Open Access Article
Open Access ArticleCompetitive biosorption behavior of Pt(IV) and Pd(II) by Providencia vermicola
Hang Xua,
Ling Tan a,
Haigang Dongb,
Jia Hea,
Xinxing Liua,
Guanzhou Qiua,
Qianfeng Hecd and
Jianping Xie*a
a,
Haigang Dongb,
Jia Hea,
Xinxing Liua,
Guanzhou Qiua,
Qianfeng Hecd and
Jianping Xie*a
aSchool of Minerals Processing and Bioengineering, Central South University, Changsha, 410083, China. E-mail: Xiejianping@csu.edu.cn; Fax: +86-0731-88836943; Tel: +86-0731-88836943
bState Key Laboratory of Advanced Technologies for Comprehensive Utilization of Platinum Metals, Kunming Institute of Precious Metals, Kunming, 650106, China
cSchool of Chemistry and Chemical Engineering, Central South University, Changsha, 410083, China
dHunan Yonker Research Institute of Environmental Protection Co., Ltd, Changsha, 410330, China
First published on 23rd June 2017
Abstract
Biosorption is an effective way to recover or remove metal ions from wastewater; however, the biosorption process in a multiple metal ion solution is still unclear. In this paper, we investigated the simultaneous biosorption in a Pt(IV) and Pd(II) binary system using Providencia vermicola. Kinetics, isotherms, thermodynamics, TEM, and FT-IR were carried out to elucidate the competition behavior and adsorption mechanisms in the process. The results revealed that Pd(II) had a relevant effect on the Pt(IV) biosorption, but the interference of Pt(IV) on the adsorption of Pd(II) was considerably less intense. The maximum adsorption capacity of P. vermicola was 30.20 mg g−1 for Pt(IV) and 119.00 mg g−1 for Pd(II) in the binary system, which corresponded to 3.94 times larger selective adsorption toward Pd(II). The TEM results revealed that P. vermicola could adsorb Pt(IV) and Pd(II) both extracellularly and intracellularly. The FT-IR results indicated that amine groups played major roles in adsorbing Pt(IV), but amine, carboxyl, hydroxyl, and phosphate groups were critical to adsorb Pd(II). This study demonstrates that P. vermicola is a promising biosorbent and could selectively adsorb Pd(II) in the Pt(IV)–Pd(II) binary system.
1. Introduction
Biosorption is a promising technology for the removal and recovery of metals from waste solutions, because of its low-cost, high effectiveness at low concentrations, and environmentally friendly nature.1–4 The removal of the metal ions by biomass can occur through any or a combination of natural chemical mechanisms such as ion exchange, complexation, chelation, and precipitation.5–7Nevertheless, biosorption has not been applied in practical industrial processes.8 A major reason is that the adsorption capacities of most biosorbents are not very high.9,10 Another great challenge is that single metal species rarely exist in the natural streams and industrial wastewater.11,12 Therefore, more effective and selective adsorbents are needed to be developed.10,13 Meanwhile, it is necessary to evaluate the simultaneous biosorption behavior and interactions of two or more metal species.14
There are three possible interactive effects of a mixture of metal ions: synergism, antagonism, and non-interaction. Aksu et al.15 studied binary adsorption of iron(III) and iron(III)–cyanide complex ions on Rhizopus arrhizus. The results showed the combined action of two components was synergistic. Mahamadi et al.11 used Eichhornia crassipes to adsorb Pb2+, Cd2+, and Zn2+ in binary and ternary systems. It showed that the combined effect among the three metals was antagonistic, and the metal adsorption followed the order: Pb2+ > Cd2+ > Zn2+. Meanwhile, the interactions between metal ions and adsorption sites were also reported. Sok Kim16 investigated selective adsorption behavior of E. coli in Pt(IV)–Pd(II) binary solution. Results showed that primary amines were the binding sites for Pt(IV) and Pd(II), and had higher affinity toward Pd(II).
Despite significant achievements in the multiple biosorption, the interactions between metal ions and functional groups in cell surface are still unclear. Therefore, this study investigated the adsorption properties and mechanisms of Pt(IV) and Pd(II) in bicomponent solutions by P. vermicola. Kinetic, isothermal, and thermodynamic models were applied to evaluate the competitive behavior of Pt(IV) and Pd(II). Furthermore, transmission electron microscopy (TEM) and fourier transform infrared spectroscopy (FT-IR) was performed to explore the competitive biosorption mechanisms of Pt(IV) and Pd(II).
2. Materials and methods
2.1. Materials
The P. vermicola strain was isolated from YueLu Mountain (Changsha, China). The microorganism was cultivated in Lysogeny Broth medium at 30 °C for 24 h. The biomass was collected by centrifugation at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min. The 500 mg L−1 of Pt(IV) and Pd(II) stock solutions were prepared by dissolving PtCl4 or PdCl2 in 0.1 M hydrochloric acid.
000 rpm for 10 min. The 500 mg L−1 of Pt(IV) and Pd(II) stock solutions were prepared by dissolving PtCl4 or PdCl2 in 0.1 M hydrochloric acid.
2.2. Adsorption experiments
For comparison of adsorption behavior of Pt(IV) and Pd(II), 0.40 g of wet biomass (equaled to 0.075 g dry biomass) was added to 50 mL of metal solution. The ratios of the metal concentrations (Pt(IV)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pd(II)) were adjusted to about 1
Pd(II)) were adjusted to about 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Pt(IV)
1 (Pt(IV)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pd(II) = 85.13
Pd(II) = 85.13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 92.64 mg L−1), about 2
92.64 mg L−1), about 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Pt(IV)
1 (Pt(IV)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pd(II) = 170.26
Pd(II) = 170.26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 92.64 mg L−1), and about 3
92.64 mg L−1), and about 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Pt(IV)
1 (Pt(IV)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pd(II) = 255.39
Pd(II) = 255.39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 92.64 mg L−1). The biomass-containing solutions were stirred for 3 h at 170 rpm, 30 °C, and pH 4.0.
92.64 mg L−1). The biomass-containing solutions were stirred for 3 h at 170 rpm, 30 °C, and pH 4.0.
Binary adsorption kinetics: the initial concentrations of Pt(IV) and Pd(II) were 86.67 and 97.10 mg L−1, respectively. The mixture was stirred at 170 rpm and 30 °C. The samples were collected at predetermined time intervals for analysis.
Binary isotherm adsorption: the initial concentrations of Pt(IV) and Pd(II) were adjusted to 50, 100, 200, 300, and 400 mg L−1. The mixtures were stirred for 3 h at 170 rpm, 30 °C, and pH 4.0.
Binary thermodynamics: the initial concentrations of Pt(IV) and Pd(II) were 90.08 and 109.5 mg L−1. The mixture was stirred for 3 h at 30 °C, 40 °C, and 50 °C, respectively.
The initial and final concentrations of Pt(IV) and Pd(II) were determined by Inductively Coupled Plasma Optical Emission Spectrometer (ICP-OES). The adsorption capacities of the metals on the biosorbent were calculated as follows:17
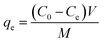 | (1) |
2.3. Transmission electron microscopy (TEM) and fourier transform infrared spectroscopy (FT-IR)
The precipitates of Pt(IV) and Pd(II) were characterized by TEM. After the biosorption experiment was finished, the bacterial suspension was washed three times with distilled water. The ultrathin sections of biomass samples were obtained using an ultramicrotome and then placed onto a copper grid. Finally, the samples were observed using a JEM-2100F transmission electron microscopy at 200 kV.The functional groups of P. vermicola were identified by FT-IR. The spectra were recorded within the range of 4000–400 cm−1 with samples prepared as KBr discs.
3. Results and discussion
3.1. Binary biosorption of P. vermicola
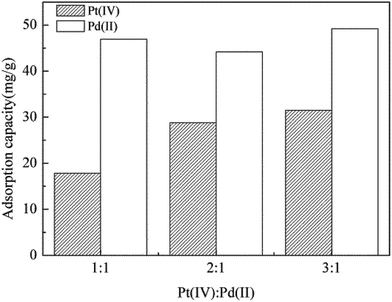
To compare adsorption behavior of Pt(IV) and Pd(II), binary batch experiments with different initial concentration ratios were carried out. When initial concentration ratio (Pt(IV)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pd(II)) was 1
Pd(II)) was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the adsorption capacity of Pd(II) (46.92 mg g−1) was almost 3 times to that of Pt(IV) (17.83 mg g−1); when the ratio was 2
1, the adsorption capacity of Pd(II) (46.92 mg g−1) was almost 3 times to that of Pt(IV) (17.83 mg g−1); when the ratio was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the adsorption capacity of Pd(II) (44.19 mg g−1) was about 2 times to that of Pt(IV) (28.82 mg g−1); even when the ratio was 3
1, the adsorption capacity of Pd(II) (44.19 mg g−1) was about 2 times to that of Pt(IV) (28.82 mg g−1); even when the ratio was 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the adsorption capacity of Pd(II) (49.22 mg g−1) was still higher than that of Pt(IV) (31.51 mg g−1) (Fig. 1). These results showed that in the Pt(IV)–Pd(II) binary system, P. vermicola preferred to adsorb Pd(II).
1, the adsorption capacity of Pd(II) (49.22 mg g−1) was still higher than that of Pt(IV) (31.51 mg g−1) (Fig. 1). These results showed that in the Pt(IV)–Pd(II) binary system, P. vermicola preferred to adsorb Pd(II).
Compared to the three different ratios, it showed that the adsorption capacity of Pd(II) was almost unchanged, while the adsorption capacity of Pt(IV) increased with increasing of the initial concentration of Pt(IV). These results suggested that the existence of Pt(IV) did not influence the adsorption of Pd(II), which implied the mechanisms of Pt(IV) and Pd(II) adsorption by P. vermicola was different.
3.2. Adsorption kinetics
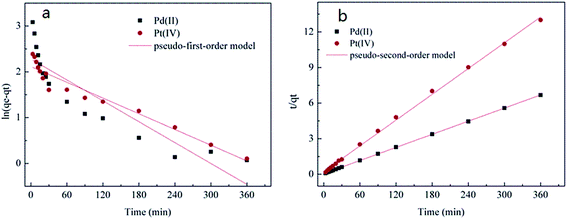
The kinetic experiment was carried out not only to estimate the equilibrium time but also to investigate the controlling step and mechanism of the adsorption process.18 It was observed that the adsorption process was very rapid at the first 30 minutes, which could attribute to an initial non-special adsorption process between biomass and metal ions. And then reached the equilibrium slowly at around 3 h for both Pt(IV) and Pd(II), which were identified as a chemical sorption process such as covalent bonding or microprecipitation.19The experimental data were described by linear pseudo-first-order and linear pseudo-second-order models. These models were expressed as follows:
Linear pseudo-first-order model:
ln(qe − qt) = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe − k1t qe − k1t
| (2) |
Linear pseudo-second-order model:
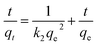 | (3) |
Fig. 2 showed the kinetic models for Pt(IV) and Pd(II) adsorption, and kinetic parameters were presented in Table 1. From Table 1, the correlation coefficients (R2) of the pseudo-second-order model for Pt(IV) and Pd(II) were higher than that of pseudo-first-order model. Moreover, the fitted curve of the pseudo-second-order model showed better agreement with the experimental data. Therefore, the pseudo-second-order model was more fitted with the adsorption process of Pt(IV) and Pd(II). This demonstrated that the controlling step might be chemical adsorption, but not mass transfer in solution.20 The adsorption process may also involve valence forces through sharing electrons between biomass and metal ions.18,21
| Pseudo-first-order model | Pseudo-second-order model | |||||
|---|---|---|---|---|---|---|
| qe | K1 | R2 | qe | K2 | R2 | |
| Pt | 8.28 | 0.0057 | 0.94 | 27.61 | 0.16 | 0.99 |
| Pd | 9.77 | 0.0076 | 0.80 | 54.29 | 0.34 | 0.99 |
In the case of the pseudo-second-order model, the estimated equilibrium uptake (qe) values were 27.61 mg g−1 for Pt(IV) and 54.29 mg g−1 for Pd(II). The equilibrium rate constants (k2) were 0.16 g mg−1 min−1 for Pt(IV) and 0.34 g mg−1 min−1 for Pd(II). Both qe and k2 of Pd(II) were higher than those values of Pt(IV). These results confirmed that P. vermicola could selectively bind with Pd(II).
3.3. Adsorption isotherm
Isotherm adsorption experiments were carried out to calculate the maximum adsorption capacity and evaluate the affinity in the bicomponent condition.The linear Langmuir model, linear Freundlich model, and linear Langmuir competitive model (LCM) were applied to Pt(IV) and Pd(II) isotherm data. These models were expressed as follows:
Linear Langmuir model:
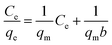 | (4) |
Linear Freundlich model:
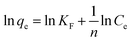 | (5) |
Linear LCM:
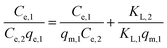 | (6) |
The linear curves of three isotherm models were shown in Fig. 3, and isotherm parameters were presented in Table 2. The results showed that the correlation coefficients of Langmuir were 0.97 for Pt(IV) and 0.95 for Pd(II), which were higher than those values of other isotherm models. Therefore, the experimental data of Pt(IV) and Pd(II) adsorption in binary system could fit best to the Langmuir isotherm model. This indicated that a monolayer adsorption process might dominate the present adsorption process rather than a multiple adsorption one.18
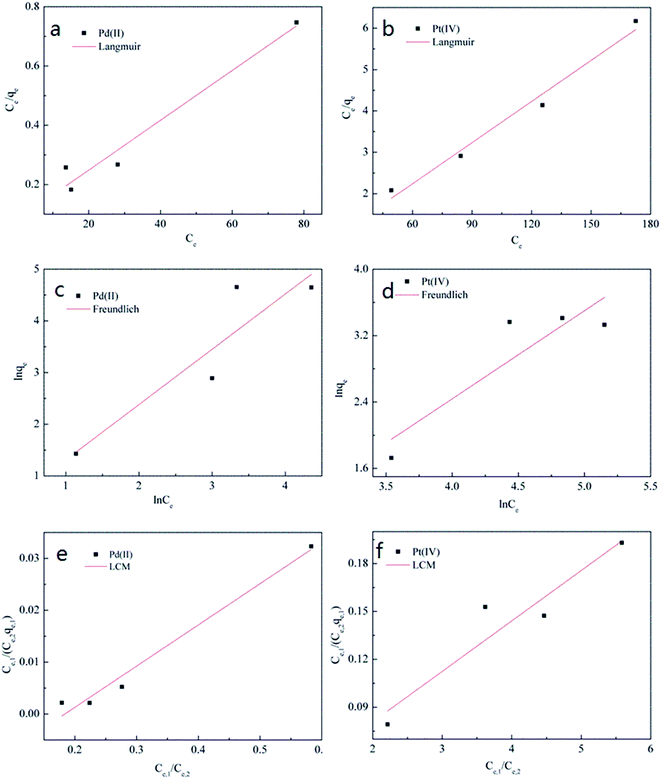 | ||
| Fig. 3 Isotherms of Pt(IV) and Pd(II) by P. vermicola in the binary system: the fitted curves of the Langmuir (a and b), Freundlich (c and d), and LCM (e and f). | ||
| qe/(mg g−1) | Langmuir | Freundlich | LCM | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| qmax/(mg g−1) | b/(L mg−1) | R2 | KF | 1/n | R2 | qmax/(mg g−1) | KL,2/KL,1 | R2 | ||
| Pt | 30.30 | 30.20 | 0.13 | 0.97 | 0.16 | 1.06 | 0.72 | 31.64 | 0.56 | 0.86 |
| Pd | 105.10 | 119.00 | 0.10 | 0.95 | 1.27 | 1.07 | 0.78 | 12.58 | −0.18 | 0.97 |
In the case of the LCM model, we found that the Pt(IV) adsorption process could be described well, but the Pd(II) adsorption process did not. Because the value of KL,2/KL,1 was negative, which was irrational. These results probably implied that the presence of Pd(II) caused competitive effect on Pt(IV) sorption, while Pt(IV) hardly influenced the Pd(II) adsorption in binary system.
Isotherm model constants, which express the surface properties and affinity of the biosorbent, can be used to compare the biosorption capacity of biomass for different adsorbate.22 One of the most important parameters is the maximum adsorption capacity. According to the Langmuir model, the maximum adsorption capacity of Pd(II) (119.00 mg g−1) was higher than that of Pt(IV) (30.20 mg g−1). This could be another evidence to prove that P. vermicola had greater affinity to Pd(II) than Pt(IV).
3.4. Adsorption thermodynamics
The thermodynamic parameters, including Gibbs free energy change (ΔG0), enthalpy change(ΔH0), and entropy change (ΔS0), were shown in Table 3. The negative values of ΔG0 demonstrated the spontaneous nature of Pt(IV) and Pd(II) adsorption by P. vermicola in binary system. The positive values of ΔH0 reflected that the present adsorption was endothermic and might exist strong bonding between metal ions and biosorbent. The positively values of ΔS0 reflected the increased randomness at the solid–solution interface during the biosorption of Pt(IV) and Pd(II) onto P. vermicola.22,23| T/°C | ΔG0/(kJ mol−1) | ΔH0/(kJ mol−1) | ΔS0/[J (K mol)−1] | R2 | |
|---|---|---|---|---|---|
| Pt | 20 | −0.09 | 14.98 | 49.54 | 0.91 |
| 30 | −0.42 | ||||
| Pd | 40 | −1.12 | 6.67 | 38.54 | 0.47 |
| 20 | −4.94 | ||||
| 30 | −5.55 | ||||
| 40 | −5.71 |
3.5. TEM analysis
The ultrathin sections of bacteria were investigated to identify the presence, spatial distribution, and morphology of Pt(IV) and Pd(II) precipitates by TEM.24 As shown in Fig. 4, the P. vermicola could sequester Pt(IV) and Pd(II) both extracellularly and intracellularly. After Pt(IV) and Pd(II) uptake for 3 h, numerous small particles were distributed uniformly on the cell wall. At the same time, a certain amount of precipitates could be observed in the cell, which clustered together to bigger agglomerates. It was possible that intracellular precipitates were isolated in particular area or bound to intracellular proteins in the cytoplasm.25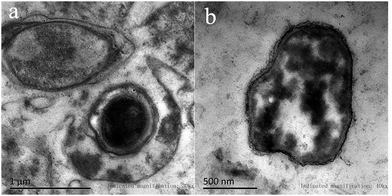 | ||
| Fig. 4 TEM analysis of P. vermicola before and after adsorption: (a) image of blank biomass; (b) image of metal-loaded biomass after 3 h of biosorption in Pt(IV) and Pd(II) binary system. | ||
Palladium precipitates were formed in a previous study by P. vermicola, which showed similarities with our results.26 Kevin Deplanche et al.27 revealed that the Pd(0) particles were located on both sides of the cytoplasmic membrane using E. coil mutant strains, which involved in the Pd(II) reduction process of the particular hydrogenases. Synthia Maes et al.28 also reported that both intra- and extracellular platinum precipitates were formed using halophilic bacteria. Therefore, the bacterial interaction in these biosorption processes is believed to consist of two steps: (1) the initial adsorption of metal ions to the cell, which is followed by (2) the transportation of the adsorbed metal ions into the cytoplasm, and gradually formed complexes.26,29
3.6. FT-IR analysis
To evaluate possible reactions between metal ions and surface functional groups, the FT-IR spectra of P. vermicola before and after adsorption were recorded. As shown in Fig. 5a, the strong and broad band around 3421.38 cm−1 represented the stretching of the N–H and O–H of the amine groups. The peak at 1650.11 cm−1 indicated C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching in the amide I bond of peptides. The peak around 1542.25 cm−1 was assigned to N–H bending and C–N stretching in amide II bond of peptides. The peak around 1396.88 cm−1 was caused by C–O stretching from carboxyl. The band at 1236.32 cm−1 resulted from the stretching vibration of P
O stretching in the amide I bond of peptides. The peak around 1542.25 cm−1 was assigned to N–H bending and C–N stretching in amide II bond of peptides. The peak around 1396.88 cm−1 was caused by C–O stretching from carboxyl. The band at 1236.32 cm−1 resulted from the stretching vibration of P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of phosphate. The peak around 1079.37 cm−1 can be attributed to stretching vibration of C–O and P–O from peptidoglycan.30–32 The results of FT-IR suggested that P. vermicola possessed many surface functional groups such as nitrogen-, oxygen- and phosphoryl-containing groups.
O of phosphate. The peak around 1079.37 cm−1 can be attributed to stretching vibration of C–O and P–O from peptidoglycan.30–32 The results of FT-IR suggested that P. vermicola possessed many surface functional groups such as nitrogen-, oxygen- and phosphoryl-containing groups.
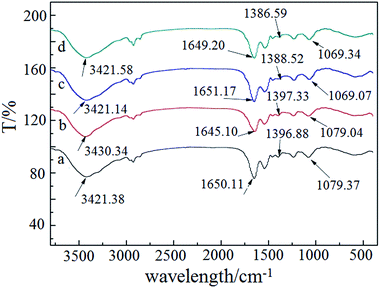 | ||
| Fig. 5 FT-IR spectra of the P. vermicola (a), Pt adsorption (b), Pd adsorption (c), binary adsorption (d). | ||
After Pt(IV) adsorption (Fig. 5b), the peak at 3421.38 cm−1 shifted to 3430.34 cm−1, which indicated that amine groups played important roles in adsorbing Pt(IV). The shifts of the peaks at 1650.11 cm−1 and 1542.25 cm−1 showed that amide I and amide II bonds were also involved in binding with Pt(IV). After Pd(II) adsorption (Fig. 5c), the shifts of the peaks from 1396.88 cm−1 to 1386.59 cm−1 and from 1386.59 cm−1 to 1069.34 cm−1 suggested that P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–O or P–O groups played major roles in adsorbing Pd(II). Amine and phosphate groups were also engaged because of the slight shifts of the peaks at 1542.25 cm−1 and 1236.32 cm−1. From Fig. 5d, the FT-IR spectrum of binary adsorption was similar to that of Pd(II) adsorption. The peaks, which largely shifted, were almost associated with Pd(II) adsorption. The shifts of the peaks, which related to Pt adsorption, were not obvious.
O and C–O or P–O groups played major roles in adsorbing Pd(II). Amine and phosphate groups were also engaged because of the slight shifts of the peaks at 1542.25 cm−1 and 1236.32 cm−1. From Fig. 5d, the FT-IR spectrum of binary adsorption was similar to that of Pd(II) adsorption. The peaks, which largely shifted, were almost associated with Pd(II) adsorption. The shifts of the peaks, which related to Pt adsorption, were not obvious.
These results verified that the surface functional groups of P. vermicola binding Pt(IV) and Pd(II) might have several differences. Nitrogen-containing groups played major roles in adsorbing Pt(IV), and nitrogen-, oxygen- and phosphoryl-containing groups were critical to adsorb Pd(II).
3.7. Adsorption mechanisms
In order to explain the differences of Pt(IV) and Pd(II) adsorption behavior by P. vermicola, it is useful to consider the possible adsorption mechanisms. PH is one of the most important factors to influence the biosorption process, because pH could affect not only the solution chemistry of metal ions, but also site dissociation of the cell surface.33At pH 4.0, when the chloride concentration is high (>10 mmol dm−3), platinum should be found as PtCl62−, and the predominant species of palladium are PdCl42− and hydroxyl complexes such as Pd(OH)+, Pd(OH)2 or Pd(OH)4.2–34 In addition, The pKa values are 7–10 for amine groups and 4.8 for carboxyl groups in the cell wall.34,35 Therefore, neutral amine groups and acidic carboxyl groups were protonated under the experimental conditions.
According to these, electrostatic attraction and anion exchange might play important roles to adsorb metal ions. Positive amine groups could bind anionic metal–chloride complex ions by electrostatic attraction. Anionic metal–chloride complex ions could be bound to the cell wall by anion exchange mechanism as below.16
| B − NH2 + H+Cl− → B − NH+3Cl− | (7) |
| B − NH+3Cl− + PtCl2−6 ↔ B − NH+3PtCl2−6 + Cl− | (8) |
| B − NH+3Cl− + PdCl2−4 ↔ B − NH+3PdCl2−4 + Cl− | (9) |
Except electrostatic attraction and anion exchange mechanism, another mechanism might be also involved in Pd(II) sorption. For example, Pd(OH)+ was possibly bound to carboxyl groups by exchanging with hydrogen ions. In addition, hydroxyl and phosphate groups could bind palladium complexes through complexation or chelation.34 Phosphate groups might be also engaged in modification of the extracellular protein.
As a result, the mechanisms of Pt(IV) adsorption were mainly electrostatic attraction and anion exchange. Pd(II) adsorption could be achieved by electrostatic attraction, anion and cation exchange, complexation and chelation. The difference of adsorption mechanisms could be a reason for selectively adsorbing Pd(II) by P. vermicola.
4. Conclusions
This study explored the biosorption properties and mechanisms of Pt(IV) and Pd(II) onto P. vermicola in binary system.(1) In Pt(IV)–Pd(II) binary system, P. vermicola preferred to adsorb Pd(II); furthermore, the presence of Pt(IV) could hardly influence on Pd(II) biosorption.
(2) Kinetic and isotherm models confirmed that P. vermicola had greater affinity to Pd(II) and selectively adsorbed Pd(II). Chemical adsorption was the controlling step of the adsorption process.
(3) The mechanism of Pd(II) adsorption was more complicated than that of Pt(IV). Amine groups were important to both Pt(IV) and Pd(II) adsorption. In addition, carboxyl, hydroxyl, and phosphate groups were also involved in Pd(II) adsorption. It was a main reason for selectively adsorbing Pd(II) onto P. vermicola in Pt(IV)–Pd(II) binary system.
In conclusion, P. vermicola could be used as an effective biosorbent to recover Pd(II) in a mixture solution of Pt(IV) and Pd(II). This study could also provide theoretical guidance for the treatment of multiple industrial wastewater.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (51104189), the 53rd China Postdoctoral Science Foundation (2013M531814), the China Postdoctoral Science Foundation (2015T80880), and the State Key Laboratory of Advanced Technologies for Comprehensive Utilization of Platinum Metals (SKL-SPM-201508).References
- J. Park, S. W. Won, J. Mao, I. S. Kwak and Y. S. Yun, J. Hazard. Mater., 2010, 181, 794–800 CrossRef CAS PubMed.
- A. Mishra, B. D. Tripathi and A. K. Rai, Ecotoxicol. Environ. Saf., 2016, 132, 420–428 CrossRef CAS PubMed.
- Z. Chen, X. Pan, H. Chen, Z. Lin and X. Guan, World J. Microbiol. Biotechnol., 2015, 31, 1729–1736 CrossRef CAS PubMed.
- E. Alipanahpour Dil, M. Ghaedi, G. R. Ghezelbash, A. Asfaram and M. K. Purkait, J. Ind. Eng. Chem., 2017, 48, 162–172 CrossRef CAS.
- F. Veglio and F. Beolchini, Hydrometallurgy, 1997, 44, 301–316 CrossRef CAS.
- S. W. Won, P. Kotte, W. Wei, A. Lim and Y. S. Yun, Bioresour. Technol., 2014, 160, 203–212 CrossRef CAS PubMed.
- S. W. Won, J. Park, J. Mao and Y. S. Yun, Bioresour. Technol., 2011, 102, 3888–3893 CrossRef CAS PubMed.
- X. Ju, K. Igarashi, S. Miyashita, H. Mitsuhashi, K. Inagaki, S. Fujii, H. Sawada, T. Kuwabara and A. Minoda, Bioresour. Technol., 2016, 211, 759–764 CrossRef CAS PubMed.
- C. P. Okoli, P. N. Diagboya, I. O. Anigbogu, B. I. Olu-Owolabi and K. O. Adebowale, Environ. Earth Sci., 2017, 76, 33 CrossRef.
- A. Asfaram, M. Ghaedi, G. R. Ghezelbash and F. Pepe, Ecotoxicol. Environ. Saf., 2017, 139, 219–227 CrossRef CAS PubMed.
- C. Mahamadi and T. Nharingo, Bioresour. Technol., 2010, 101, 859–864 CrossRef CAS PubMed.
- Y. Sağ and T. Kutsal, Process Biochem., 1996, 31, 561–572 CrossRef.
- W. Wei, S. Lin, D. H. Reddy, J. K. Bediako and Y. S. Yun, J. Hazard. Mater., 2016, 318, 79–89 CrossRef CAS PubMed.
- L. Li, F. Liu, X. Jing, P. Ling and A. Li, Water Res., 2011, 45, 1177–1188 CrossRef CAS PubMed.
- Z. Aksu and H. Gülen, Process Biochem., 2002, 38, 161–173 CrossRef CAS.
- S. Kim, M. H. Song, W. Wei and Y. S. Yun, J. Hazard. Mater., 2015, 283, 657–662 CrossRef CAS PubMed.
- H. Xie, Q. Zhao, Z. Zhou, Y. Wu, H. Wang and H. Xu, RSC Adv., 2015, 5, 33478–33488 RSC.
- K. Fujiwara, A. Ramesh, T. Maki, H. Hasegawa and K. Ueda, J. Hazard. Mater., 2007, 146, 39–50 CrossRef CAS PubMed.
- C. L. Mack, B. Wilhelmi, J. R. Duncan and J. E. Burgess, Miner. Eng., 2008, 21, 31–37 CrossRef CAS.
- Y. S. Ho and G. Mckay, Process Biochem., 1999, 34, 451–465 CrossRef CAS.
- A. Özer, D. Özer and H. İ. Ekiz, Adsorption, 2005, 10, 317–326 CrossRef.
- G. Uslu and M. Tanyol, J. Hazard. Mater., 2006, 135, 87–93 CrossRef CAS PubMed.
- S. Muthusamy and S. Venkatachalam, RSC Adv., 2015, 5, 45817–45826 RSC.
- S. Maes, R. Props, J. P. Fitts, R. D. Smet, R. Vilchezvargas, M. Vital, D. H. Pieper, F. Vanhaecke, N. Boon and T. Hennebel, Environ. Sci. Technol., 2016, 50, 2619 CrossRef CAS PubMed.
- J. Bai, X. Yang, R. Du, Y. Chen, S. Wang and R. Qiu, J. Environ. Sci., 2014, 26, 2056–2064 CrossRef PubMed.
- L. Tan, H. Dong, X. Liu, J. He, H. Xu and J. Xie, RSC Adv., 2017, 7, 7060–7072 RSC.
- K. Deplanche, I. Caldelari, I. P. Mikheenko, F. Sargent and L. E. Macaskie, Microbiology, 2010, 156, 2630 CrossRef CAS PubMed.
- S. Maes, R. Props, J. P. Fitts, S. R. De, R. Vilchezvargas, M. Vital, D. Pieper, F. Vanhaecke, N. Boon and T. Hennebel, Environ. Sci. Technol., 2016, 50, 2619 CrossRef CAS PubMed.
- S. Maes, R. Props, J. P. Fitts, R. D. Smet, F. Vanhaecke, N. Boon and T. Hennebel, PLos One, 2017, 12, e0169093 Search PubMed.
- R. Aravindhan, F. Aafreen, M. Selvamurugan, J. Raghava Rao and U. N. Balachandran, Clean Technol. Envir., 2011, 14, 727–735 CrossRef.
- J. Bai, X. Yang, R. Du, Y. Chen, S. Wang and R. Qiu, Clean Technol. Environ. Policy, 2014, 26, 2056–2064 Search PubMed.
- Y.-G. Liu, T. Liao, Z.-B. He, T.-T. Li, H. Wang, X.-J. Hu, Y.-M. Guo and Y. He, Trans. Nonferrous Met. Soc. China, 2013, 23, 1804–1814 CrossRef CAS.
- A. Esposito, F. Pagnanelli and F. Vegliò, Chem. Eng. Sci., 2002, 57, 307–313 CrossRef CAS.
- I. de Vargas, L. E. Macaskie and E. Guibal, J. Chem. Technol. Biotechnol., 2004, 79, 49–56 CrossRef CAS.
- J. B. Fein, C. J. Daughney, N. Yee and T. A. Davis, Geochim. Cosmochim. Acta, 1997, 61, 3319–3328 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |