Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Intracellular free Ca2+ signals antibiotic exposure in cyanobacteria†
M. González-Pleiter,
F. Leganés and
F. Fernández-Piñas *
*
Department of Biology, Facultad de Ciencias, Universidad Autónoma de Madrid, 28049 Madrid, Spain. E-mail: francisca.pina@uam.es
First published on 14th July 2017
Abstract
Intracellular free Ca2+, [Ca2+]i, is a key element of the cellular response to many abiotic and biotic stresses. Cyanobacteria are ancient organisms which have evolved signal transduction systems in response to an ever changing environment. Cyanobacteria have been found to respond to specific and quick Ca2+ signatures (unique combinations of changes in [Ca2+]i) when exposed to environmental stress. Antibiotics are bioactive molecules which are being considered as pseudopersistent contaminants as they are being continuously released to the environment; although non-target organisms, cyanobacteria as prokaryotes are sensitive to antibiotics. In order to check whether [Ca2+]i could be involved in sensing antibiotic exposure, we have recorded the Ca2+ signatures induced by antibiotics belonging to different classes at two effective toxicity concentrations, the EC10 and the EC50. For that, we have used a recombinant bioluminescent strain of the cyanobacterium Anabaena sp. PCC7120 that constitutively expresses the calcium-binding indicator protein, apoaequorin. We found that Ca2+ signatures upon exposure to antibiotics were induced quickly (2–3 seconds) and that they differed between antibiotics and antibiotic concentration. As different classes of antibiotics have been detected simultaneously in the aquatic environment, we also recorded and analyzed the Ca2+ signatures induced by binary mixtures and a multiantibiotic mixture; we found that these Ca2+ signatures could determine the nature of the interaction between antibiotics in the mixture, whether synergistic or antagonistic, well before toxicity was evident. To our knowledge, this study is the first report of intracellular free Ca2+ as an early biomarker of antibiotic exposure in the environment.
Introduction
Cyanobacteria are ancient organisms dating from the Precambrian era which constitute a phylogenetically diverse group of photosynthetic prokaryotes.1,2 Increasing evidence indicates that they are the ancestors of chloroplasts of photoautotrophic eukaryotes.3 They are ubiquitous organisms occupying a diverse range of habitats;2 they have a crucial role both in the N and C cycles and as primary producers; and any negative impact on this group may also negatively affect organisms of higher trophic levels.As any other living being, cyanobacteria possess signal transduction systems to sense and respond to changes in their environment. Intracellular messengers are basic components of the cellular signal transduction toolkits. Intracellular free Ca2+, [Ca2+]i, is probably the most universal of second messengers in living organisms including prokaryotes.4–6 A prerequisite for [Ca2+]i to act as a second messenger is a tight homeostasis of its basal levels which is regulated by Ca2+-binding proteins, Ca2+ ATPases, Ca2+/H+ antiporters and Ca2+ pumps in order to avoid the formation of toxic intracellular calcium phosphate precipitates.
Our group was the first to construct a recombinant strain of the freshwater nitrogen-fixing cyanobacterium Anabaena sp. PCC7120 constitutively expressing apoaequorin, Anabaena sp. PCC7120 (pBG2001a), which allows continuous and in vivo monitoring of [Ca2+]i; with this strain we demonstrated that cyanobacteria were also able to tightly regulate their basal [Ca2+]i levels with values in the range of 100–200 nM;7 similar to the levels found in other bacteria5,6 and eukaryotic cells.8–11 Cells respond to different abiotic and biotic stimuli by transient changes in [Ca2+]i, the specificity of the induced Ca2+ signals relies not only in the change of the intracellular calcium concentration. A combination of changes in all Ca2+ parameters of the signal such as amplitude, duration, frequency, rise time, final Ca2+ resting levels, recovery time and source of the signal induced by a specific stimulus is referred to as a “Ca2+ signature”.10–14 The Ca2+ signatures encode information relating to the nature and strength of stimuli in their spatio-temporal dynamics.10,11 In cyanobacteria, we have been able to record and analyse a variety of calcium signatures induced by specific environmental stimuli in this strain as well as in a unicellular cyanobacterium also expressing apoaequorin.7,15–18
There are numerous studies reporting that a wide range of pollutants affect Ca2+ homeostasis/signalling in a number of organisms19–23 with microorganisms being under represented.24–30 Regarding specifically antibiotics, there are few studies reporting that antibiotics (mostly aminoglycosides and tetracycline) significantly alter Ca2+ homeostasis in animal and human cell lines.31–35
Our group using the apoaequorin expressing cyanobacterial Anabaena strain, Anabaena sp. PCC7120 (pBG2001a), has recorded and analysed Ca2+ signatures triggered by a variety of pollutants: cationic an anionic heavy metals, the metalloid As, naphthalene, organic solvents and some pharmaceuticals; we also recorded Ca2+ signatures induced by some binary mixtures of the pollutants as well as that induced by a real wastewater sample.24 It was found that, in general, each group of tested pollutants induced a specific Ca2+ signature in a reproducible and dose-dependent way. We hypothesized that the recorded Ca2+ signatures might be early biomarkers of exposure to pollutants.
In the present study, we wish to deepen this hypothesis by recording Ca2+ signatures induced by antibiotics both applied singly and in combination to the apoaequorin expressing Anabaena strain, Anabaena sp. PCC7120 (pBG2001a). The applied antibiotics were from different classes and mode of action: amoxicillin (β-lactam, AMO); erythromycin (macrolide, ERY); tetracycline (tetracycline, TET) and the quinolones norfloxacin (NOR) and levofloxacin (LEV). All of them highly used in human and veterinary medicine. In a previous study, we investigated the individual and combined toxicity of these five antibiotics in the transgenic bioluminescent cyanobacterium Anabaena sp. PCC7120 CPB4337.36 The Combination Index (CI) method was used to identify and quantify the toxicological interactions between the antibiotics in the mixtures finding a heterogeneous patterns of interactions whether synergistic or antagonistic. Thus, in the present study, we aimed at determining the role of [Ca2+]i in the cellular perception of the presence of antibiotics both singly and combined in the external medium and whether the recorded Ca2+ signatures are related with the toxicity and pattern of interactions observed in our previous toxicological study.
Materials and methods
Chemicals
Amoxicillin (AMO), erythromycin (ERY) and levofloxacin (LEV) were purchased from TCI Chemicals; tetracycline (TET) was purchased from Sigma-Aldrich; and norfloxacin (NOR) purchased from Fluka.The purity of each antibiotic was AMO ≥90%, ERY ≥98%, LEV ≥98%, TET ≥98%, NOR ≥98%. Except ERY, which was dissolved in methanol, stock solutions of antibiotics were made in high purity water obtained from a Millipore Milli-Q system with a resistivity of at least 18 MΩ cm at 25 °C. The antibiotics were stored in the dark and −20 °C.
The dilutions of each antibiotic and their corresponding mixtures were freshly prepared. For the case of ERY, the final concentration of methanol in the assay media was always below 0.005% (v/v) which did not affect the performance of Anabaena sp. PCC7120 (pBG2001a) (ESI Fig. S1†).
Injection of culture medium induced a quite smaller Ca2+ transient in amplitude (peak height of 0.30 ± 0.05 μM) and duration (11 ± 1 s) as shown in ESI Fig. S1† which has been attributed to a small mechanically induced Ca2+ increase in Anabaena sp. PCC7120 (pBG2001a).7 Native coelenterazine, the apoaequorin cofactor, was purchased from Biotium.
Organism and growth conditions
Anabaena sp. PCC7120 (pBG2001a) expressing apoaequorin was routinely grown in 250 mL conical flasks containing 100 mL of BG11 medium with 2 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES)/NaOH, pH 7 and 2.5 μg mL−1 spectinomycin, with the standard calcium concentration (0.25 mM). Cell cultures were incubated on a rotatory shaker at 28 °C under 65 μE m−2 s−1 fluorescent white light.In vivo aequorin reconstitution and luminescence measurements
For aequorin luminescence measurements, in vivo reconstitution of aequorin was performed by the addition of 2.5 μM coelenterazine to cell suspensions (15 μg mL−1 chlorophyll) and incubation for 4 h in darkness and shaking at 28 °C. Before Ca2+ measurements were made, cells were washed twice, firstly in Ca2+-depleted BG11 medium buffered with 2 mM HEPES, pH 7.5, containing 0.5 mM EGTA, and later in Ca2+-depleted BG11 medium to remove excess coelenterazine.7 Cells were finally resuspended in BG11 medium (calcium concentration of 0.25 mM) to carry out the bioassays.Luminescence measurements were made using a digital luminometer with a photomultiplier (BioOrbit 1250). Reconstituted cell suspensions (0.5 mL) in a transparent polystyrene cuvette were placed in the luminometer and luminescence was recorded every 1 s over the duration of the experiment.
Calibration of the [Ca2+]i changes requires knowledge of the total available amount of reconstituted aequorin in cell suspensions (Lmax) at any one point in time of the experiment, as well as the running luminescence (L0). For estimation of total aequorin luminescence, the remaining aequorin was discharged at the end of the experiment by the addition of 0.3 mL of 1 M CaCl2 and 10% (v/v) Triton X-100. Rate constants of luminescence (L0 × Lmax−1), and [Ca2+]i were calculated by using calibration curves obtained for aequorin extracted from the recombinant strain of Anabaena sp. PCC7120 (pBG2001a) according to Torrecilla et al.7
Cell lysis check
For each of the treatments used in the present study, the occurrence of cell lysis was checked by the following methods: (a) examination by optical microscopy; (b) measurement of luminescence after addition of Ca2+ to the medium in which reconstituted cells were present after removing the cells by centrifugation at 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g at room temperature for 15 min; (c) measurements of phycobiliproteins in the medium in which reconstituted cells were present after removing the cells by centrifugation at 23
000g at room temperature for 15 min; (c) measurements of phycobiliproteins in the medium in which reconstituted cells were present after removing the cells by centrifugation at 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g at room temperature for 15 min.
000g at room temperature for 15 min.
Statistical analysis
All data were obtained from a minimum of three replicates for each assay situation. The Ca2+ traces represented in the figures represent the mean ± S.D. from at least three independent experiments.Results and discussion
Ca2+ signatures induced in Anabaena sp. PCC7120 (pBG2001a) in response to exposure to antibiotics applied singly
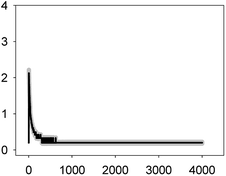
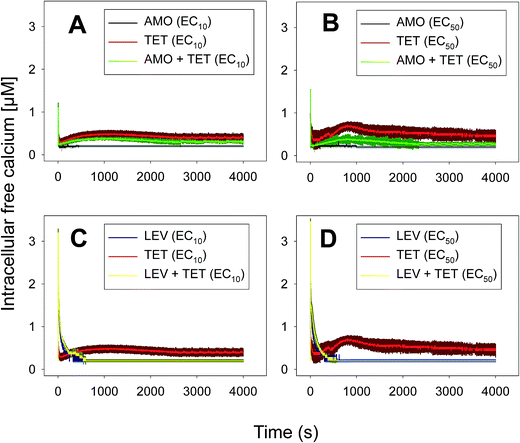
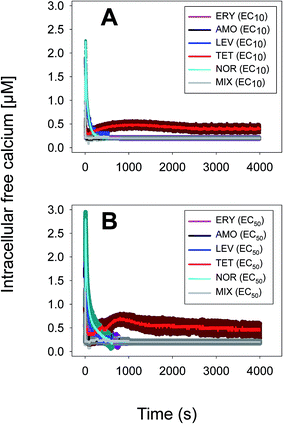
Anabaena sp. PCC7120 (pBG2001a) cells expressing apo-aequorin were incubated with the cofactor coelenterazine in order to reconstitute a functional aequorin. Then, the cells were placed in a luminometer cuvette, the selected antibiotics were injected and subsequent changes (if any) in [Ca2+]i were recorded. Antibiotics were tested at their EC10 (as a surrogate of the NOEC: very low or negligible effect on growth) and EC50 (as sublethal concentration: 50% growth inhibition) according to our previous 72 h toxicity study.36 All the antibiotics at the tested concentrations induced a calcium transient (Table 1). The Ca2+ signal in all cases was very quick, within seconds of injection. Transient shape, amplitude, rise time (time from application of the stimulus to maximum amplitude of the response), total transient duration (length of the transient form zero time to the recovery of the resting [Ca2+]i) were the characteristics of the Ca2+ signatures recorded.As shown in Table 1, all tested antibiotics triggered quick Ca2+ transients with the same rise time (2–3 s) but which differed in the amplitude (ranging from 0.36 ± 0.06 μM for ERY at its EC10 to 2.80 ± 0.15 μM for NOR at its EC50) and total transient duration (from 32 ± 5 s for AMO at its EC10 to >4200 s for TET at its EC50). Regarding shape, in most cases it was characterized by a quick burst of [Ca2+]i, followed by a quick decline reaching a plateau that restored the level of [Ca2+]i back to resting values (Table 1); in the case of TET applied at both concentrations, the Ca2+ signature consisted of two consecutive Ca2+ transients: one quick and steep spike followed by a bell-shaped transient with lower amplitude but higher duration than the first transient; it should be noted that the amplitude of the second transient was higher for the EC50 than for the EC10. In the case of ERY applied at its EC50, there were also two consecutive transients; the second transient had slightly lower amplitude but also higher duration than the first transient.
Although no correlation was found between any of the characteristics of the recorded Ca2+ signature and the order of toxicity of the tested antibiotics which was: ERY > LEV > NOR > TET > AMO.36 It should be noted that antibiotics at the higher concentration tested, EC50, which already inhibited growth by 50%, triggered Ca2+ signatures with higher amplitudes, longer transient duration (meaning a longer time to restore [Ca2+]i basal levels) and the appearance of secondary bell-shaped transients with much longer duration times (case of TET and ERY). This means that [Ca2+]i may sense differences in antibiotic concentrations even at concentrations causing negligible or very low toxicity (i.e. EC10); this could be essential for the cellular response to the pollutant.
Taken altogether these results show that [Ca2+]i responds very quickly, within seconds, to antibiotic exposure, the Ca2+ signature of each antibiotic differs in amplitude, shape and total transient duration and within each antibiotic also differs with concentration; this suggests that the presence of antibiotics is signalled through immediate [Ca2+]i transients in the cyanobacterial cytoplasm that may trigger the subsequent cellular response to the pollutant.
In a previous work,24 our group recorded the Ca2+ signatures triggered in Anabaena sp. PCC7120 (pBG2001a) when exposed to 17 chemicals grouped in cations (Zn2+, Pb2+, Cd2+, Cu2+ and Hg2+), anions (arsenate and chromate), pharmaceuticals such as lipid regulators (bezafibrate, gemfibrozil, fenofibric acid and clofibric acid) and quinolones (ciprofloxacin and ofloxacin), the PAH naphthalene and the organic solvents acetone, ethanol and toluene.24 For easy comparison, chemicals were tested at the same concentration, 5 mg L−1, finding that, in general, each group of chemicals triggered a specific Ca2+ signature; in fact the two tested quinolones ciprofloxacin and ofloxacin showed a signature very similar to the quinolones assayed in the present study: LEV and NOR; in fact, chemicals of the same group induced very similar Ca2+ signatures, it was proposed that the observed similarity might correlate with a similar perception by the organism of the pollutants and eventually similar toxic mode of action. In the present study, five antibiotics belonging to four different classes [β-lactam (AMO); macrolide (ERY); tetracycline (TET) and quinolone (LEV and NOR)] have been used, the four classes differ in their mechanisms of toxic action: β-lactams inhibit bacterial cell wall synthesis; macrolides irreversibly bind to the 50S subunit of the bacterial ribosome impairing protein synthesis; tetracycline also inhibits bacterial protein synthesis but by a different mechanism as it prevents the association of aminoacyl-tRNAs with the bacterial ribosome; quinolones act at the level of DNA replication by inhibiting bacterial gyrase. The lack of correlation between the order of toxicity of the tested antibiotics and the specific features of Ca2+ signatures might then be due to Ca2+ signalling discriminating between chemicals or pollutants with different mode of toxic action and not the intensity of the toxicity which is mostly related with the concentration of the pollutants used. In this context, in our previous study as well as in this one, chemicals were tested at higher concentrations (shown to be toxic) and they induced Ca2+ signatures characterized by broad bell-shaped transients (incapacity to rapidly restore basal cytosolic [Ca2+]i) or by two consecutive transients being the second also broad and bell-shaped. Besides, it was found that Ca2+ signatures preceded toxicity in the lights-off bioreporter Anabaena CPB4337 as Ca2+ signatures were induced within seconds at concentrations much lower than their EC50 values. It was concluded that the specific Ca2+ signature subsequently induces a signaling pathway which results in an appropriate cellular response to the pollutant which may be adaptation and survival or, when the pollutant is at high concentration, or, the exposure is long, may cause deregulation of Ca2+ homeostasis leading to cellular toxicity. In this regard, Ruta et al.28,29 have suggested that the features of the Ca2+ signatures may have a significant role in the final cellular response with sudden and sharp Ca2+ transients allowing the adaptation to high concentrations of Cd and Cu in apoaequorin-expressing Saccharomyces cerevisiae cells while the absence of Ca2+ signals or broad ones lead the cells to hypersensitivity and toxicity. They concluded that cells prepared to face high and rapid Ca2+ cytosolic waves were fitter for survival. Kozlova et al.26 also tried to correlate Ca2+ signatures triggered by Cr6+, Zn2+ and 3,5-dichlorophenol in apoaequorin-expressing fungus Aspergillus awamori with toxicity; for this, they chose length of the Ca2+ transients (LT50) to compare with the EC50 at 5 and 30 min of the Aliivibrio fischeri classical bioluminescent toxicity assay. All toxicants provoked a response in one or more of the studied parameters, but LT50 was not the best parameter to compare with the A. fischeri bioassay. Therefore, the authors concluded that assessment of a different parameter was needed.
All these results suggest that Ca2+ signatures between different pollutants may not correlate with their order of toxicity but that specific Ca2+ signatures may serve to discriminate between pollutants and that, within a single pollutant and depending on the concentration, they may serve to tune the cellular response, whether tolerant or hypersensitive.
Ca2+ signatures induced by selected mixtures of antibiotics in Anabaena sp. PCC7120 (pBG2001a)
Different classes of antibiotics co-occur in environmental compartments.37–40 Aquatic organisms are thus exposed to the combined action of antibiotics. An important issue of chemical mixtures is the determination of potential interactions between pollutants in a mixture; in this regard, antibiotics in a mixture may not interact (zero interaction or additive effect) or may interact synergistically when the effect of the mixture is greater than that expected from the sum of their individual effects (more-than-additive effects) or antagonistically when the effect of the mixture is less than expected from the sum of individual effects (less-than-additive effect).In our previous toxicity study, we determined the nature of the interactions between the five antibiotics in binary mixtures and in a multiantibiotic mixture in the bioluminescent strain Anabaena sp. PCC7120 CPB4337 after 72 h of exposure.36 As [Ca2+]i responds very quickly to individual antibiotics (as shown in Table 1) we evaluated whether [Ca2+]i could also respond to antibiotic binary mixtures and a multiantibiotic mixture so that by comparing the Ca2+ signature of the mixture with those of antibiotics when applied singly, patterns of interactions might become evident. We also wanted to assess whether Ca2+ signatures of the mixtures could anticipate/predict the toxicological interactions observed at longer times of exposure.
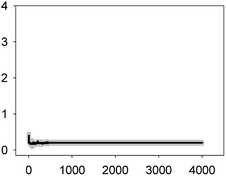
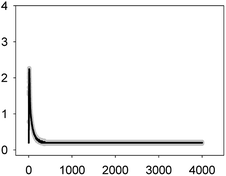
For this set of experiments, we selected four binary mixtures that represented different types of toxicological interactions and the five-antibiotic or multiantibiotic mixture:36 two synergistic mixtures at both very low (10%) and median effect levels (50%) which were AMO–TET and LEV–TET; TET–ERY which was synergistic at very low effect level (10%) but turned into antagonistic at effect levels higher than 60% and AMO–ERY which was antagonistic at both very low (10%) and median effect levels (50%). The multiantibiotic mixture was antagonistic at very low (10%) effect levels, nearly additive at 20–30% effect level, slightly synergistic at 50% effect level and clearly synergistic above this level. For comparison, the Ca2+ signatures induced by the antibiotics when applied individually at the same concentrations (EC10 and EC50) as those in the mixtures are shown in Fig. 1 and 2. Amplitude of the first transient (some but not all tested antibiotics/concentrations induced a second transient; see Table 1) was chosen as the most representative parameter to discern potential interactions between antibiotics in the binary mixtures. Both, the AMO–TET and LEV–TET mixtures, tested at the EC10 and EC50, induced a Ca2+ signature whose amplitude was higher than the sum of the Ca2+ signatures when both pollutants were applied individually, suggesting a more-than-additive effect or synergism; synergism was clearer in the case of the LEV–TET combination (Fig. 1).
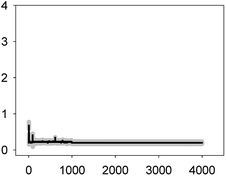
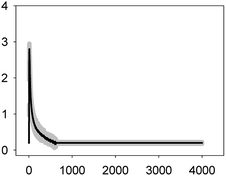
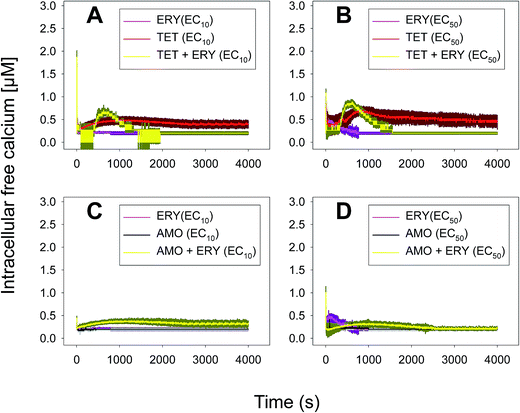
In the case of the TET–ERY combination, the mixture tested at EC10 induced a Ca2+ signature with an amplitude higher than the sum of the individual Ca2+ signatures suggesting synergism; however, the interaction was clearly antagonistic when tested at the EC50. The AMO–ERY combination, tested at the EC10 and EC50, induced a Ca2+ signature whose amplitude was lower than the sum of the individual Ca2+ signatures suggesting a less-than-additive effect or antagonism (Fig. 2).
Although amplitude was chosen as the reference parameter, it should be noted that the shape of some binary mixtures clearly changed with respect to the individual Ca2+ signatures; in the AMO–TET combination, the amplitude of the second TET transient clearly diminished when mixed at their EC50s; in the LEV–TET mixture, the second TET transients disappeared in both combination concentrations (Fig. 1). Interestingly, in the TET–ERY combination, the second TET transient was clearly sharper and shorter in both combination concentrations and in the AMO–ERY mixture, a second transient appeared at both combination concentrations that was larger and with lower amplitude than that of ERY applied at its EC50. The found differences did not clearly correlate with the differences seen when considering the amplitude as reference parameter and, in some cases, they were independent of the tested concentration; so, these differences in shape could indicate that cells are just detecting the specific mixture.
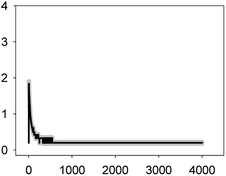
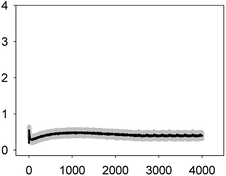
The multiantibiotic mixture, tested at the EC10 and EC50, induced Ca2+ signatures whose amplitudes were lower than the sum of the individual Ca2+ signatures suggesting a less-than-additive effect or antagonism (Fig. 3 and Table 1). Regarding the shape and for both concentrations, only one quick transient which rapidly returned to basal levels was recorded.
As thoroughly discussed by many scientists, the toxicity and, thereby, the risk associated with environmental pollutants is the result of the many interactions of co-occurring pollutants in a given environmental compartment.41,42 Ca2+ signatures in response to mixtures of pollutants could serve as an excellent biomarker for prediction of potential types of interaction, whether synergistic or antagonistic, in exposed organisms. Our group previously reported that the analysis of the Ca2+ signatures induced by binary mixtures of Zn2+ plus Cu2+; Zn2+ plus the lipid regulator fenofibric acid, Zn2+ plus arsenate and the two fibrates fenofibric acid plus bezafibrate could anticipate the toxicological interactions found in toxicity experiments.24,43,44 Recently, Ruta et al.28 reported, in apoaequorin-expressing Saccharomyces cerevisiae, that the binary mixture Cu2+ and H2O2 induced a Ca2+ signature sharper and with an amplitude higher than that of individual Ca2+ signatures indicating a synergistic interactions between both chemicals in signaling the stress conditions; the authors indicated that the stronger and sharper Ca2+ signature induced by the mixture increased the cell chances of survival. In this study, we have found that binary mixtures of antibiotics triggered quick Ca2+ signatures that, based on amplitude as the reference parameter to compare with those of the individual Ca2+ signatures, could also predict the toxicological interactions found for these antibiotics.36 In the case of the multiantibiotic mixture only antagonism was found (Fig. 3) although in the toxicological experiments a clear synergism was found at medium to high effect levels, this could be explained by the fact that the toxicological experiment was done after 72 h of exposure and cells might be already strongly affected by the antibiotic mixture.36 This would imply that Ca2+ signatures are not only early biomarkers/sensors of antibiotic exposure both singly and in mixtures but also that they seem to anticipate the toxicological outcome of the mixture; besides, as suggested by Ruta et al.,28 the specific features of the Ca2+ signatures induced by mixtures might perhaps signal whether the cells may survive or not; in this study, we also found that the Ca2+ signatures of the mixture were quite different from those of the individual antibiotics although at present we cannot give a clear explanation for these differences, it might occur that, in order for the cell to distinguish between singly and combined antibiotic exposure, distinct Ca2+ influx or efflux systems might be involved in the generation of the recorded Ca2+ signatures. In most organisms, [Ca2+]i homeostasis is tightly regulated by the so-called Ca2+-signalling toolkit which includes different Ca2+ transporters (Ca2+ channels, Ca-ATPases or Ca2+ exchangers such as Ca2+/H+ antiporters) and Ca2+-binding proteins (both intracellular Ca2+ buffers as well as Ca2+ sensors which activate downstream target proteins involved in the cellular response).6 Many of these components are involved in the generation of the Ca2+ signals as well as in restoring the [Ca2+]i to resting values (around 100–200 nM in most organisms). In cyanobacteria, just a few proteins involved in Ca2+ transport have been characterized so far and all of them in unicellular cyanobacteria: the mechanosensitive channel encoded by the mscL gene in Synechococcus sp. PCC7942; the two Ca2+/H+ antiporters ApCAX in Aphanothece halophytica and SynCAX in Synechocystis sp. Strain PCC6803 and two Ca2+-ATPases, PMA1, an E1–E2 ATPase in Synechocystis sp. Strain PCC6803 and PacL, Ca2+ transporting P-ATPase in Synechococcus sp. PCC7942; regarding Ca2+-binding proteins, the only reported so far is CcbP from the filamentous Anabaena sp. PCC7120, the organism used in this study (reviewed in Dominguez et al.6). There is much to be done regarding the identification and characterization of the Ca2+-signalling toolkit in cyanobacteria; this will aid in a better understanding of the Ca2+ signatures induced by different environmental pollutants and to decipher the signalling pathway between the perception of the pollutants by Ca2+ and the final cellular response whether tolerance or hypersensitivity to the pollutant. By using cyanobacterial strains mutated in these Ca2+ signalling components as done by Ruta et al.28,29 in yeast, it might be determined whether the Ca2+-mediated response to pollutants depends predominantly on extracellular Ca2+ influx or internal Ca2+ stores, how intracellular resting Ca2+ levels are restored and which role may have the Ca2+-binding proteins in such response.
To gain insight into the correct interpretation of the Ca2+ signatures triggered by mixtures, a careful and thorough research is needed in more organisms and with more pollutants mixed in realistic scenarios; in this regard, in our previous study by Barran-Berdon et al.,24 we recorded a Ca2+ signature triggered by a real wastewater sample that could be mimicked by mixing its main components at their measured environmental concentration.
Conclusions
To our knowledge, this is the first report on intracellular free Ca2+, [Ca2+]i, as an early biomarker of antibiotic exposure in the environment. Upon antibiotic exposure, quick (within 2–3 s) and specific Ca2+ signatures were recorded. Signatures induced by the different antibiotics differed in amplitude, shape and total transient duration and within the same antibiotic also with the tested concentration with higher amplitude, longer transient duration and in some case the appearance of a second bell-shaped Ca2+ signal at the higher concentration tested, EC50. Ca2+ signatures induced by binary mixtures and a multiantibiotic mixture were clearly different from those of the antibiotics applied singly and, using amplitude as a reference parameter, potential interactions between antibiotics in the mixtures could be determined; the Ca2+ signatures induced by the mixtures seemed to anticipate the toxicological interactions found at larger times of exposure; thus, well before toxicity is evident.Conflict of interest
There are no conflicts to declare.Acknowledgements
This research was supported by CTM2013-45775-C2-2-R and CGL2010-15675 grants from MINECO/FEDER EU. MGP holds an FPI grant from MINECO/FEDER EU.Notes and references
- R. Rippka, J. Deruelles, J. B. Waterbury, M. Herdman and R. Y. Stanier, Microbiology, 1979, 111, 1–61 Search PubMed.
- M. Potts, Microbiol. Rev., 1994, 58, 755–805 CAS.
- L. I. Falcón, S. Magallón and A. Castillo, ISME J., 2010, 4, 777–783 CrossRef PubMed.
- D. E. Clapham, Cell, 1995, 80, 259–268 CrossRef CAS PubMed.
- D. C. Dominguez, Mol. Microbiol., 2004, 54, 291–297 CrossRef CAS PubMed.
- D. C. Dominguez, M. Guragain and M. Patrauchan, Cell Calcium, 2015, 57, 151–165 CrossRef CAS PubMed.
- I. Torrecilla, F. Leganes, I. Bonilla and F. Fernandez-Pinas, Plant Physiol., 2000, 123, 161–176 CrossRef CAS PubMed.
- M. Brini, R. Marsault, C. Bastianutto, J. Alvarez, T. Pozzan and R. Rizzuto, J. Biol. Chem., 1995, 270, 9896–9903 CrossRef CAS PubMed.
- M. R. Knight, A. K. Campbell, S. M. Smith and A. J. Trewavas, FEBS Lett., 1991, 282, 405–408 CrossRef CAS PubMed.
- J. Kudla, O. Batistic and K. Hashimoto, Plant Cell, 2010, 22, 541–563 CrossRef CAS PubMed.
- M. R. McAinsh and J. K. Pittman, New Phytol., 2009, 181, 275–294 CrossRef CAS PubMed.
- M. J. Berridge, M. D. Bootman and H. L. Roderick, Nat. Rev. Mol. Cell Biol., 2003, 4, 517–529 CrossRef CAS PubMed.
- M. J. Berridge, P. Lipp and M. D. Bootman, Nat. Rev. Mol. Cell Biol., 2000, 1, 11–21 CrossRef CAS PubMed.
- J. J. Rudd and V. E. Franklin-Tong, New Phytol., 2001, 151, 7–33 CrossRef CAS.
- F. Leganes, K. Forchhammer and F. Fernandez-Pinas, Microbiology, 2009, 155, 25–34 CrossRef CAS PubMed.
- I. Torrecilla, F. Leganes, I. Bonilla and F. Fernandez-Pinas, Microbiology, 2004, 150, 3731–3739 CrossRef CAS PubMed.
- I. Torrecilla, F. Leganés, I. Bonilla and F. Fernández-Piñas, Plant, Cell Environ., 2001, 24, 641–648 CrossRef CAS.
- I. Torrecilla, F. Leganés, I. Bonilla and F. Fernández-Piñas, Plant, Cell Environ., 2004, 27, 810–819 CrossRef CAS.
- C.-C. Huang, R. S. Aronstam, D.-R. Chen and Y.-W. Huang, Toxicol. In Vitro, 2010, 24, 45–55 CrossRef CAS PubMed.
- O. A. Ogunbayo, P. F. Lai, T. J. Connolly and F. Michelangeli, Toxicol. In Vitro, 2008, 22, 943–952 CrossRef CAS PubMed.
- H. Wang, Z.-K. Wang, P. Jiao, X.-P. Zhou, D.-B. Yang, Z.-Y. Wang and L. Wang, Toxicology, 2015, 333, 137–146 CrossRef CAS PubMed.
- Z. Xie, Y. Zhang, A. Li, P. Li, W. Ji and D. Huang, Toxicol. Lett., 2010, 192, 115–118 CrossRef CAS PubMed.
- H. Zou, X. Liu, T. Han, D. Hu, Y. Yuan, J. Gu, J. Bian and Z. Liu, J. Toxicol. Sci., 2015, 40, 469–477 CrossRef CAS PubMed.
- A. L. Barran-Berdon, I. Rodea-Palomares, F. Leganes and F. Fernandez-Pinas, Anal. Bioanal. Chem., 2011, 400, 1015–1029 CrossRef CAS PubMed.
- F. Dondero, H. Jonsson, M. Rebelo, G. Pesce, E. Berti, G. Pons and A. Viarengo, Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol., 2006, 143, 150–157 CrossRef PubMed.
- O. Kozlova, M. Zwinderman and N. Christofi, BMC Microbiol., 2005, 5, 40 CrossRef PubMed.
- M. Ohta and T. Suzuki, Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol., 2007, 146, 525–530 CrossRef PubMed.
- L. L. Ruta, C. V. Popa, I. Nicolau and I. C. Farcasanu, Environ. Sci. Pollut. Res., 2016, 23, 24514–24526 CrossRef CAS PubMed.
- L. L. Ruta, V. C. Popa, I. Nicolau, A. F. Danet, V. Iordache, A. D. Neagoe and I. C. Farcasanu, FEBS Lett., 2014, 588, 3202–3212 CrossRef CAS PubMed.
- S. Schäfer, U. Bickmeyer and A. Koehler, Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol., 2009, 150, 261–269 CrossRef PubMed.
- S. D. Bird, R. J. Walker and M. J. Hubbard, In Vitro Cell. Dev. Biol.: Anim., 1994, 30, 420–424 CrossRef.
- C. Dai, D. Zhang, J. Li and J. Li, Toxicol. Mech. Methods, 2013, 23, 281–288 CrossRef CAS PubMed.
- D. Dulon, G. Zajic, J. M. Aran and J. Schacht, J. Neurosci. Res., 1989, 24, 338–346 CrossRef CAS PubMed.
- P. D. Holohan, P. P. Sokol, C. R. Ross, R. Coulson, M. E. Trimble, D. Laska and P. Williams, J. Pharmacol. Exp. Ther., 1988, 247, 349–354 CAS.
- S. J. McLarnon, D. Holden, D. T. Ward, M. N. Jones, A. C. Elliott and D. Riccardi, Biochem. Biophys. Res. Commun., 2002, 297, 71–77 CrossRef CAS PubMed.
- M. González-Pleiter, S. Gonzalo, I. Rodea-Palomares, F. Leganés, R. Rosal, K. Boltes, E. Marco and F. Fernández-Piñas, Water Res., 2013, 47, 2050–2064 CrossRef PubMed.
- D. W. Kolpin, M. Skopec, M. T. Meyer, E. T. Furlong and S. D. Zaugg, Sci. Total Environ., 2004, 328, 119–130 CrossRef CAS PubMed.
- B. Li, T. Zhang, Z. Xu and H. H. P. Fang, Anal. Chim. Acta, 2009, 645, 64–72 CrossRef CAS PubMed.
- S. Nazaret and R. Aminov, Front. Microbiol., 2014, 5, 520 Search PubMed.
- A. Watkinson, E. Murby, D. Kolpin and S. Costanzo, Sci. Total Environ., 2009, 407, 2711–2723 CrossRef CAS PubMed.
- I. Rodea-Palomares, M. Gonzalez-Pleiter, S. Gonzalo, R. Rosal, F. Leganes, S. Sabater, M. Casellas, R. Muñoz-Carpena and F. Fernández-Piñas, Sci. Adv., 2016, 2, e1601272 Search PubMed.
- I. Rodea-Palomares, M. González-Pleiter, K. Martín-Betancor, R. Rosal and F. Fernández-Piñas, Toxics, 2015, 3, 342–369 CrossRef.
- I. Rodea-Palomares, F. Fernández-Piñas, C. González-García and F. Leganés, Handbook on Cyanobacteria: Biochemistry, Biotechnology and Applications, 2009, pp. 283–304 Search PubMed.
- I. Rodea-Palomares, A. L. Petre, K. Boltes, F. Leganés, J. A. Perdigón-Melón, R. Rosal and F. Fernández-Piñas, Water Res., 2010, 44, 427–438 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra03001k |
| This journal is © The Royal Society of Chemistry 2017 |