Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Boosting visible light photo-/Fenton-catalytic synergetic activity of BiOIO3 by coupling with Fe2O3†
Hehua Zeng*a,
Xiu Liua,
Ting Weia,
Xueqin Lia,
Tianxiang Liua,
Xuemei Mina,
Qiuhui Zhua,
Xiufeng Zhaoa and
Jianhui Li *ab
*ab
aDepartment of Chemistry and Applied Chemistry, Changji University, Changji 831100, Xinjiang, China. E-mail: hhzeng@cjc.edu.cn
bNational Engineering Laboratory for Green Chemical Productions of Alcohols–Ethers–Esters, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen 361005, Fujian, China. E-mail: jhli@xmu.edu.cn
First published on 3rd May 2017
Abstract
The combination of a semiconductor photocatalyst with guest transition metal oxides can extend its light absorption property in the visible region, leading to the emergence of potential catalysts for solar photocatalytic degradation. In the present study, Fe2O3/BiOIO3 nanosheets with heterojunction couples have been fabricated in one-step by a simple hydrothermal method and used as a heterogeneous Fenton-like photocatalyst for the oxidative degradation of p-nitrophenol (PNP) under visible light. The absorption range of BiOIO3 extended to the visible light region with the introduction of Fe, while the band gap is dramatically decreased from 3.00 eV for pure BiOIO3 to 1.81 eV for 30% Fe2O3/BiOIO3. The recombination of photogenerated electron–hole pairs can also be prevented effectively. The intrinsic relationship between structure and catalytic activity was fully studied and a possible catalytic mechanism was proposed. The enhanced photocatalytic activity can be attributed to the synergistic effect of heterojunction semiconductor photocatalysis and heterogeneous Fenton catalysis caused by BiOIO3 and Fe2O3. This highly active heterogeneous catalyst has the potential for efficient utilization of solar energy in the visible range and can be applied in environmental remediation and waste water treatment.
1. The introduction
Semiconductor photocatalysis, based on the promotion of redox reactions by photoexcited electrons and holes under light irradiation, is a green technique that can be used for the oxidation of organic pollutants into CO2 and H2O without secondary pollution. In the past several years, bismuth-based layered photocatalysts, such as Bi2S3,1 Bi2WO6,2 BiOX (X = Cl, Br, I),3–8 and BiIO4,9 have been a hot area of research because of their special structure that can effectively separate photogenerated carriers. Recently, nanostructured BiOIO3 possessing two lone-pair cations and displaying an Aurivillius-type (BiO)22+ layer has been reported to exhibit high photocatalytic activity.10–12 However, it can only be excited by ultraviolet light that occupies only about 5% of the solar light due to its wide bandgap, which restricts the practical applications. Therefore, further researches for the broadening of the absorption spectrum of BiOIO3 have been carried out, including the synthesis of BiOIO3/g-C3N4,13 Bi/I−/BiOIO3,14 Ag/AgCl/BiOIO3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 and BiOIO3/BiOI.16 Nonetheless, the photocatalytic activity of BiOIO3 is far from efficient for practical applications.
15 and BiOIO3/BiOI.16 Nonetheless, the photocatalytic activity of BiOIO3 is far from efficient for practical applications.
Modification using transition metals to the bismuth-based photocatalysts may also be a good way to obtain the enhanced semiconductor photocatalytic activity. The studies on Mn decorated BiOCl and Fe decorated BiOBr have indicated that the doping of transition metals can reduce the band gap of bismuth oxyhalides and extend the wavelength response toward the visible range.17,18 Therefore, it is believed that BiOIO3 heterojunctions with transition metal oxides can be constructed to increase the response to visible light as well as the photocatalytic activity.
Fenton reagent, which can rapidly convert hydrogen peroxide (H2O2) into a stoichiometric amount of hydroxyl free radical (·OH) with high oxidative ability in acidic medium between pH 2 and pH 4, is known for more than a century and considerable as a successful treatment for advanced oxidation process (AOP).19 Conventional homogeneous Fenton process occurrs in Fe2+/H2O2 aqueous system, in which organic pollutants are oxidized by the ·OH produced from H2O2 decomposition catalyzed by Fe2+. However, its applications are limited due to the generation of excessive amounts of ferric hydroxide sludge that requires additional separation process. Heterogeneous Fenton-like system employing solid catalyst, which could provide a promising alternative for producing a large amount of hydroxyl free radical, may be available over a wide pH range and prevent ferric hydroxide sludge effectively.20 Recent years, many heterogeneous catalysts such as zero-valent iron,21,22 iron oxides23,24 and other iron-containing materials19,20,25,26 have been studied. Fe2O3, the most stable iron oxide under ambient conditions, is widely used in catalysts and water treatment. The band gap energy of Fe2O3 is 2.2 eV, and can be activated with visible light (<560 nm). In recent years, the combination of Fe2O3 and different nanospecies for photocatalytic reactions has received much attention.27–29
Herein, a novel BiOIO3 nanosheets married with various doping level of Fe2O3 were constructed in a one-pot route by the facile hydrothermal method. The binary photocatalysts were employed as heterogeneous catalyst to oxidize p-nitrophenol (PNP) in the presence of H2O2. The trapping experiment and photoluminescence probing technique were used to verify the active species of photocatalytic process. The effect of initial parameters on the oxidation of PNP was investigated in detail, and the catalytic mechanism was also preliminarily studied.
2. Materials and methods
2.1 Reagents
All the reagents were of analytical grade and used without further purification. Potassium iodate (KIO3), iron nitrate (Fe(NO3)3·9H2O), bismuth nitrate (Bi(NO3)3·5H2O) were provided by Sinopharm Chemical Reagent Beijing Co., Ltd. PNP and hydrogen peroxide (H2O2, 30 wt%) was supplied by Beijing Chemical Factory. The other reagents were analytically pure, ultrapure water was used throughout the study.2.2 Preparation and characterization of catalysts
Firstly, 1 mmol of Bi(NO3)3·5H2O was dispersed in 20 mL deionized water by ultrasonic method to form solution A. Simultaneously, 1 mmol KIO3 was fully dissolved in 20 mL deionizer water to form solution B. Then the solution B was dropped into the solution A. After magnetic stirring for 30 min, a certain amount of NaOH solution was added to adjust the pH value of the precursor to 3. Then, another 20 mL water solution containing a stoichiometric Fe(NO3)3·9H2O was dropped into the above suspension under the magnetic stirring condition, the concentration of Fe ions was chosen as 5%, 10%, 15%, 20%, 25%, and 30% (Fe2O3 and BiOIO3 in the mass percent). The mixture was transferred into a 100 mL Teflon-lined stainless autoclave to perform hydrothermal process at 180 °C for 12 h and allowed to cool down to room temperature naturally. The precipitate was washed using distilled water and absolute ethanol for three times and then dried at 80 °C for 12 h. The obtained samples were named as x% Fe2O3/BiOIO3, where x represents the percent concentration of Fe2O3.Fourier transform infrared spectrometry (FT-IR, IR Affinity-1), UV-vis diffuse reflection spectrometry (UV-vis DRS, UV-2550), X-ray powder diffraction (XRD, XD-2 Cu Kα radiation), field emission scanning electron microscopy (FE-SEM, 55VP SUPRA), transmission electron microscopy (TEM, JEOL JSM-2010), X-ray photoelectron spectrometer (XPS, ESCALAB MK II), and fluorescence spectrophotometer (PL, RF-5301) were performed to characterize the resultant catalysts.
2.3 Photocatalytic evaluation
The photocatalytic activities of the products were evaluated by degradation of PNP at room temperature. The reaction was performed in a photochemical reactor (BL-GHX-V, Shanghai Bilon Instruments Co., Ltd, China), equipped with a 250 W metal halide lamp combined with a 400 nm cut-off filter as light source. In a typical experiment, 50 mg catalyst was dispersed into 100 mL of 25 mg L−1 PNP solution with magnetic stirring to maintain a uniform suspension. The initial pH of the suspension was adjusted to a certain value using dilute aqueous H2SO4 or NaOH solution. After 30 min of stirring in the dark to reach the adsorption/desorption equilibrium, the reaction was timed with addition of H2O2 solution. At given time intervals, 5 mL of the suspension was continually collected. The solution and the catalyst were separated by centrifuging and the concentration of the remaining PNP was determined at 316 nm using a UV/Vis spectrophotometer. Total organic carbon (TOC) was obtained by a TOC analyzer (Shimadzu TOC-V CPH). Experiments were carried out in triplicate, and the results were expressed as mean values.3. Results and discussion
3.1 Textural and structural properties
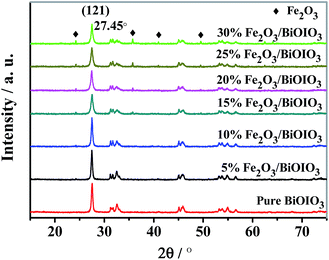
The crystal quality of the as-synthesized samples was analyzed by X-ray diffraction (XRD) as showed in Fig. 1. All patterns of samples exhibit sharp diffraction peaks, indicating that all samples were highly crystalline. It can be seen that BiOIO3 exhibits an orthorhombic crystal structure according to data files (ICSD #262019). With increasing Fe ions concentration, the intensity of (121) peak of BiOIO3 gradually decreased, while the peaks of Fe2O3 phase were simultaneously enhanced, which suggesting that the formation of Fe2O3/BiOIO3 composites.The FT-IR spectra are presented in Fig. S1.† All the modified samples possess similar FT-IR spectra to BiOIO3. But the absorption at 516 cm−1 becomes weaker upon introduction of Fe2O3 with little change in the peak shape and peak position. Meanwhile there is an obvious new absorption peak at 480 cm−1 due to Fe–O vibrations of Fe2O3.28 The new weak absorption peak (889 cm−1) could be associated with the stretching vibration of the Bi–O–Fe bond formed by interactions of BiOIO3 and Fe2O3. These results combined with the XRD characterizations further corroborates that Fe2O3 has been successfully combined with BiOIO3.
The surface composition and chemical states of related elements of 15% Fe2O3/BiOIO3 were analyzed by XPS. The shift of peak position on the charge effect was calibrated using the binding energy of C 1s at 284.6 eV.30 Fig. 2a shows the survey spectra and Fig. 2b–e shows high-resolution XPS spectra of the four primary elements. Clearly, the peak at 529.7 (Fig. 2b) corresponds to O 1s, which is related to the Bi–O bond in BiOIO3 lattice. Two strong peaks with binding energies at 634.9 and 623.4 eV are observed in the I 3d high-resolution XPS spectrum (Fig. 2c), which are separately attributed to the I5+ 3d3/2, and I5+ 3d5/2. The Bi 4f XPS spectra are shown in Fig. 2d, revealing Bi 4f5/2 and Bi 4f7/2 characteristic peaks at 164.5 and 159.0 eV, respectively. The peaks located at 711.5 and 724.8 eV can be assigned to Fe 2p3/2 and Fe 2p1/2 (Fig. 2e), respectively, which are in agreement with the value reported for Fe2O3.28
 | ||
| Fig. 2 XPS spectra of the 15% Fe2O3/BiOIO3 sample: (a) a wide-scan spectrum, the spectra in the energy regions of (b) O 1s, (c) I 3d, (d) Bi 4f, and (e) Fe 2p states. | ||
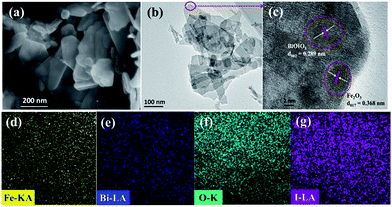
SEM and TEM revealed the morphologies of the as-prepared samples. Apparently, the sample exhibits irregular thin nanosheets with several hundred nanometers in size (Fig. 3a and b). The HRTEM image shown in Fig. 3c further verified the presence of Fe2O3. The lattice spacing of pure BiOIO3 is 0.289 nm, corresponding well to the (002) plane of the orthorhombic BiOIO3.23 Another distinct lattice fringe with a spacing of 0.368 nm was found on the edge of the Fe2O3/BiOIO3 flake, this lattice spacing matches well with the (012) plane of Fe2O3. EDX mapping was conducted to inspect the elemental composition and distribution (Fig. 3d–g). It is obvious that there is a homogeneous distribution of Fe, Bi, O and I elements in 15% Fe2O3/BiOIO3. These results above showed successful formation of Fe2O3/BiOIO3 heterojunction.
The optical properties of the as-prepared samples were measured by a UV-Vis spectrometer. As can be seen in Fig. S2a,† BiOIO3 has a clear edge around 400 nm without obvious absorption in the visible-light region. After Fe2O3/BiOIO3 heterojunction is formed, the optical absorption exhibits a systematical red shift with a wide and strong light absorption in the visible light range. The corresponding hν = (Ahν)1/2 plot for the samples are shown in Fig. S2b.† The band gaps were estimated to be about 3.00 eV and 1.81 eV for pure BiOIO3 and 30% Fe2O3/BiOIO3 by the linear extrapolation, respectively.
The photoluminescence (PL) emission spectra is usually employed to investigate the migration, transfer and recombination processes of photogenerated charge carriers in semiconductors, which plays a crucial role in photocatalytic reaction. Generally, the lower PL intensity, the lower recombination rate of photogenerated electron–hole pairs, thus there are more oxidative species and higher photocatalytic activity. Fig. 4 exhibits the PL spectra of the as-prepared samples under the excitation wavelength of 300 nm, and all the PL spectra exhibit similar peak shape and peak position. Obviously, the PL intensity of various Fe2O3/BiOIO3 samples is lower than that of pure BiOIO3, indicating that the recombination of photogenerated electron–hole pairs can be efficiently prevented by the introduction of Fe2O3. The 15% Fe2O3/BiOIO3 sample possesses the lowest PL intensity, suggesting the highest separation efficiency of photogenerated electrons and holes. However, when Fe2O3 concentration further increased, the PL emission intensity of samples increased, leading to inferior separation of the electron–hole pairs.
3.2 Photocatalytic performance
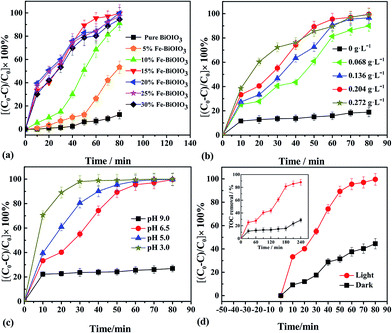
The effects of various reaction parameters on the oxidation of p-nitrophenol are presented in Fig. 5. Except for the investigated parameter, others are fixed on the following conditions: irradiation (λ > 400 nm), PNP 25 mg L−1, H2O2 0.204 g L−1, 15% Fe2O3/BiOIO3 0.5 g L−1, pH 6.5 and room temperature. From the results, it is clear that all x% Fe2O3/BiOIO3 samples exhibit much higher oxidation ability for PNP than pure BiOIO3 (Fig. 5a). Among these samples, 15% Fe2O3/BiOIO3 sample possesses the highest oxidation ability toward PNP, which is 4 times than that of pure BiOIO3. Excessive amounts of Fe over the catalysts with higher Fe2O3 concentration may act as recombination centers for photogenerated carriers and decrease the photocatalytic performance, which is consistent with the PL spectra results in Fig. 4.H2O2 is the source of ·OH, which is regarded as the main oxidative species to degrade organics in photocatalytic process. The influence of initial concentration of H2O2 on the degradation is presented in Fig. 5b. The result shows that when H2O2 concentration increased from 0 to 0.204 g L−1, the degradation of PNP is remarkably enhanced. Nevertheless, the increase of degradation rate becomes less significant when the concentration of H2O2 further increased to 0.272 g L−1. This could be attributed to the scavenging of ·OH which occurred through the following reaction:31,32
| H2O2 + ·OH → H2O + HO2·/O2−· |
They (HO2·/O2−·) are much less reactive.33 Therefore, the optimum H2O2 concentration appeared to be around 0.204 g L−1 under the conditions used in this study.
As shown in Fig. 5c, the degradation of PNP became very slow under alkaline conditions with pH value of 9.0. Moreover, the degradation efficiency increased significantly with lowering pH value. This increased degradation efficiency can be ascribed to the higher oxidation potential of ·OH and the higher stability of H2O2 that cannot decompose immediately to H2O and O2 at a lower pH.20,34 However, under strong acidic condition (pH ≤ 3.0), homogeneous Fenton reaction, which was caused by the releasing of iron ions, could shorten the life of catalyst.
The catalytic activity of catalyst was significantly enhanced under irradiation condition compared with that in dark, as showed in Fig. 5d. The degradation efficiency reached to 99.9% after 80 min under irradiation, while it was only 45% in the dark under the same conditions. The similar feature can be observed in TOC removal processes (inset in Fig. 5d). This is a further proof that the synergistic effect of photocatalysis and Fenton-like catalysis of catalyst would be responsible for the degradation of PNP.
The effect of catalyst dosage on degradation of PNP is illustrated in Fig. S3.† The result indicates that higher degradation efficiency was achieved when the catalyst dosage increased from 0 to 0.5 g L−1. However, less obvious change was observed when catalyst dosage was further increased to 1.0 g L−1, which could be ascribed to the agglomeration of particles which led to decline of surface area.35
Fig. S4† shows the absorption variation of PNP on the 15% Fe2O3/BiOIO3 system, the peak at 316 nm decreased gradually as the irradiation time increased, indicating the destruction of the conjugated structure of the PNP.
3.3 Stability and reusability of the catalyst
It is important to evaluate the stability and reusability of catalyst for practical application of a heterogeneous catalytic system. Therefore, leaching test was determined by ICP-AES by measuring the amount of iron leached into the solution after a reaction time of 80 min. The result shows that only 0.0072 mg L−1 of Fe3+ leached into the solution for the 15% Fe2O3/BiOIO3 sample. A homogeneous Fenton reaction was carried out using the same concentration of Fe3+ (prepared from Fe(NO3)3·9H2O) under the same operating conditions. Interestingly, there was not obvious degradation of PNP observed during the catalytic process. Thus, homogeneous contribution of leached Fe3+ to degradation of PNP can be ignored. Meanwhile, the reusability was investigated during five consecutive cycles under irradiation and fixed reaction time of 80 min. The degradation efficiency of the reused catalyst was still close to fresh catalyst, as showed in Fig. S5a.† Additionally, the XRD pattern of the catalyst after five times reuse is also shown in Fig. S5b.† No obvious differences in the diffraction peaks was observed, confirming a very stable crystal structure. These results indicate that the as-prepared catalysts exhibit high stability and reusability as a photocatalyst in the AOP process.3.4 Analysis of the active species
To verify the active species of photocatalytic process, the effect of photogenerated holes (h+), superoxide radical (·O2−) and hydroxyl radical (·OH) on the degradation of PNP was investigated. Triethanolamine (TEA), p-benzoquinone (BQ) and isopropyl alcohol (IPA) were served as the scavengers of h+, ·O2−, and ·OH, respectively. After being trapped, the active species will be efficiently quenched, and the degradation efficiency of PNP thus became lower. As shown in Fig. 6, the addition of IPA resulted in a remarkable decrease in the degradation of PNP, indicating that ·OH is the main active species in the photocatalytic process. Only slight decrease in the degradation efficiency was observed in the presence of TEA and BQ, revealing that h+, and ·O2− made negligible contribution to the photocatalytic reaction.The formation of ·OH on the surface of catalysts was further confirmed by PL technique using coumarin as probe molecule. Coumarin can be rapidly oxidized by photogenerated ·OH in the presence of Fe2O3/BiOIO3 and H2O2 under irradiation to form hydroxycoumarin, which shows high fluorescence intensity. As illustrated in Fig. S6,† the PL intensity was enhanced rapidly as the reaction time increases. In other words, coumarin can be rapidly oxidized to form hydroxycoumarin by the synergistic effect of irradiation and Fenton catalyst and the PL intensity was increased significantly with reaction time (inset curve 1), which was much higher than that in the dark (inset curve 2). However, the PL intensity was very low under the conditions when single catalyst or H2O2 was used (inset curves 3 and 4). On the other hand, the fluorescence for the hydroxycoumarin was completely quenched (inset curve 5) once IPA was added to the system, meaning that ·OH is the principle oxidative species that participate in the heterogeneous Fenton-like photocatalytic reaction.
3.5 Possible photocatalytic mechanism
It is generally believed that the photocatalytic activity can be affected by many factors, which cooperate with each other to determine the photocatalytic activity. The reasons for the enhanced catalytic activities of Fe2O3/BiOIO3 samples was explained as follows.Firstly, in the photocatalytic process, iron ion of the x% Fe2O3/BiOIO3 samples can serve as a mediator of the interfacial charge transfer, which can trap photogenerated electrons and holes to form corresponding FeII and FeIV.36 Once FeII and FeIV contact with O2 or OH− in the solution, they will re-transfer into FeIII together with the formation of ·O2− or ·OH.37 Secondly, Fe2O3/BiOIO3 sample exhibited good photocatalytic activity under irradiation because of the heterojunction structure between BiOIO3 and Fe2O3. The photogenerated electrons in the CB (∼0.28 eV) of the Fe2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27–29 migrated to the CB (∼1.00 eV) of the BiOIO3, while the photogenerated holes in the VB (∼4.08 eV) of BiOIO3 (ref. 10–12) moved to the VB (∼2.48 eV) of Fe2O3. As a result, the photogenerated electrons and holes were spatially isolated, which greatly inhibited their undesirable recombination (eqn (1) in Fig. 7). Then the photogenerated holes directly reacted with H2O or OH− and converted it into ·OH (eqn (2)). Meanwhile, H2O2 chemisorbed on the catalyst surfaces by hydrogen bonding was favorable to trap electrons to produce ·OH (eqn (3)). Whereas excessive Fe2O3 would reduce the activity due to its low efficiency. The 15% Fe2O3/BiOIO3 sample may be the most satisfying to these requirements. Thirdly, Fenton-like process can generate hydroxyl free radical, this proposal involves the redox cycle of FeIII ↔ FeII on the surface, similar to the process occurred in homogeneous Fenton system24,38 (eqn (4) and (5)). Thus, the synergistic effect of photocatalysis of x% Fe2O3/BiOIO3 semiconductor and Fenton-like catalysis would be responsible for the enhanced hydroxyl free radical yield.
27–29 migrated to the CB (∼1.00 eV) of the BiOIO3, while the photogenerated holes in the VB (∼4.08 eV) of BiOIO3 (ref. 10–12) moved to the VB (∼2.48 eV) of Fe2O3. As a result, the photogenerated electrons and holes were spatially isolated, which greatly inhibited their undesirable recombination (eqn (1) in Fig. 7). Then the photogenerated holes directly reacted with H2O or OH− and converted it into ·OH (eqn (2)). Meanwhile, H2O2 chemisorbed on the catalyst surfaces by hydrogen bonding was favorable to trap electrons to produce ·OH (eqn (3)). Whereas excessive Fe2O3 would reduce the activity due to its low efficiency. The 15% Fe2O3/BiOIO3 sample may be the most satisfying to these requirements. Thirdly, Fenton-like process can generate hydroxyl free radical, this proposal involves the redox cycle of FeIII ↔ FeII on the surface, similar to the process occurred in homogeneous Fenton system24,38 (eqn (4) and (5)). Thus, the synergistic effect of photocatalysis of x% Fe2O3/BiOIO3 semiconductor and Fenton-like catalysis would be responsible for the enhanced hydroxyl free radical yield.
4. Conclusions
A series of Fe2O3 modified BiOIO3 photocatalysts were prepared using a simple hydrothermal method. There is a strong cooperative effect of bandgaps between these two components, which makes the apparent band gap decrease greatly from 3.00 eV for pure BiOIO3 to 1.81 eV for 30% Fe2O3/BiOIO3. The visible light photocatalytic activity for the oxidative degradation of PNP under visible light could be obviously improved. The enhanced catalytic activities of Fe2O3/BiOIO3 samples can mainly be ascribed to three factors: Fe ions acted as a mediator of the interfacial charge transfer, the heterojunction structure between BiOIO3 and Fe2O3 greatly improved the efficiency of charge separation, the synergistic effect of Fenton-like catalysis. After consecutive catalytic procedures, the catalyst was still stable without obvious deactivation. Coumarin PL experiment confirmed that ·OH was the dominating active species of oxidation process. As a kind of heterogeneous Fenton-like photocatalyst, the Fe2O3/BiOIO3 heterojunction is a promising alternative semiconductor for efficient removal of organic pollutants from water.Acknowledgements
This work was financially supported by the Fundamental Research Funds for the Central Universities (20720170030), and the Educational Commission of XinJiang (XJEDU2014S066). We thank all the graduate students for their assistance with the fieldwork.References
- L. Cademartiri, R. Malakooti, P. G. O'Brien, A. Migliori, S. Petrov, N. P. Kherani and G. A. Ozin, Angew. Chem., Int. Ed., 2008, 47, 3814–3817 CrossRef CAS PubMed.
- X. F. Cao, L. Zhang, X. T. Chen and Z. L. Xue, CrystEngComm, 2011, 13, 1939–1945 RSC.
- L. Zhao, X. Zhang, C. Fan, Z. Liang and P. Han, Phys. Rev. B: Condens. Matter Mater. Phys., 2012, 407, 3364–3370 CrossRef CAS.
- H. Li, Y. Cui and W. Hong, Appl. Surf. Sci., 2013, 264, 581–588 CrossRef CAS.
- K. Zhao, L. Zhang, J. Wang, Q. Li, W. He and J. J. Yin, J. Am. Chem. Soc., 2013, 135, 15750–15753 CrossRef CAS PubMed.
- X. Zhang, C. Y. Wang, L. W. Wang, G. X. Huang, W. K. Wang and H. Q. Yu, Sci. Rep., 2016, 6, 22800 CrossRef CAS PubMed.
- J. Jiang, K. Zhao, X. Xiao and L. Zhang, J. Am. Chem. Soc., 2012, 134, 4473–4476 CrossRef CAS PubMed.
- J. Jiang, L. Zhang, H. Li, W. He and J. J. Yin, Nanoscale, 2013, 5, 10573–10581 RSC.
- H. Huang, S. Wang, N. Tian and Y. Zhang, RSC Adv., 2014, 4, 5561–5567 RSC.
- S. D. Nguyen, J. Yeon, S. H. Kim and P. S. Halasyamani, J. Am. Chem. Soc., 2011, 133, 12422–12425 CrossRef CAS PubMed.
- W. Wang, B. Huang, X. Ma, Z. Wang, X. Qin, X. Zhang and Y. Dai, Chem.–Eur. J., 2013, 19, 14777–14780 CrossRef CAS PubMed.
- X. M. Qi, M. L. Gu, X. Y. Zhu, J. Wu, H. M. Long, K. He and Q. Wu, Chem. Eng. J., 2016, 285, 11–19 CrossRef CAS.
- W. Wang, H. Cheng, B. Huang, X. Liu, X. Qin, X. Zhang and Y. Dai, J. Colloid Interface Sci., 2015, 442, 97–102 CrossRef CAS PubMed.
- T. Xiong, F. Dong, Y. Zhou, M. Fu and W. K. Ho, J. Colloid Interface Sci., 2015, 447, 16–24 CrossRef CAS PubMed.
- T. Xiong, H. Zhang, Y. Zhang and F. Dong, Chin. J. Catal., 2015, 36, 2155–2163 CrossRef CAS.
- H. Huang, K. Xiao, K. Liu, S. Yu and Y. Zhang, Cryst. Growth Des., 2016, 16, 221–228 CAS.
- B. Pare, B. Sarwan and S. B. Jonnalagadda, Appl. Surf. Sci., 2011, 258, 247–253 CrossRef CAS.
- Z. Liu, B. Wu, Y. Zhu, D. Yin and L. Wang, Catal. Lett., 2012, 142, 1489–1497 CrossRef CAS.
- L.-Z. Zhang, H. Chen, X.-F. Zhao, Q. Zhai, D.-J. Yin, Y.-F. Sun and J.-H. Li, Appl. Catal., B, 2016, 193, 47–57 CrossRef CAS.
- H. Chen, L.-Z. Zhang, H.-H. Zeng, D.-J. Yin, Q. Zhai, X.-F. Zhao and J.-H. Li, J. Mol. Catal. A: Chem., 2015, 406, 72–77 CrossRef CAS.
- C. M. Rui, A. F. Rossi and R. M. Quinta-Ferreira, J. Hazard. Mater., 2010, 180, 716–721 CrossRef PubMed.
- C. M. Rui, D. V. Lopes, M. J. Quina and R. M. Quinta-Ferreira, Chem. Eng. J., 2012, 192, 219–225 CrossRef.
- F. Duarte, F. J. Maldonado-Hódar and L. M. Madeira, Appl. Catal., B, 2013, 129, 264–272 CrossRef CAS.
- L. Xu and J. Wang, Appl. Catal., B, 2012, 123–124, 117–126 CrossRef CAS.
- N. Wang, L. Zhu, M. Lei, Y. She, M. Cao and H. Tang, ACS Catal., 2011, 1, 1193–1202 CrossRef CAS.
- Q. Zhai, L.-Z. Zhang, X.-F. Zhao, H. Chen, D.-J. Yin and J.-H. Li, Appl. Surf. Sci., 2016, 377, 17–22 CrossRef.
- M. B. Sassin, A. N. Mansour, K. A. Pettigrew, D. R. Rolison and J. W. Long, ACS Nano, 2010, 4, 4505–4514 CrossRef CAS PubMed.
- P. L. Lee, Y. K. Chiu, Y. C. Sun and Y. C. Ling, Carbon, 2010, 48, 1397–1404 CrossRef CAS.
- L. L. Peng, T. F. Xie, Y. C. Lu, H. M. Fan and D. J. Wang, Phys. Chem. Chem. Phys., 2010, 12, 8033–8041 RSC.
- J. Zhu, F. Chen, J. Zhang, H. Chen and M. Anpo, J. Photochem. Photobiol., A, 2006, 180, 196–204 CrossRef CAS.
- Z. Tao, Y. Li, J. Jing, F. S. Wong and X. Lu, Sep. Purif. Technol., 2008, 62, 551–558 CrossRef.
- L. Xu and J. Wang, J. Hazard. Mater., 2011, 186, 256–264 CrossRef CAS PubMed.
- Q. Liao, J. Sun and L. Gao, Colloids Surf., A, 2009, 345, 95–100 CrossRef CAS.
- N. K. Daud and B. H. Hameed, J. Hazard. Mater., 2010, 176, 1118–1121 CrossRef CAS PubMed.
- T. A. Gad-Allah, M. E. M. Ali and M. I. Badawy, J. Hazard. Mater., 2011, 186, 751–755 CrossRef CAS PubMed.
- S. Guo, X. Li, H. Wang, F. Dong and Z. Wu, J. Colloid Interface Sci., 2012, 369, 373–380 CrossRef CAS PubMed.
- W. Choi, A. Termin and M. R. Hoffmann, J. Phys. Chem., 1994, 98, 13669–13679 CrossRef.
- Y. Gao, H. Gan, G. Zhang and Y. Guo, Chem. Eng. J., 2013, 217, 221–230 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra02761c |
| This journal is © The Royal Society of Chemistry 2017 |