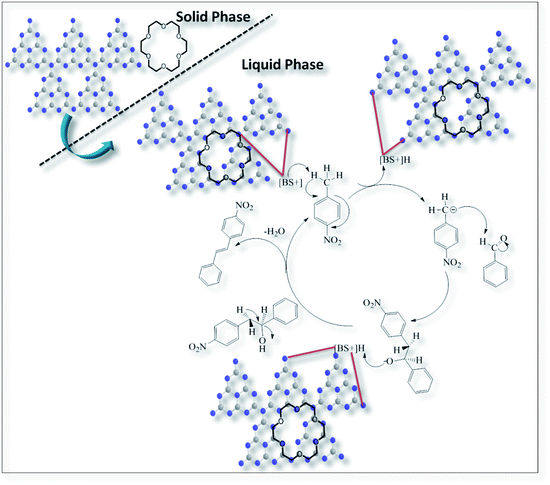
After systematic protocol assigning for Knoevenagel condensation, we tried to explore a suitable mechanism involved in the system. According to the earlier reported mechanism, Knoevenagel condensation initiated with the deprotonation (either at higher temperature or with PTC) step from acidic site of substrate (p-nitro toluene) with the help of base.50,51,64 In the present study g-C3N4 catalyst with 18Crown ether 6 (PTC) is first time studied for the Knoevenagel condensation. The described reaction most probably might have followed the mechanism as shown in the Scheme 4. However recently Junjie Guo et al. and co workers described the flexibility of graphene with 18Crown ether 6 and claims crown ethers (highly flexible, good binding affinity, and selective) embedded in graphene structure.65 With the good agreement to the literature that graphene is analogous to g-C3N4 structure;66–69 therefore in the present study 18Crown ether 6 might have coordinated with g-C3N4 structure via owning rich flexibility and binding affinity for the easy availability of basic site (BS+) in organic medium at room temperature. Further Lewis basic site of g-C3N4 deprotonate the acidic (H+) from 4-nitrotoluene followed by attack of the carbanion on the electrophilic site of the benzaldehyde to yield the alcohol intermediate resulting subsequently to dehydration to form E-stilbene.
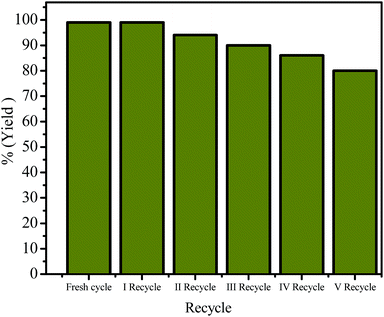
Nearly constant product yield was observed (>84%) even after five recycling experiments, confirmed that the catalyst is stable and the basic nature of the synthesized g-C3N4 heterogeneous catalyst remains active (ESI: S10†) (Fig. 5). For further catalyst stability confirmation, FT-IR analyses of fresh g-C3N4 and spent g-C3N4 catalyst (five recycles) was carried out to probe the change of g-C3N4 at molecular level. The Fig. S8† shows the major peaks of g-C3N4 is well preserved even after the multiple reuses (1625, 1550, 1410 and 1250, 810, 3100 cm−1). Further BET analysis result of spent g-C3N4 catalyst are as follows; BET surface area (SBET), total pore volume (Vp) and mean pore size (radius) Dv(r) is, 23.22 m2 g−1, 0.018 cm3 g−1 17.356 nm respectively. The observed increase in the surface area and pore size in spent catalyst, might be due to the transformation into monolithic sheets from bulk g-C3N4 via continues reuse (Fig. ESI 9†). Both the performed characterization suggesting that the spent g-C3N4 catalyst remains unchanged at molecular level even after multiple reuses.
Conclusions
In a concluding remark, here we have demonstrated first time the use of g-C3N4 owning the basic and heterogeneous nature, with phase transfer catalyst for Knoevenagel condensation reaction. The catalytic performance is surprisingly enhanced due to use of phase transfer catalyst precisely 18Crown ether 6 (PTC) upto 99% yield within very short reaction time period (30 min) at room temperature with standard substrate. The newly established protocol for Knoevenagel condensation reaction is successfully screened with different substrate and demonstrated excellent product yield (>99%) at room temperature. The conducted experimental results demonstrate the basic nature of g-C3N4 efficiently activates the substrate at room temperature with PTC, which is responsible for the Knoevenagel condensation initiation. The exclusive salient feature of the successive protocol via g-C3N4 basic heterogeneous catalyst and phase transfer catalyst are; room temperature, short reaction time period (30 min), screening capability of various reactant, remains active under mild conditions and even after several reaction cycles.Experimental
For graphitic carbon nitride (g-C3N4) synthesis procedure sees ESI† for detail.General procedure for the Knoevenagel condensation reaction
The reaction was carried out in a 25 mL oven dried, round bottom flask, with high magnetic stirring at ∼800 rpm at room temperature. In a typical run, substrate 1 (4-nitrotoluene or similar kind) (1 mmol) and substrate 2 (benzaldehyde or similar kind) (1 mmol), at room temperature (25 °C) with PTC (0.1 mmol), solvent toluene (5 mL), g-C3N4 (30 mg) were allowed to stir. The reaction mixture samples were withdrawn periodically at the measured time intervals and analyzed by gas chromatography. The samples were diluted and filtered with whatman paper before injecting into a gas chromatograph.Acknowledgements
Author gratefully acknowledges Hani Gnayem & Ofer for their help to accomplish the present research work.Notes and references
- R. J. Davis, J. Catal., 2003, 216, 396–405 CrossRef CAS.
- M. J. Climent, A. Corma, R. Guil-Lopez, S. Iborra and J. Primo, Catal. Lett., 1999, 59, 33–38 CrossRef CAS.
- R. Radhakrishan, D. M. Do, S. Jaenicke, Y. Sasson and G.-K. Chuah, ACS Catal., 2011, 1, 1631–1636 CrossRef CAS.
- R. A. Sheldon, J. Mol. Catal. A: Chem., 1996, 107, 75–83 CrossRef CAS.
- X. Liu, X. Piao, Y. Wang, S. Zhu and H. He, Fuel, 2008, 87, 1076–1082 CrossRef CAS.
- H. Dabbagh and B. H. Davis, J. Mol. Catal., 1988, 48, 117–122 CrossRef CAS.
- H. Teterycz, R. Klimkiewicz and B. W. Licznerski, Appl. Catal., A, 2001, 214, 243–249 CrossRef CAS.
- A. Corma and S. Iborra, in Advances in Catalysis, ed. C. G. Bruce and K. Helmut, Academic Press, 2006, vol. 49, pp. 239–302 Search PubMed.
- S. K. Kundu and A. Bhaumik, RSC Adv., 2015, 5, 32730–32739 RSC.
- N. Linares, A. M. Silvestre-Albero, E. Serrano, J. Silvestre-Albero and J. Garcia-Martinez, Chem. Soc. Rev., 2014, 43, 7681–7717 RSC.
- A. Corma, V. Fornés, R. M. Martín-Aranda, H. García and J. Primo, Appl. Catal., 1990, 59, 237–248 CrossRef CAS.
- V. Calvino-Casilda, R. M. Martín-Aranda, A. J. López-Peinado, I. Sobczak and M. Ziolek, Catal. Today, 2009, 142, 278–282 CrossRef CAS.
- J. Roggenbuck and M. Tiemann, J. Am. Chem. Soc., 2005, 127, 1096–1097 CrossRef CAS PubMed.
- L.-B. Sun, X.-Q. Liu and H.-C. Zhou, Chem. Soc. Rev., 2015, 44, 5092–5147 RSC.
- M.-A. Courtemanche, M.-A. Légaré, L. Maron and F.-G. Fontaine, J. Am. Chem. Soc., 2013, 135, 9326–9329 CrossRef CAS PubMed.
- V. B. Saptal and B. M. Bhanage, ChemSusChem, 2016, 9, 1980–1985 CrossRef CAS PubMed.
- B. List, Chem. Rev., 2007, 107, 5413–5415 CrossRef CAS.
- X. Chen, J. Zhang, X. Fu, M. Antonietti and X. Wang, J. Am. Chem. Soc., 2009, 131, 11658–11659 CrossRef CAS PubMed.
- F. Su, S. C. Mathew, G. Lipner, X. Fu, M. Antonietti, S. Blechert and X. Wang, J. Am. Chem. Soc., 2010, 132, 16299–16301 CrossRef CAS PubMed.
- S. Cao and J. Yu, J. Phys. Chem. Lett., 2014, 5, 2101–2107 CrossRef CAS PubMed.
- J. Wen, J. Xie, X. Chen and X. Li, Appl. Surf. Sci., 2017, 391, 72–123 CrossRef CAS.
- Y. Wang, X. Wang and M. Antonietti, Angew. Chem., Int. Ed., 2012, 51, 68–89 CrossRef CAS PubMed.
- S. Yin, J. Han, T. Zhou and R. Xu, Catal. Sci. Technol., 2015, 5, 5048–5061 CAS.
- S. Patnaik, S. Martha, S. Acharya and K. M. Parida, Inorg. Chem. Front., 2016, 3, 336–347 RSC.
- X. Jin, V. V. Balasubramanian, S. T. Selvan, D. P. Sawant, M. A. Chari, G. Q. Lu and A. Vinu, Angew. Chem., 2009, 121, 8024–8027 CrossRef.
- X. Jin, V. V. Balasubramanian, S. T. Selvan, D. P. Sawant, M. A. Chari, G. Q. Lu and A. Vinu, Angew. Chem., Int. Ed., 2009, 48, 7884–7887 CrossRef CAS PubMed.
- S. van Dommele, K. P. de Jong and J. H. Bitter, Top. Catal., 2009, 52, 1575–1583 CrossRef.
- M. B. Ansari, H. Jin, M. N. Parvin and S.-E. Park, Catal. Today, 2012, 185, 211–216 CrossRef CAS.
- L. Zhang, H. Wang, W. Shen, Z. Qin, J. Wang and W. Fan, J. Catal., 2016, 344, 293–302 CrossRef CAS.
- Y. Wang, H. Li, J. Yao, X. Wang and M. Antonietti, Chem. Sci., 2011, 2, 446–450 RSC.
- F. Su, S. C. Mathew, L. Möhlmann, M. Antonietti, X. Wang and S. Blechert, Angew. Chem., Int. Ed., 2011, 50, 657–660 CrossRef CAS PubMed.
- X.-H. Li, J.-S. Chen, X. Wang, J. Sun and M. Antonietti, J. Am. Chem. Soc., 2011, 133, 8074–8077 CrossRef CAS PubMed.
- F. Su, M. Antonietti and X. Wang, Catal. Sci. Technol., 2012, 2, 1005–1009 CAS.
- K. B. Becker, Synthesis, 1983, 1983, 341–368 CrossRef.
- T. P. Schultz, Q. Cheng, W. D. Boldin, T. F. Hubbard Jr, L. Jin, T. H. Fisher and D. D. Nicholas, Phytochemistry, 1991, 30, 2939–2945 CrossRef CAS.
- T. P. Schultz, T. F. Hubbard Jr, L. Jin, T. H. Fisher and D. D. Nicholas, Phytochemistry, 1990, 29, 1501–1507 CrossRef CAS.
- A. Roviello and A. Sirigu, Makromol. Chem. Rapid Comm., 1982, 183, 409–415 CrossRef CAS.
- E. C. Buruiana, T. Buruiana, G. Strat and M. Strat, J. Polym. Sci., Part A: Polym. Chem., 2002, 40, 1918–1928 CrossRef CAS.
- F. Silva, A. Figueiras, E. Gallardo, C. Nerín and F. C. Domingues, Food Chem., 2014, 145, 115–125 CrossRef CAS PubMed.
- C. J. Lion, C. S. Matthews, M. F. G. Stevens and A. D. Westwell, J. Med. Chem., 2005, 48, 1292–1295 CrossRef CAS PubMed.
- G. J. Soleas, E. P. Diamandis and D. M. Goldberg, Clin. Biochem., 1997, 30, 91–113 CrossRef CAS PubMed.
- T.-C. Hsieh and J. M. Wu, BioFactors, 2010, 36, 360–369 CrossRef CAS PubMed.
- K. Ferré-Filmon, L. Delaude, A. Demonceau and A. F. Noels, Coord. Chem. Rev., 2004, 248, 2323–2336 CrossRef.
- C. M. Kormos and N. E. Leadbeater, J. Org. Chem., 2008, 73, 3854–3858 CrossRef CAS PubMed.
- F. Alonso, P. Riente and M. Yus, Eur. J. Inorg. Chem., 2009, 2009, 6034–6042 CrossRef.
- T. Banno, Y. Hayakawa and M. Umeno, J. Organomet. Chem., 2002, 653, 288–291 CrossRef CAS.
- S. Yousefi and A. R. Kiasat, RSC Adv., 2015, 5, 92387–92393 RSC.
- N. Qafisheh, S. Mukhopadhyay and Y. Sasson, 2002.
- S. Mukhopadhyay, G. Rothenberg, N. Qafisheh and Y. Sasson, Tetrahedron Lett., 2001, 42, 6117–6119 CrossRef CAS.
- M. Chidambaram, S. U. Sonavane, J. de la Zerda and Y. Sasson, Tetrahedron, 2007, 63, 7696–7701 CrossRef CAS.
- Y. Sasson and G. Rothenberg, in Handbook of Green Chemistry and Technology, Blackwell Science Ltd, 2007, ch. 10, pp. 206–257, DOI:10.1002/9780470988305.
- G. Barak and Y. Sasson, J. Org. Chem., 1989, 54, 3484–3486 CrossRef CAS.
- J. Liu, T. Zhang, Z. Wang, G. Dawson and W. Chen, J. Mater. Chem., 2011, 21, 14398–14401 RSC.
- F. Dong, L. Wu, Y. Sun, M. Fu, Z. Wu and S. C. Lee, J. Mater. Chem., 2011, 21, 15171–15174 RSC.
- X. Chen, L. Zhang, B. Zhang, X. Guo and X. Mu, Sci. Rep., 2016, 6, 28558 CrossRef PubMed.
- A. Kumar, P. Kumar, C. Joshi, S. Ponnada, A. K. Pathak, A. Ali, B. Sreedhar and S. L. Jain, Green Chem., 2016, 18, 2514–2521 RSC.
- P. Sharma and Y. Sasson, Green Chem., 2017, 19, 844–852 RSC.
- L. Song, S. Zhang, X. Wu, H. Tian and Q. Wei, Ind. Eng. Chem. Res., 2012, 51, 9510–9514 CrossRef CAS.
- S. Verma, R. B. Nasir Baig, M. N. Nadagouda and R. S. Varma, ACS Sustainable Chem. Eng., 2016, 4, 2333–2336 CrossRef CAS.
- F. Bigi, L. Chesini, R. Maggi and G. Sartori, J. Org. Chem., 1999, 64, 1033–1035 CrossRef CAS PubMed.
- L. F. Tietze, Chem. Rev., 1996, 96, 115–136 CrossRef CAS PubMed.
- J. C. Hogan and R. D. Gandour, J. Am. Chem. Soc., 1980, 102, 2865–2866 CrossRef CAS.
- J. C. Hogan and R. D. Gandour, J. Org. Chem., 1992, 57, 55–61 CrossRef CAS.
- N. Taha, Y. Sasson and M. Chidambaram, Appl. Catal., A, 2008, 350, 217–224 CrossRef CAS.
- J. Guo, J. Lee, C. I. Contescu, N. C. Gallego, S. T. Pantelides, S. J. Pennycook, B. A. Moyer and M. F. Chisholm, Nat. Commun., 2014, 5, 5389 CrossRef CAS PubMed.
- Y. Zhang, Q. Pan, G. Chai, M. Liang, G. Dong, Q. Zhang and J. Qiu, Sci. Rep., 2013, 3, 1943 CrossRef PubMed.
- G. Dong, Y. Zhang, Q. Pan and J. Qiu, J. Photochem. Photobiol., C, 2014, 20, 33–50 CrossRef CAS.
- X. Dong and F. Cheng, J. Mater. Chem. A, 2015, 3, 23642–23652 CAS.
- V. N. Khabashesku, J. L. Margrave and J. L. Zimmerman, Chem. Mater., 2002, 12, 3264–3270 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Physical appearance of g-C3N4, SEM-EDX images g-C3N4, TEM-EDX images g-C3N4, TEM images of g-C3N4 with different magnification & SAED pattern, characterization, experimental, heterogeneous study of catalyst g-C3N4. See DOI: 10.1039/c7ra03051g |
| This journal is © The Royal Society of Chemistry 2017 |