Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Exploring the pathways of aromatic carboxylic acids in ozone solutions
Xin Zhong a,
Chongwei Cui*a and
Shuili Yub
a,
Chongwei Cui*a and
Shuili Yub
aSchool of Municipal & Environmental Engineering, Harbin Institute of Technology, Harbin 150090, China
bSchool of Environmental Science and Engineering, Tongji University, Shanghai 200433, China
First published on 10th July 2017
Abstract
The reaction between ozone and natural organic matter (NOM) generates a certain amount of aromatic carboxylic acids (ACAs). Previous studies report that the pre-ozonation of fulvic acid can produce protocatechuic acid (PA), 3-hydroxybenzoic acid (3-OHBA), phthalic acid (PhA), and benzoic acid (BA). Ozonation of ACAs may result in diverse, low-molecular-weight carbonyl compounds, significantly affecting the formation of disinfection by-products (DBPs) following chlorination. The purpose of this study was to determine the degradation mechanisms, possible ozonation reaction pathways, ozonation intermediates and DBPs of four ACAs, and evaluate the ability of degrading micro-pollutants with different structures by ozonation, as well as to make up for the knowledge gap of DBPs generated. The results showed that two stages pseudo-first-order reaction for four ACAs and six intermediates accompanied the ozonation of ACAs, namely formaldehyde, glyoxal, methylglyoxal, fumaric acid, formic acid, and acetic acid. Whether the hydroxyl radical was involved or not, the ozonation of ACAs contained three steps; first step being the BA hydroxylation. Second, hydroxylation products of BA were oxidized to generate ring-opened compounds such as unsaturated carbonyl compounds. Third, short-chain aldehydes and carboxylic acids were formed. As for mineralization, the short-chain aldehydes and carboxylic acids were finally transformed into CO2 and H2O. In addition, not only ACAs themselves were the main DBPs precursors, but the intermediates of ACAs ozonation could also be the DBPs precursors, and they produced high yields of trihalomethanes (THMs) and haloacetic acids (HAAs). Therefore, the ACA levels should be kept under control in drinking water treatment plants.
1. Introduction
Aromatic carboxylic acids (ACAs) represent a wide range of chemicals used as raw materials or generated in various industries such as printing and dyeing, pharmaceuticals, food, cosmetics and paper mills.1 All compounds are toxic and display low biodegradability.2,3 Benzoic acid (BA) is detected in the effluents of the pharmaceutical industries.4 In printing and dyeing industries, phthalic acid (PhA) is generally used as the main raw material for acylation and esterification reactions. Consequently, these ACAs can be found in diverse media, such as surface water, sediments, landfill leachate water and even in atmospheric aerosols.5,6 BA (from 1.1 to 4.5 μg l−1) has been detected in surface water.7 PhA (12.2 ± 0.8 μg l−1) has been found in tap water disinfected by ozonation combined with chlorination.8Ozone (O3), an electrophile with high selectivity, is commonly used for degrading organic pollutants in water treatment plants.9 However, organic compounds containing electron-rich functional groups (e.g. double bonds, activated aromatic ring) are selectively attacked by molecular O3, while ˙OH non-selectively reacts with organic compounds.10 O3 oxidation mechanism includes molecular O3 direct reactions and ˙OH indirect reactions that are produced by O3 decomposition. Currently, some electron-rich moieties and hydroxylation products are intermediately formed during ozonation.11–14 On the contrary, aromatics structures containing electron-withdrawing functional groups how activated O3 as well as intermediate products received little attention. For one thing, aromatic compounds containing electron-withdrawing functional groups (e.g. –Cl, –COOH, –NO2, –CN) are generally O3 resistant. For another thing, they could be effectively oxidized by ˙OH, with second-order rate constants ranging from 108 to ∼1010 M−1 s−1.15
Some researchers have reported the selectivity of compounds with similar chemical structures during ozonation process. Zhang and Croué selected six carboxylic acids, namely acetic acid, oxalic acid, citric acid, pyruvic acid, malonic acid and succinic acid, to study their reactivity with molecular O3, and the results demonstrated that their reactivity was low.16 Their results clearly demonstrated that ozonation could not completely degrade the carboxylic acids. Huang et al., reported that though BA is recalcitrant to O3 attack, it undergoes efficient degradation at an acidic pH (2.3).15 The degradation of BA and O3 decomposition were both enhanced on increasing the BA concentration. Our previous studies showed that the pre-ozonation of fulvic acid can produce protocatechuic acid (PA), 3-hydroxybenzoic acid (3OHBA), PhA, and BA, which could continue to react with O3 during the main ozonation step in the treatment of drinking water. It is noteworthy that their chemical structures are closely similar (containing different numbers of –COOH and –OH groups). However, considering the course of degradation and mineralization, the knowledge about diverse chemical structures of ACAs was not adequate, probably due to the lack of a suitable analytical method. ACAs should be converted into weaker polar and stable derivates so that they can be applicable for GC determination. In order to avoid the complex procedures of derivatization, our team employed solid phase extraction-ultra high performance liquid chromatography (SPE-UPLC) to determine the level of ACAs. Moreover, ozonation of ACAs may result in diverse low-molecular-weight carbonyl by-products, significantly affecting the formation of disinfection by-products (DBPs) following chlorination.
Although O3 has long been used for disinfection and oxidation in water treatments, there is a critical gap in information related to the transformation of organic compounds. This has become increasingly important in recent years because there is a considerable concern about the formation of potentially harmful degradation products and oxidation products from the reaction with the matrix components. Thus, the purpose of this study was to investigate the degradation mechanism, possible ozonation reaction pathways, ozonation intermediates and DBPs of 4 ACAs, evaluate the ability of degrading micro-pollutants with different structures by ozonation, and make up for the knowledge gap of the generated DBPs.
2. Materials and methods
2.1 Materials
The analytical standard including 15 carbonyl compounds (formaldehyde, acetaldehyde, propanal, butanal, pentanal, hexanal, cyclohexanone, crotonaldehyde, heptanal, octanal, benzaldehyde, nonanal, decanal, glyoxal and methyl glyoxal) and the derivatization reagent O-(2,3,4,5,6-pentafluorobenzyl) hydroxylamine (PFBOA) were purchased from Acc Standard (New Haven, USA). HPLC grade n-hexane from Anpel (Shanghai, China) was used as a solvent for the liquid–liquid extraction. The Synergy UV-Ultrapure Water System (Millipore, Molsheim, France) provided organic-free water.Standards of the 7 carboxylic acids (>95% purity), formic acid, acetic acid, fumaric acid, PA, 3-OHBA, PhA and BA, were purchased from J&K (Beijing, China). Hydrochloric acid, sodium hypochlorite, sodium hydroxide, potassium dihydrogenphosphate and orthophosphoric acid were analytically pure and supplied by Shanghai (Shanghai, China). Silica-reverse phase sorbent (Supelclean ENVI-18) containing octadecyl functional groups, was purchased from Supelco (Bellefonte, PA, USA). LiChrolut EN (particle size 40–120 μm) was provided by Merck (Darmstadt, Germany).
2.2 Experimental procedures
Stock solutions of 4 ACAs were prepared by dissolving 1.0 g ACAs in 1000 ml purified water and mixing for 24 h, individually. Working solutions at 1.0 mg l−1 concentration were obtained by water dilution. All these solutions were stored at 4 °C.All ozonation experiments were operated on 500 ml SIMAX bottles fitted with a magnetic stirring bar. COM-AD-01 O3 generator (4 g h−1, ANSEROS, Germany) produced O3 from pure O2 (≥99.2% purity). Subsequently, through a disperser placed at the bottom of the reactor, O3 was scattered rapidly into ultrapure water. According to the direct UV absorbance method,17 the concentration of O3 stock solution (15–20 mg l−1) was measured. Phosphate buffer solution was added to ensure a uniform solution pH of 7 (±0.1) in all experiments. The ozonation reactions were terminated by sodium nitrite (200 μl, 6.9 g l−1, J&K, Beijing, China).18 The ozonated samples were stored at 4 °C for not more than 24 h. The experimental scheme included the following conditions: O3 contact time 1, 2, 5, 10, 20, 30 min, O3 1.0 mg l−1 and pH 7.
Chlorination was conducted on ozonated samples using 100 ml chlorine-free bottles. Chosen Cl2 dosage guaranteed that a sufficient amount of Cl2 remained in solution after incubation for 24 h, and hence reactions were not limited by chlorine concentration. After chlorine was added, samples were stored headspace-free, at a pH 7 and 25 ± 1 °C in the dark for 24 h. Ascorbic acid was used to quench the residual chlorine (100 μl, 10 g l−1, Anpel, Shanghai, China).
2.3 Analytical methods
According to Standard Method 5310,17 the total organic carbon analyzer (OI, Aurora1030) was used to measure the decay of total organic carbon (TOC) during treatment.The EPA 556 method and the EPA 552.2 method were individually used for the analysis of aldehydes and dichloroacetic acid (DCAA) in water samples (EPA, 1998, EPA, 2003). Aldehydes and DCAA were analyzed by 7890B gas chromatograph fitted with an electron capture detector (ECD) (Agilent Technologies, Palo Alto, USA). The DB-5MS capillary column (30 m × 0.25 mm I.D. × 0.25 μm film thickness, Agilent Technologies, Bellefonte, PA, USA) was applied for the separation. The carrier gas was He (1 ml min−1) and the detector make-up gas was N2 (30 ml min−1). The aldehydes temperature program was as follows: 50 °C hold for 1 min, program at 4 °C min−1 up to 220 °C, program at 20 °C min−1 up to 250 °C and hold at 250 °C for 10 min. The DHAA temperature program was as follows: 35 °C hold for 10 min, program at 2 °C min−1 up to 40 °C, program at 5 °C min−1 up to 75 °C and hold at 75 °C for 15 min, program at 40 °C min−1 up to 100 °C and hold at 100 °C for 15 min, program at 40 °C min−1 up to 135 °C.
According to the purge and trap gas chromatographic method, chloroform was measured using 4660-7890B-5077A gas chromatograph (Agilent Technologies, Palo Alto, USA) equipped with a mass spectrometer. The operating conditions were as follows: magnetic mass analyzer scans from 35 to 200 m/z, 70 eV electron energy, ion source 250 °C, carrier gas: He (1 ml min−1), temperature program: 30 °C hold for 10 min, program at 7 °C min−1 up to 72 °C and hold at 72 °C for 1 min, program at 40 °C min−1 up to 220 °C and hold at 220 °C for 1 min.
The 4 ACAs and 1 aliphatic carboxylic acid (fumaric acid, PA, 3-OHBA, PhA and BA) were analyzed by SPE-UPLC.19 The SPE system (Anpel, Shanghai, China) was assembled from a GAST pump (MICH, USA). The SPE cartridges were conditioned using 1 ml acetonitrile–methanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and 1 ml ultrapure water. Chromatographic analyses were carried out on a Waters H-class Ultra high performance liquid chromatography system (Waters, USA). The column was ACQUITY UPLC BEH C18 (2.1 × 50 mm I.D., particle size 1.7 μm, Waters, USA). A PHS-2C pH meter (Shanghai, China) was used to adjust the pH value. In brief, 100 ml standard solution or ACAs water sample at pH ∼ 1.3 (adjusted with 5 M HCl) flowed past the SPE cartridge at 2–3 ml min−1. The SPE cartridge was filled with 80 mg of the mixture of LiChrolut EN/Supelclean ENVI-18 (1
1) and 1 ml ultrapure water. Chromatographic analyses were carried out on a Waters H-class Ultra high performance liquid chromatography system (Waters, USA). The column was ACQUITY UPLC BEH C18 (2.1 × 50 mm I.D., particle size 1.7 μm, Waters, USA). A PHS-2C pH meter (Shanghai, China) was used to adjust the pH value. In brief, 100 ml standard solution or ACAs water sample at pH ∼ 1.3 (adjusted with 5 M HCl) flowed past the SPE cartridge at 2–3 ml min−1. The SPE cartridge was filled with 80 mg of the mixture of LiChrolut EN/Supelclean ENVI-18 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) sorbents. Later, ACAs were eluted by 1 ml methanol and collected in a 2 ml glass GC vial.
1) sorbents. Later, ACAs were eluted by 1 ml methanol and collected in a 2 ml glass GC vial.
Formic acid and acetic acid were analyzed by ICS-2100 Ion chromatograph (Thermo Scientific, USA) equipped with a Dionex IonPac AS-19 capillary column (0.4 mm × 250 mm) and a Dionex IonPac AS-19 guard column (0.4 mm × 50 mm). Dionex RFIC-EG eluent generator provided the mobile phase, with the following conditions: flow 10 μl min−1, 0–10 min 10 mM KOH, 10–42 min linear ramp to 52 mM KOH, 42–45 min linear ramp to 70 mM KOH, 45–50 min 10 mM KOH.
2.4 Quality control
Calibration curves prepared for each compound were linear (R2 > 0.980). The method of aldehydes detection limits varied from 0.2 μg l−1 to 4.0 μg l−1. The method of carboxylic acids detection limits varied from 0.8 μg l−1 to 5.0 μg l−1. The recovery rates of each compound were greater than 80%. Experiments were conducted in duplicates. Relative standard deviation of the two measurements was below 15% in general. The SPSS software (IBM SPSS, version 17) was employed for statistical data analysis.3. Results and discussion
3.1 ACAs degradation by ozone
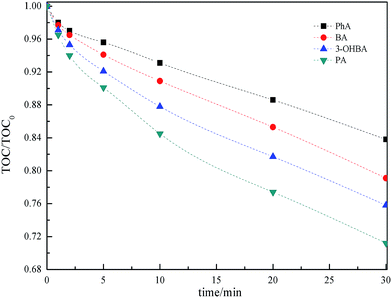
It is generally recognized that under acidic conditions, molecule O3 is the primary active substance, whereas under basic conditions, ˙OH is the primary active substance. In addition, O3 not only depletes by reaction with ACAs, but also by self-degradation in the ultrapure water. Hence, first-order reactions were not observed during the monitoring period. On the contrary, the reactions could be expressed in two steps pseudo-first-order reactions, as shown in Fig. 1. It is clearly noticed that the reactivity was in the order PA > 3-OHBA > BA > PhA. Previous studies have indicated that O3 directed oxidation was effective for degradation of organic pollutants bearing active groups, such as –OH, –NH2, and double bond.10,11 The existence of active groups was advantageous for O3 for the electrophilic attack on the aromatic ring. Thus, this was more significant for PA (bearing two electron-donating groups), resulting in a rapid degradation. In addition, carboxylate group enhanced the nucleophilicity of reaction site, further activating the reaction with O3.
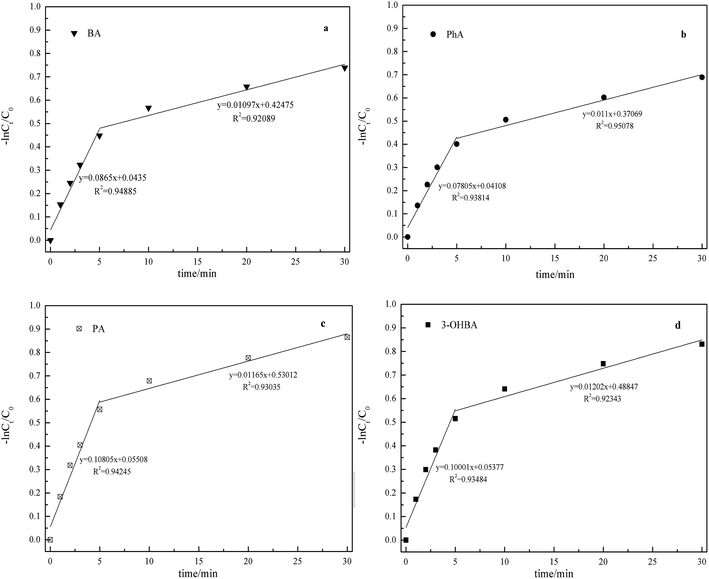 | ||
| Fig. 1 Degradation of BA, PA, PhA and 3-OHBA at different contact times. Conditions: pH 7, 25 °C, [O3]0 = 1.0 mg l−1, and [BA, PA, PhA, 3-OHBA]0 = 1.0 mg l−1; (a) BA; (b) PhA; (c) PA; (d) 3-OHBA. | ||
These reaction rates are not only the function of the concentration of O3 and ˙OH radicals, but are also dependent on other oxidative species, therefore these reaction rates cannot be regarded as fundamental degradation mechanisms. It should be pointed out that although different aromatic groups result in different reaction rates, the degradation trends of ACAs are found to be similar. It can be inferred that the ozonation pathway for degrading ACAs could form similar by-products before the mineralization step.
There was about 39.7% of PhA, 43.3% of BA, 46.2% of 3-OHBA and 49.3% of PA degradation observed in 10 min. As the initial reaction stage proceeds rapidly, it could be advantageous to regulate ozonation treatment in water treatment plants, particularly for O3 active components.
During ozonation, although ACAs were degraded, the mineralization degree was not high, as evidenced by the TOC measurement (Fig. 2). The mineralization degree of PA, 3-OHBA, BA, and PhA was 28.8%, 24.2%, 20.9%, 16.2% at 30 min, respectively. This implied the formation of ozonation by-products. Although O3 is a strong oxidizer with selectivity, it cannot mineralize the inert organics in a given time.20 The transformation products detected during the ozonation processes are discussed in the next section.
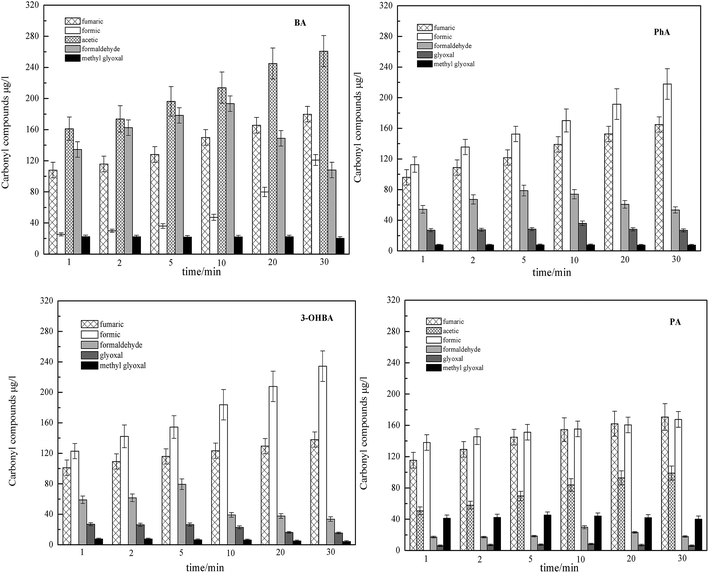 | ||
| Fig. 3 The concentration of intermediates during ozonation of ACAs at different contact times. Conditions: pH 7, 25 °C, [O3]0 = 1.0 mg l−1, and [BA, PA, PhA, 3-OHBA]0 = 1.0 mg l−1. | ||
For PhA and 3-OHBA, formic acid was the main by-product. However, for BA and PA, the main by-products were formic acid and acetic acid. Due to the different concentration levels of formic acid and acetic acid, the amount of formed formic and acetic acids relates to the chemical structure of the ACAs. In addition, it should be noted that, during the initial phase of O3 oxidation of BA, 3-OHBA was detected with a concentration ranging from 6.2 μg l−1 to 23.6 μg l−1 and then quickly disappeared. Since 3-OHBA could be further oxidized by excess O3, it was not easily detected. Pillar et al., also reported that the reaction rate constants of hydroxybenzoic acids with O3 and ˙OH were 5.2 × 105 M−1 s−1 and 1.1 × 1010 M−1 s−1, respectively.24 Moreover, Huang et al., found a significant increase in ˙OH production in the presence of hydroxybenzoic acids.15 Their results indicated that hydroxybenzoic acids could promote O3 decomposition to generate ˙OH. These results indicated that BA containing hydroxyl groups was easily eliminated with O3. It can be concluded that hydroxylation products were the main products of ozonation transformation organics, and the formation of formic acid, acetic acid and fumaric acid may have resulted from further oxidation of these hydroxylation products.
Carboxylic acids are an important type of newly formed assimilable organic carbon (AOC), which are a significant part of organic DBPs in drinking water ozonation treatment plants.7,25 Generally, compared to the precursors, the short-chain carboxylic acids are easily biodegradable, and they have been proven to be associated with bacterial re-growth and bio-film formation in water distribution systems. Therefore, certain measures should be adopted to control the source of carboxylic acids in drinking water treatment plants.
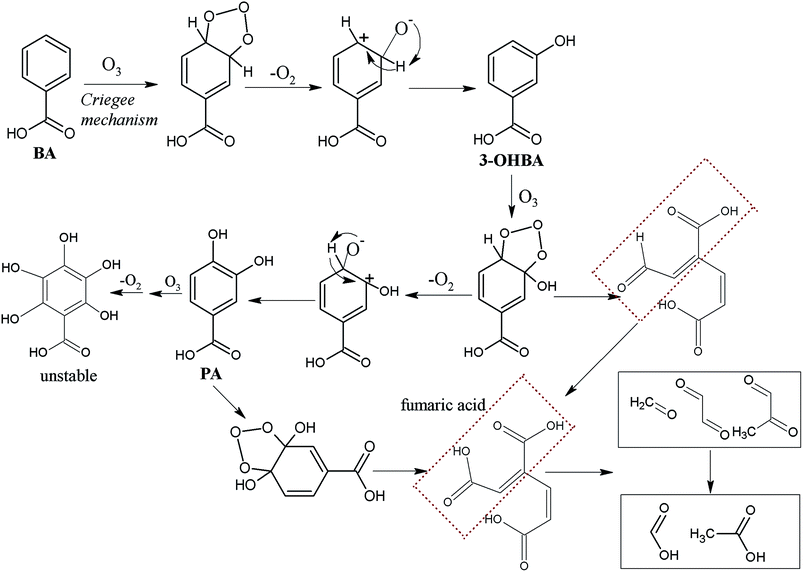
In O3 solution, the reaction between O3 and aromatic compounds hinged largely on the electron-donating/withdrawing properties of the functional groups. –COOH being a strong electron-withdrawing group, results in a more electron-deficient ortho/para-position as compared to the meta-position, and therefore meta-position is the first to undergo addition. Therefore, in the reaction between O3 and BA, the electrophilic group generally attacks the meta-position, which leads to the formation of 3-OHBA. This could explain why 3-OHBA was detected in the process of BA ozonation. On the contrary, –OH results in a more electron-rich ortho/para-position compared to meta-position, at the same time making the benzene ring more nucleophilic. In summary, the combined action of –COOH and –OH on the electron distribution may lead to a more electron-rich at position 5 than position 4 and 6 in 3-OHBA. This could presumably result in the formation of polyhydroxylated aromatic products. As described above, hydroxylation aromatic intermediates react rapidly with O3. Therefore, PA was not detected during 3-OHBA ozonation. Moreover, the effect of –COOH steric hindrance should be taken into consideration. Several researchers also reported that the first step of the ozonation degradation pathway, the formation of polyhydroxylated aromatic products, was a predominant route.1,11,15,26–28
At the same time, these hydroxylation aromatic intermediates were further oxidized, leading to the ring opening by cycloaddition and formation of a compound containing two carboxylic groups. Through Criegee mechanism, this compound could be further decomposed into short-chain aldehydes (formaldehyde, glyoxal, methyl glyoxal) and carboxylic acids (formic acid and acetic).29 Finally, aldehydes were constantly oxidized to form the corresponding carboxylic acid through a nucleophilic mechanism, and some of them could be completely mineralized to CO2. Instead, due to the stability of carboxylic acids in O3 solution, particularly formic and acetic acids, they would be accumulated in the solution.
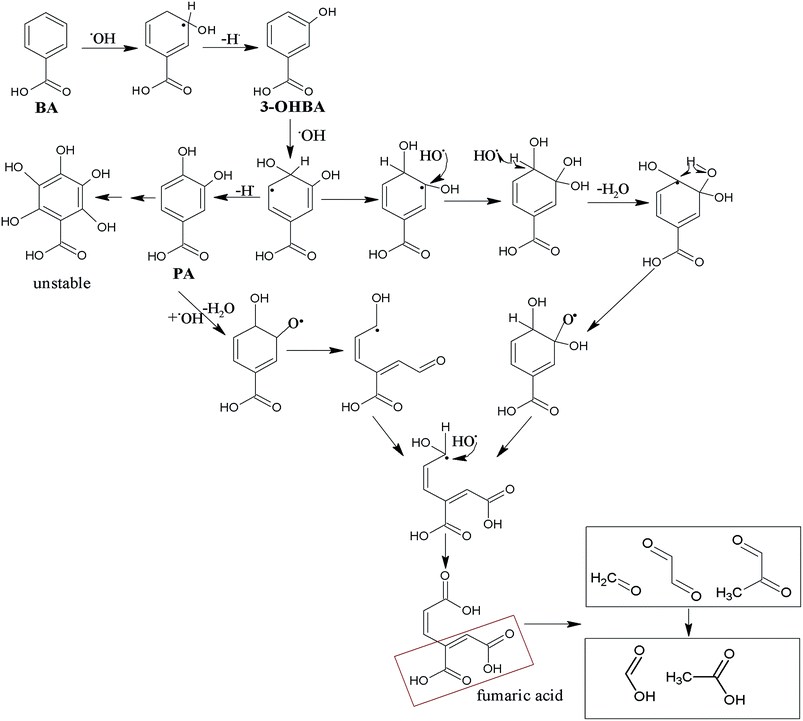
Beyond that, ˙OH radical, which was generated from O3 decomposition in water, may play an important role during the ozonation processes; the reaction of ˙OH with BA was proposed and is illustrated in Fig. 5. Most ˙OH reactions are analogous to diffusion-controlled reactions and generally have the following three mechanisms: H-atom abstraction, ˙OH addition to C–C, and ˙OH interaction with compounds containing S, N, or P atoms. In addition, ˙OH is more susceptible to add to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C rather than C
C rather than C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, as the carbon atom is electron deficient. Since most radical reactions are composed of chain reactions, the generation of other radicals and electron transfer reactions occur rarely. Thus, these intermediates may not experience addition and H-abstraction.11
O, as the carbon atom is electron deficient. Since most radical reactions are composed of chain reactions, the generation of other radicals and electron transfer reactions occur rarely. Thus, these intermediates may not experience addition and H-abstraction.11
BA mainly reacting with ˙OH may generate radical intermediates through addition reaction, resulting in the generation of hydroxylation aromatic products such as 3-OHBA and PA. Subsequently, O3, ˙OH and other oxidizers would further decompose hydroxylation aromatic products, resulting in the formation of ring-open products through ˙OH addition reactions and Criegee mechanism that is similar to the reaction illustrated in Fig. 4.
3.2 DBPs formation by ozonation of ACAs
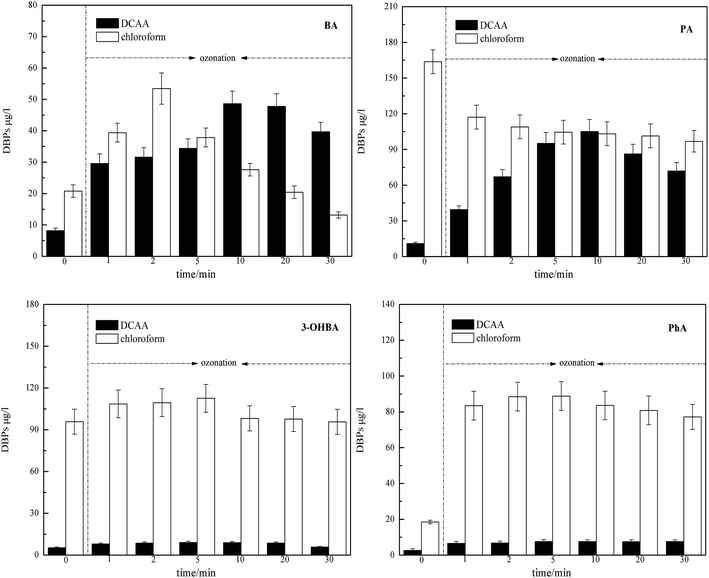
As shown in Fig. 6, direct chlorination disinfection and ozonation–chlorination disinfection of ACAs generated a certain amount of chloroform and DCAA, and the chlorination conditions were as follows: pH 7, 25 ± 1 °C in the dark for 24 h. ACAs ozonation promoted the production of DCAA, and the increasing levels of BA and PA were found to be higher than 3-OHBA and PhA. A consistent trend was observed for chloroform formation; ACAs ozonation also promoted the production of chloroform, whereas PA exhibited chloroform yield reductions. This result was in agreement with the results of our previous study on the raw water of Taihu Lake, where the formation potential of THMs and HAAs was improved through pre-ozonation step. Thus, this phenomenon suggested that the amount of DOC was not a proper indicator for determining the relative abundance of the precursors of chloroform and DCAA in these experiments. In addition, the concentrations of chloroform and DCAA reach a maximum when O3 reacts with ACAs at 2–5 min and then decrease over time following O3 application. The effect of ozonation on the reduction of chloroform and DCAA was significant initially and then slowed down. It also should be noted that chloroform was the main product of ozonation–chlorination reactions of ACAs; the more reactive groups it contained, the greater the amount of DBPs generated.It is widely assumed that chlorine reacts with the electron-donating group of aromatic compounds through electrophilic substitution and ortho/para-position stepwise chlorination, resulting in the formation of THMs. However, in the presence of an electron-withdrawing group, meta-position is progressively substituted by chlorine, resulting in the formation of THMs. Besides, resorcinol structures are popularly regarded as the most important THMs precursors.30,31 Although O3 can decompose THMs or HAA precursors, O3 intermediate products have a higher potential to form DBPs compared to the precursors.32 Combining the results of the aforementioned research studies and literature, it can be assumed that during ACAs ozonation, the formed hydroxylation aromatic intermediates could be the main precursors. In addition, ACAs ozonation leads to short-chain aldehydes and carboxylic acids formation (e.g. formaldehyde, glyoxal, methyl glyoxal, formic acid, acetic acid and fumaric acid). During decarboxylation, the structure of these short-chain aldehydes and carboxylic acids tend to be converted into that of β-ketoacids and give high yields of THMs at solutions with pH around 7. As far as the DCAA formation was concerned, hydroxyl and carboxyl groups were found to be prone to generate DCAA.33
Not only were the ACAs primary DBPs precursors, the intermediates of ACAs ozonation also could be DBPs precursors, and they produced high yields of THMs and HAAs. Previous study demonstrated the ubiquitous presence of fulvic acid in the terrestrial environment and that it generated a certain amount of ACAs and low-molecular-weight carbonyl compounds during ozonation. Therefore, the ACA levels should be kept under control in drinking water treatment plants.
4. Conclusions
Previous studies suggest that fulvic acid ozonation can produce ACAs, such as protocatechuic acid, 3-hydroxybenzoic acid, phthalic acid, and benzoic acid, to investigate ozonation kinetics, possible ozonation reaction pathways, ozonation intermediates and DBPs of 4 ACAs. The following conclusions were obtained:(1) Two stages pseudo-first-order reaction for 4 ACAs was carried out at pH 7, 25 °C, [O3]0 = 1.0 mg l−1 and [BA, PA, PhA, 3-OHBA]0 = 1.0 mg l−1.
(2) Six intermediates accompanied the ozonation of ACAs, namely fumaric acid, formic acid, acetic acid, formaldehyde, glyoxal and methyl glyoxal.
(3) Independent of the involvement of the hydroxyl radical, the ozonation of ACAs comprised three steps; first step was BA hydroxylation. Second, hydroxylation products of BA were oxidized to generate ring-opened compounds, and unsaturated carbonyl compounds were generated. Third, short-chain aldehydes and carboxylic acids were formed. As for mineralization, the short-chain aldehydes and carboxylic acids were finally transformed into CO2 and H2O.
(4) Not only ACAs themselves were the main DBPs precursors, but the intermediates of ACAs ozonation could also be the DBPs precursors, and they produced high yields of THMs and HAAs. Therefore, the ACA levels should be kept under control in drinking water treatment plants.
Acknowledgements
This study was performed with financial support from the National Water Pollution Control and Major Projects of Science and Technology Management, Item No. 2012ZX07403001. The authors gratefully acknowledge the assistance of Wujiang Hua-Yan Water Co. Ltd.References
- N. Milovac, D. Juretic, H. Kusic, J. Dermadi and A. L. Bozic, Ind. Eng. Chem. Res., 2014, 53, 10590–10598 CrossRef CAS.
- T. Velegraki, E. Nouli, A. Katsoni, I. V. Yentekakis and D. Mantzavinos, Appl. Catal., B, 2011, 101, 479–485 CrossRef CAS.
- M. M. Abdel Daiem, J. Rivera-Utrilla, R. Ocampo-Pérez, J. D. Méndez-Díaz and M. Sánchez-Polo, J. Environ. Manage., 2012, 109, 164–178 CrossRef CAS PubMed.
- V. G. Gandhi, M. K. Mishra, M. S. Rao, A. Kumar, P. A. Joshi and D. O. Shah, J. Ind. Eng. Chem., 2011, 17, 331–339 CrossRef CAS.
- S. Garcia-Segura, R. Salazar and E. Brillas, Electrochim. Acta, 2013, 113, 609–619 CrossRef CAS.
- T. Velegraki, G. Balayiannis, E. Diamadopoulos, A. Katsaounis and D. Mantzavinos, Chem. Eng. J., 2010, 160, 538–548 CrossRef CAS.
- B. Jurado-Sánchez, E. Ballesteros and M. Gallego, Water Res., 2014, 51, 186–197 CrossRef PubMed.
- B. Jurado-Sánchez, E. Ballesteros and M. Gallego, J. Chromatogr. A, 2010, 1217, 7440–7447 CrossRef PubMed.
- A. Papageorgiou, D. Voutsa and N. Papadakis, Sci. Total Environ., 2014, 481, 392–400 CrossRef CAS PubMed.
- U. von Gunten, Water Res., 2003, 37, 1443–1467 CrossRef CAS PubMed.
- R. Hu, L. Zhang and J. Hu, Chemosphere, 2016, 153, 394–404 CrossRef CAS PubMed.
- G. Wen, J. Ma, Z. Liu and L. Zhao, J. Hazard. Mater., 2011, 195, 371–377 CrossRef CAS PubMed.
- K. S. Tay, N. A. Rahman and M. R. B. Abas, Chemosphere, 2010, 81, 1446–1453 CrossRef CAS PubMed.
- K. S. Tay, N. A. Rahman and M. R. B. Abas, Chemosphere, 2009, 76, 1296–1302 CrossRef CAS PubMed.
- X. Huang, X. Li, B. Pan, H. Li, Y. Zhang and B. Xie, Water Res., 2015, 73, 9–16 CrossRef CAS PubMed.
- T. Zhang and J. Croué, Appl. Catal., B, 2014, 144, 831–839 CrossRef CAS.
- APHA (American Public Health Association), American Water Works Association, Water Environment Federation, Standard Methods for the Examination of Water and Wastewater, American Public Health Association, Washington, DC, 20th edn, 1999 Search PubMed.
- F. Hammes, E. Salhi, O. Köster, H. Kaiser, T. Egli and U. von Gunten, Water Res., 2006, 40, 2275–2286 CrossRef CAS PubMed.
- X. Zhong, C. W. Cui and S. L. Yu, China Water Wastewater, 2016, 32, 107–112 Search PubMed.
- D. Magallanes, J. L. Rodriguez, T. Poznyak, M. A. Valenzuela, L. Lartundo and I. Chairezd, New J. Chem., 2015, 39, 7839–7848 RSC.
- M. Pariente, F. Martinez, J. Melero, J. Botas, T. Velegraki, N. Xekoukoulitakis and D. Mantzavinos, Appl. Catal., B, 2008, 85, 24–32 CrossRef CAS.
- D. Shahidi, A. Moheb, R. Abbas, S. Larouk, R. Roy and A. Azzouz, J. Hazard. Mater., 2015, 298, 338–350 CrossRef CAS PubMed.
- C. Liu, X. Tang, J. Kim and G. V. Korshin, Chemosphere, 2015, 125, 182–190 CrossRef CAS PubMed.
- E. A. Pillar, R. C. Camm and M. I. Guzman, Environ. Sci. Technol., 2014, 48, 14352–14360 CrossRef CAS PubMed.
- T. Zhang, J. Lu, J. Ma and Z. Qiang, Water Res., 2008, 42, 1563–1570 CrossRef CAS PubMed.
- C. Y. Chang, Y. H. Hsieh, K. Y. Cheng, L. L. Hsieh, T. C. Cheng and K. S. Yao, Water Sci. Technol., 2008, 58, 873–879 CrossRef CAS PubMed.
- D. M. Theodora Velegraki, Chem. Eng. J., 2008, 140, 15–21 CrossRef.
- J. Wenk, M. Aeschbacher, E. Salhi, S. Canonica, U. von Gunten and M. Sander, Environ. Sci. Technol., 2013, 47, 11147–11156 CrossRef CAS PubMed.
- R. Criegee, Angew. Chem., Int. Ed., 1975, 14, 745–752 CrossRef.
- T. Bond, O. Henriet, E. H. Goslan, S. A. Parsons and B. Jefferson, Environ. Sci. Technol., 2009, 43, 5982–5989 CrossRef CAS PubMed.
- K. K. Philippe, C. Hans, J. Macadam, B. Jefferson, J. Hart and S. A. Parsons, Chemosphere, 2010, 81, 1509–1516 CrossRef CAS PubMed.
- F. Qi, B. Xu, Z. Chen, L. Feng, L. Zhang and D. Sun, Chem. Eng. J., 2013, 219, 527–536 CrossRef CAS.
- A. Li, X. Zhao, H. Liu and J. Qu, Water Res., 2011, 45, 6131–6140 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2017 |