Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Grandiflodines A and B, two novel diterpenoid alkaloids from Delphinium grandiflorum†
Neng-Hua Chenab,
Yu-Bo Zhangab,
Wen Liab,
Pan Liab,
Li-Feng Chenab,
Yao-Lan Liab,
Guo-Qiang Li*c and
Guo-Cai Wang*ab
aInstitute of Traditional Chinese Medicine & Natural Products, College of Pharmacy, Jinan University, Guangzhou 510632, P. R. China. E-mail: twangguocai@jnu.edu.cn
bGuangdong Province Key Laboratory of Pharmacodynamic Constituents of TCM and New Drugs Research, Jinan University, Guangzhou 510632, P. R. China
cFoshan University, Foshan, 528000, P. R. China. E-mail: liguoqiang@jnu.edu.cn
First published on 3rd May 2017
Abstract
Two novel diterpenoid alkaloids, grandiflodines A and B (1 and 2), were isolated from Delphinium grandiflorum. Compound 1 represents a rare hetisine-type C20-diterpenoid alkaloid in which the bond between the atoms of N and C-17 is broken. Compound 2 features an unusual lycoctonine-type C19-diterpenoid alkaloid skeleton with the cleavage of N–C19 and C7–C17 bonds, and the construction of the N–C7 bond. Structural elucidations of the isolates were performed by spectroscopic analysis, X-ray diffraction and comparison with the literature. These compounds were tested for their antiviral and anti-inflammatory activities.
Introduction
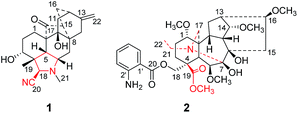
The genus Delphinium belongs to the family Ranunculaceae and consists of about 300 species distributed throughout the northern hemisphere.1,2 Among the 300 species, more than 113 ones are endemic to China and about 18 ones are used as folk medicines.1,2 As an important medicinal plant, Delphinium plants are used to treat traumatic injury, analgesia and rheumatism, etc.1 Recent investigations showed that the diterpenoid alkaloids are the main components of Delphinium plants, and the alkaloids possess complex structure skeletons and exhibit a wide spectrum of pharmacological activities.3–7 Thus, the diterpenoid alkaloids have become an increasing, attractive target for medicinal chemists.8Delphinium grandiflorum is a perennial herb mainly distributed in the Northwest of China and some regions of Siberia and People's Republic of Mongolia.9 As a folk medicine, the D. grandiflorum is applied for the treatment of toothache, and used as native pesticide as well.9 As part of our ongoing research on the bioactive natural products from Delphinium plants,10 an extensive phytochemical investigation on D. grandiflorum was undertaken, leading to the isolation of two novel diterpenoid alkaloids, grandiflodines A and B (1 and 2). Compound 1 is a rare hetisine-type C20-diterpenoid alkaloid with the cleavage of the bond between the atoms of N and C-17. Compound 2 features an unusual lycoctonine-type C19-diterpenoid alkaloid skeleton with the cleavage of N–C19 and C7–C17 bonds, and construction of the N–C7 bond. Herein, we report the isolation, structure elucidation and biological activities of 1 and 2 (Fig. 1).
Results and discussion
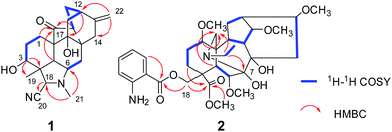
Grandiflodine A (1) was isolated as colorless block crystal. The molecular formula of 1 was established as C22H28N2O3 by its HR-ESI-MS (m/z 369.2175 [M + H]+, calcd for C22H29N2O3: 369.2173). The UV spectrum of 1 displayed the absorption maxima at 208 nm, and its IR spectrum showed the characteristic absorptions for hydroxyl groups (3479, 3423 cm−1), cyanogroup (2228 cm−1) and carbonyl group (1673 cm−1). The 1H NMR and HSQC spectroscopic data of 1 provided the resonances for two methyls [δH 1.18, 2.26 (each 3H, s); δC 25.6, 33.4], an olefinic methylene [δH 4.51, 4.67 (each 1H, d, J = 1.8 Hz); δC 103.4] and an oxygenated methine [δH 3.36 (1H, t, J = 5.8 Hz); δC 72.9]. The 13C and DEPT NMR data exhibited 22 signals of two methyls, seven methylenes, six methines and seven quaternary carbons, including a cyanogroup (δC 117.6), a pair of double bond (δC 103.4, 150.3) and a carbonyl group (δC 216.9). Detailed comparison of the 1D NMR data of 1 (Table 1) with those of anhydroignavinol11 showed that they were similar except for the presence of additional carbonyl and cyanogroup, and the absence of two oxygenated methines in 1. In the HMBC spectrum, the correlations (Fig. 2) between H-1/H-5/H-15 and the carbonyl group (δC 216.9) revealed that the carbonyl group was located at C-17. Moreover, the HMBC correlations between H-21 [δH 2.26, (3H, s)] and C-6/C-18 suggested that the methyl (δC 33.4, C-21) was connected to the nitrogen atom. The above information implied that the N–C17 bond was broken to form a unique hetidines-type C20-diterpenoid alkaloid skeleton as depicted. In addition, the cyanogroup (δC 117.6) was located at C-18 based upon the HMBC correlation between H-18 and C-20 (δC 117.6). And the HMBC correlations between H-3 (δH 3.36, 1H, t, J = 5.8 Hz) and C-4/C-18/C-19, between H-11/H-12/H-15/H-16 and C-9 (δC 76.5) indicated that the carbons at C-3 and C-9 were substituted by hydroxyls, respectively. In light of the evidences mentioned above, the planar structure of 1 was finally established.| Position | Grandiflodine A (1)a | Grandiflodine A (2)b | ||
|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | |
| a Measured at 500/125 MHz in DMSO-d6.b Measured at 300/75 MHz in CDCl3. Overlapped signals are reported without designating multiplicity. | ||||
| 1 | 23.2 | 1.62 | 88.9 | 2.88 dd (10.8, 4.1) |
| 1.06 m | ||||
| 2 | 26.6 | 2.60 m | 21.7 | 1.98 m |
| 1.62 | 1.75 m | |||
| 3 | 72.9 | 3.36 t (5.8) | 30.1 | 2.34 m |
| 1.44 m | ||||
| 4 | 47.7 | — | 49.8 | — |
| 5 | 53.5 | 2.30 | 49.9 | 2.02 d (6.7) |
| 6 | 58.6 | 3.07 m | 90.5 | 3.71 m |
| 7 | 31.2 | 2.30 | 87.8 | — |
| 1.75 dd (14.8, 4.5) | ||||
| 8 | 39.6 | — | 82.0 | — |
| 9 | 76.5 | — | 39.9 | 2.30 m |
| 10 | 51.7 | — | 51.3 | 1.89 m |
| 11 | 38.7 | 1.86 | 42.7 | — |
| 1.38 dd (14.1, 2.6) | ||||
| 12 | 35.9 | 2.16 | 29.6 | 1.83 m |
| 1.35 m | ||||
| 13 | 150.3 | — | 45.3 | 2.43 m |
| 14 | 32.7 | 1.62 | 85.3 | 3.66 t (3.9) |
| 1.56 dd (12.8, 4.3) | ||||
| 15 | 49.2 | 2.30 | 33.2 | 2.37 m |
| 1.57 dd (13.8, 8.1) | ||||
| 16 | 31.2 | 1.70 d (14.5) | 84.2 | 3.12 m |
| 2.16 | ||||
| 17 | 216.9 | — | 42.3 | 2.99 d (11.0) |
| 2.65 d (11.0) | ||||
| 18 | 55.3 | 3.81 s | 70.5 | 4.66 d (10.9) |
| 4.15 d (10.9) | ||||
| 19 | 25.6 | 1.18 s | 174.9 | — |
| 20 | 117.6 | — | 167.7 | — |
| 21 | 33.4 | 2.26 s | 43.7 | 3.15 m |
| 2.75 m | ||||
| 22 | 103.4 | 4.51 | 13.7 | 1.02 t (6.9) |
| 4.67 d (1.8) | ||||
| 3-OH | 5.00 d (5.5) | |||
| 9-OH | 4.86 s | |||
| 1-OCH3 | 56.8 | 3.24, s | ||
| 6-OCH3 | 61.3 | 3.62, s | ||
| 14-OCH3 | 57.9 | 3.38, s | ||
| 16-OCH3 | 56.4 | 3.28, s | ||
| 19-OCH3 | 52.1 | 3.72, s | ||
| 1′ | 110.7 | |||
| 2′ | 150.8 | |||
| 3′ | 117.0 | 6.64, m | ||
| 4′ | 134.4 | 7.24, m | ||
| 5′ | 116.5 | 6.58, m | ||
| 6′ | 131.0 | 7.71, dd (8.0, 1.4) | ||
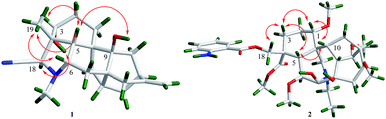
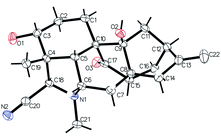
The relative configuration of 1 could be elucidated by the NOESY experiment. The correlations (Fig. 3) between 3-OH and H-18, between H-19 and H-3/H-5/H-6, as well as between 9-OH and H-5 established the relative configuration of 1. Finally, the structure and configuration were further elucidated by an X-ray diffraction analysis (Fig. 4). The final refinement of the Cu Kα data resulted in a small flack parameter of −0.05 (6) allowing the assignment of the absolute configuration of 1 as 3R, 4R, 5R, 6S, 8S, 9S, 10S, 12S, 15S, 18R.
The molecular formula of 2 was deduced as C33H48N2O10 by HR-ESI-MS at m/z 633.3387 [M + H]+ (calcd for C33H49N2O10: 633.3382). The 1H NMR spectrum of 2 displayed the signals of one ortho-substituted benzene ring at δH 7.71 (1H, dd, J = 8.0, 1.4 Hz), 7.24 (1H, m), 6.64 (1H, m), 6.58 (1H, m), and five methoxyls at δH 3.72, 3.62, 3.38, 3.28, 3.24 (each 3H, s). The 13C and DEPT NMR data displayed thirty-three carbon signals including six methyls, seven methylenes, thirteen methines and seven quaternary carbons. Detailed analysis of the 1H and 13C NMR data (Table 1) of 2 showed a number of similarities to those of anthranoyllycoctonine.12 The most notable differences were the existence of an additional carbonyl (δC 174.9) and an additional methoxyl (δC 52.1) in 2. The HMBC correlations between H-3/H-18 and C-19 (δC 174.9), and between 19-OCH3 (δC 52.1) and C-19 indicated that the N–C19 bond was broken, and the carbon at C-19 was oxidized to be carbonyl. Furthermore, the HMBC correlations between H-1/H-5/H-21 and the methylene at C-17 (δC 42.3) revealed that the C7–C17 linkage was broken. In addition, the correlation from H-21 to C-7 suggested that a new bond was constructed between the nitrogen atom and C-7. Hence, the planar structure of 2 was established. The relative configuration of 2 was the same as that of anthranoyllycoctonine by interpretation of the NOESY data (Fig. 3).12
Compounds 1 and 2 were tested for their antiviral effect against the respiratory syncytial virus (RSV), and anti-inflammatory activity on Nitric Oxide (NO) production. Both the two compounds showed no cell cytotoxicity towards the tested cells with the CC50 values more than 100 μM. Compound 2 displayed weak inhibitory effect on the growth of RSV and the production of NO in tested cells with the IC50 values of 75.3 and 72.7 μM, respectively, and 1 was virtually inactive with IC50 values more than 100 μM.
Conclusions
In summary, compounds 1 and 2, two novel diterpenoid alkaloids were isolated from D. grandiflorum. Compound 1 represents a rare hetisine-type C20-diterpenoid alkaloid, and 2 features an unusual lycoctonine-type C19-diterpenoid alkaloid skeleton, revealing that the alkaloids in Delphinium plants possess complex structure skeletons and adding the diversity of alkaloid compositions isolated from Delphinium plants. Moreover, the assays of anti-RSV and anti-inflammatory activities showed that these two compounds had little cytotoxicity towards the tested cells, providing more potentiality for further pharmacologic study.Experimental section
General
Melting point was obtained on an X-5 microscopic melting point apparatus. Optical rotations were recorded on a digital JASCO P-2000 polarimeter. UV spectra were obtained using a JASCO V-550 UV/VIS spectrophotometer. IR spectra were measured on a JASCO FT/IR-480 plus FT-IR spectrometer. NMR spectra were obtained by Bruker AV-500/300 spectrometers, with TMS as an internal standard. The chemical shifts (δ) were expressed in ppm and coupling constants (J) in Hz. HR-ESI-MS data was recorded on an Agilent 6210 ESI/TOF mass spectrometer. Analytical HPLC was performed using a Dionex ultimate 3000 system with a Cosmosil C18 analytical column (5 μm, 4.6 × 250 mm). Preparative HPLC was performed using an Agilent 1100 liquid chromatograph with a Cosmosil C18 preparative column (5 μm, 20 × 250 mm). Column chromatographies were performed with silica gel (80–100, 200–300, 300–400 mesh; Qingdao Marine Chemical Group Co. Ltd, Qingdao, China), ODS (50 μm, 120 Å; YMC) and Sephadex LH-20 (Pharmacia Biotech, Uppsala, Sweden). Silica gel GF254 plates (Yantai Chemical Industry Research Institute, Yantai, China) were used for thin-layer chromatography (TLC). Fractions were monitored by TLC, and spots were detected with modified Dragendorff's reagent.Plant material
The dried rhizomes of D. grandiflorum were purchased in Guangzhou, Guangdong Province of China, in July, 2015. The plant was authenticated by Prof. Guang-Xiong Zhou (College of Pharmacy, Jinan University). A voucher specimen (no. 150713) was deposited in the Institute of Traditional Chinese Medicine and Natural Products, Jinan University, Guangzhou, P. R. China.Extraction and isolation
The air-dried and powdered rhizome (10.0 kg) was extracted four times with 95% alcohol (4 × 35 L) at room temperature. After evaporation of alcohol, the crude extract (492.2 g) was suspended in water (2 L) and acidified with HCl to pH = 4–5, then partitioned with CHCl3 (3 × 4 L) to give a water-soluble fraction. The water-soluble fraction was basified with NH3·H2O to pH = 9–10 and then partitioned with a H2O/CHCl3 mixture to give a CHCl3-soluble fraction (63.0 g). The CHCl3-soluble fraction was chromatographed on silica gel column (300–400 mesh, 1000 g) eluted with a solvent system of CHCl3/CH3OH (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 to 0
0 to 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, v/v), yielding six fractions (Fr.A-F). Fr.B (9.2 g) was further separated on an ODS column (200 g) eluted with MeOH/H2O (30
100, v/v), yielding six fractions (Fr.A-F). Fr.B (9.2 g) was further separated on an ODS column (200 g) eluted with MeOH/H2O (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 to 100
70 to 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, v/v) to afford 9 subfractions (Fr.B1–B9). Fr.B5 (1.2 g) were purified by Sephadex LH-20 (MeOH/CHCl3, 1
0, v/v) to afford 9 subfractions (Fr.B1–B9). Fr.B5 (1.2 g) were purified by Sephadex LH-20 (MeOH/CHCl3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) and compound 1 (15.0 mg) was crystallized from the eluent. Then 2 (12.3 mg) was obtained by the preparative HPLC with MeOH/H2O (68
1, v/v) and compound 1 (15.0 mg) was crystallized from the eluent. Then 2 (12.3 mg) was obtained by the preparative HPLC with MeOH/H2O (68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32, v/v) from Fr.B5.
32, v/v) from Fr.B5.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 208.6 (3.57) nm; IR (KBr) νmax 3480, 3423, 2933, 2876, 2228, 1674, 1462, 1057, 1054, 894 cm−1; 1H and 13C NMR data see Table 1; HRESIMS m/z 369.2175 (calcd for C22H29N2O3, 369.2173).
ε) 208.6 (3.57) nm; IR (KBr) νmax 3480, 3423, 2933, 2876, 2228, 1674, 1462, 1057, 1054, 894 cm−1; 1H and 13C NMR data see Table 1; HRESIMS m/z 369.2175 (calcd for C22H29N2O3, 369.2173).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 219.5 (3.78), 250.2 (3.30), 340.5 (3.18) nm; IR (KBr) νmax 3455, 2931, 2874, 1677, 1453, 1360, 1189, 1055, 893 cm−1; 1H and 13C NMR data see Table 1; HRESIMS m/z 633.3382 (calcd for C33H49N2O10, 633.3387).
ε) 219.5 (3.78), 250.2 (3.30), 340.5 (3.18) nm; IR (KBr) νmax 3455, 2931, 2874, 1677, 1453, 1360, 1189, 1055, 893 cm−1; 1H and 13C NMR data see Table 1; HRESIMS m/z 633.3382 (calcd for C33H49N2O10, 633.3387).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 056 reflections were observed [F2 > 2σ(F2)]. The structure was solved by direct methods (SHELXS 97)13 and refined by full-matrix least-squares on F2. Final R = 0.0297, Rw = 0.0840, and S = 1.111. Crystallographic data for these structures have been deposited with the Cambridge Crystallographic Data Center as CCDC 1517870 for compound 1.
056 reflections were observed [F2 > 2σ(F2)]. The structure was solved by direct methods (SHELXS 97)13 and refined by full-matrix least-squares on F2. Final R = 0.0297, Rw = 0.0840, and S = 1.111. Crystallographic data for these structures have been deposited with the Cambridge Crystallographic Data Center as CCDC 1517870 for compound 1.Assay of anti-RSV activities on Hep-2 cells
The human larynx epidermoid carcinoma (HEp-2, ATCC CCL-23) cells and RSV A2 (ATCC VR-1540) strains were purchased from Medicinal Virology Institute, Wuhan University, China. HEp-2 cells were cultured in DMEM (Gibco) supplemented with 100 U mL−1 penicillin and streptomycin solution, and virus was propagated in HEp-2 cells and incubated in DMEM with 2 mM L-glutamine, 2% FBS, and 100 U mL−1 penicillin and streptomycin solution. All of the cells were cultured in a 95% humidified atmosphere supplied with 5% CO2 at 37 °C, and the ribavirin (Sigma, purity of 99%) was used as the positive control. The cytotoxicity of the compounds toward HEp-2 cells was detected by the MTT assay in 96-well plates (Corning) with the optical density (OD) values measured in an enzyme immunoassay reader (Thermo Labsystems Multiskan MK3) at 570 nm, and the 50% cytotoxic concentration (CC50) was estimated by regression analysis. The antiviral activities of the isolates against the RSV-A2 strain were assessed by the CPE reduction assay as reported in previous paper.14 The concentration that reduces 50% of CPE with respect to the virus control was estimated from the plots of the data and was defined as the 50% inhibitory concentration (IC50) of the tested compounds.Assay of anti-inflammatory activities on NO production toward RAW 264.7 cells
RAW 264.7 cells were provided by the Medicinal Virology Institute of Wuhan University and maintained in DMEM (Gibco) containing 10% FBS (Gibco), and supplemented with 100 U mL−1 penicillin and streptomycin solution. Cells were cultured at 37 °C in a 95% humidified atmosphere supplied with 5% CO2. The cytotoxicity of the compounds on RAW 264.7 cells was detected by the MTT assay in 96-well plates with the OD values measured at 570 nm, and the CC50 was estimated by regression analysis. The anti-inflammatory activities of the compounds were evaluated by the inhibitory effect on NO production. RAW 264.7 cells (4 × 104 cells per well) were incubated in a 96-well plate for 14 h and then pretreated with 100 ng mL−1 LPS and different concentrations of compounds (6.25–100 μM) for 24 h. Then, the Griess reagent (100 μL) was added and blended with the supernatant (100 μL), and the absorbance was measured at 540 nm with an enzyme immunoassay reader. NO levels were determined via a calibration curve constructed with NaNO2 concentrations of 3.12–100 μM. Inhibitory effects of compounds on NO production (IC50) were calculated by regression analysis of the dose–response curve generated from the data.Acknowledgements
This work was supported by the National Natural Science Foundation (No. 81473116, 81673319), and Science and Technology Planning Project of Guangdong Province (No. 2016B030301004, 2016A030303011).Notes and references
- K. J. Guan, Editorial committee of Flora of China, Science Press, Flora of China, Beijing, 1979, p. 326 Search PubMed.
- Y. Q. He, Z. Y. Ma, X. M. Wei, D. J. Liu, B. Z. Du, B. H. Yao and L. M. Gao, Chem. Biodiversity, 2011, 8, 2104–2109 CAS.
- X. L. Zhou, D. L. Chen, Q. H. Chen and F. P. Wang, J. Nat. Prod., 2005, 68, 1076–1079 CrossRef CAS PubMed.
- J. G. Diaz, J. G. Ruiz and G. D. Fuente, J. Nat. Prod., 2000, 63, 1136–1139 CrossRef CAS.
- J. Li, D. L. Chen, X. X. Jian and F. P. Wang, Molecules, 2007, 12, 353–360 CrossRef CAS PubMed.
- T. F. Xu, S. Liu, L. L. Meng, Z. F. Pi, F. R. Song and Z. Q. Liu, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2016, 1026, 56–66 CrossRef CAS PubMed.
- F. P. Wang, Q. H. Chen and X. Y. Liu, Nat. Prod. Rep., 2010, 27, 529–570 RSC.
- F. Z. Chen, D. L. Chen, Q. H. Chen and F. P. Wang, J. Nat. Prod., 2009, 72, 18–23 CrossRef CAS PubMed.
- K. J. Guan, Editorial committee of Flora of China, Science Press, Flora of China, Beijing, 1979, p. 445 Search PubMed.
- L. Yang, Y. B. Zhang, L. Zhuang, T. Li, N. H. Chen, Z. N. Wu, P. Li, Y. L. Li and G. C. Wang, Planta Med., 2017, 83, 111–116 CAS.
- S. W. Pelletier, S. W. Page and M. G. Newton, Tetrahedron Lett., 1970, 55, 4825–4827 CrossRef.
- J. Lu, H. K. Desai, S. A. Ross, H. M. Sayed and S. W. Pelletier, J. Nat. Prod., 1993, 56, 2098–2103 CrossRef CAS.
- G. M. Sheldrick, SHELXS-97: Manual of Program for the Refinement of Crystal Structures, University of Göttingen, Germany, 1997 Search PubMed.
- H. W. Geng, X. L. Zhang, G. C. Wang, X. X. Yang, X. Wu, Y. F. Wang, W. C. Ye and Y. L. Li, J. Asian Nat. Prod. Res., 2011, 13, 665–669 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: UV, IR, HRESIMS and NMR spectra of compounds 1 and 2. CCDC 1517870. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7ra02869e |
| This journal is © The Royal Society of Chemistry 2017 |