Open Access Article
Open Access ArticleSynthesis of polyoxymethylene dimethyl ethers catalyzed by sulfonic acid-functionalized mesoporous SBA-15
Zhenzhen Xue a,
Hongyan Shang*a,
Chunhua Xiongb,
Changbo Lub,
Gaojun Anb,
Zailong Zhanga,
Chuntao Cuia and
Mingjie Xua
a,
Hongyan Shang*a,
Chunhua Xiongb,
Changbo Lub,
Gaojun Anb,
Zailong Zhanga,
Chuntao Cuia and
Mingjie Xua
aCollege of Science, China University of Petroleum (East China), Qingdao 266580, Shandong, P. R. China. E-mail: catagroupsh@163.com
bChinese People's Liberation Army Oil Research Institute, Beijing 102300, P. R. China
First published on 7th April 2017
Abstract
Sulfonic acid-functionalized mesoporous SBA-15 (SO3H-SBA-15) with different sulfur loading was synthesized via a post-synthesis method with 3-mercaptopropyltrimethoxysilane (MPTMS) and was used to investigate the catalytic performance for the synthesis of polyoxymethylene dimethyl ethers (PODEn) from methylal (DMM) and trioxymethylene (TOX). X-ray diffraction, N2 adsorption–desorption, Fourier transform infrared spectra, 13C-NMR and 29Si-NMR, elemental analyses, and X-ray fluorescence were used to characterize the structures and sulfur loading of the obtained catalysts, and chemistry titration was carried out to investigate the acid amounts of the catalysts. Through the comparison of the catalysts with different sulfur loading, it was found that the acidity of the catalysts has a decisive effect on the product distribution and the chain length of the products: under the catalysis of the SO3H-SBA-15(0.8) catalyst, which has an acid amount of 0.166 mmol g−1, the highest DMM and TOX conversion and PODEn yield and selectivity were achieved and the desired chain length of PODE2–8 was obtained. The optimum reaction conditions such as reaction time, temperature, and molar ratio of DMM and TOX were investigated, and by comparison of different reaction temperatures, it was demonstrated that temperature played an important role in the TOX depolymerization rate and selectivity of the main products and by-products. By adjusting the molar ratio of DMM and TOX, it was observed that an increasing amount of TOX was beneficial for promoting the reaction to generate the products with a higher degree of polymerization. The comparison test of the reaction time confirmed that the reaction reached chemical equilibrium within 60 min and could not be promoted by further extending the time.
1. Introduction
With the increasing depletion of oil resources and environmental pressure, researchers have been developing a new diesel engine fuel or additive that could replace diesel and reduce engine emissions. According to the combustion characteristics of the diesel engine fuel, it is considered that the oxygenated compounds are very suitable to be used as diesel fuel additives. As a representative of an oxygenated compound, polyoxymethylene dimethyl ethers (PODEn) have the characteristics of high cetane number,1 high oxygen content,2 and are free of sulfur and aromatics. They can achieve highly efficient and clean combustion3,4 that effectively reduces soot emission, PM emission, and particle number without changing the engine infrastructure.3,5,6 On the other hand, the feedstock for PODEn synthesis primarily consists of methanol and methanol derivatives; therefore, the development of PODEn is of great significance in promoting the development of methanol downstream products of high added value and the healthy development of the coal chemical industry.It is well known that the synthesis of PODEn is a type of acid catalytic process, at present; there are relatively few reports on the PODEn synthesis catalysts. Several acid catalysts such as ion-exchanged resin,7–9 molecular sieve,10,11 ionic liquids,12–14 heteropoly acids,15 Zr–Alumina,16 graphene oxide,17 solid super acid,18 etc. have been studied for the synthesis of PODEn. However, only few catalysts are suitable for industrial application because of the unsatisfactory conversion rate and selectivity of PODEn. Wu et al.11 evaluated the catalytic performance of ZSM-5 molecular sieves with different Si/Al ratios for the synthesis of PODEn, and they indicated that under the catalysis of ZSM-5 with the Si/Al ratio of 580, the conversion rate of TOX and selectivity of PODEn were 85.3% and 88.5%, respectively. Li et al.18 prepared SO42−/Fe2O3–SiO2 catalysts with varied acid strength and acid sites, by which the conversion rate of TOX and selectivity of PODEn were only 81.9% and 34.4%, respectively. Zhao and coworkers10 compared the catalytic performance of HY, HZSM-5, Hβ, and HMCM-22, and they indicated that HMCM-22 showed the best catalytic performance; however, the selectivity of PODE3–8 was only 29%. Therefore, an effective catalyst with high activity is urgently required for PODEn synthesis from methylal and trioxymethylene.
Sulfonic acid mesoporous SBA-15 catalyst as a type of excellent and efficient Brønsted acid catalyst has been applied in different fields such as ethylene polymerization,19,20 biodiesel synthesis,21,22 bio-oil upgrading,23 fatty acid esterification,24–26 glycerol acetylation,27 and dimethyl ether synthesis.28 In our previous research, we synthesized a series of mesoporous Al-SBA-15 molecular sieves with different Si/Al ratios and investigated the influences of acid strength, acid amount, acid type, and pore sizes on the PODEn synthesis.29 In this study, as a continuation of our previous study, a series of sulfonic acid-functionalized mesoporous SBA-15 with different sulfur loading were synthesized by a post-synthesis method. X-ray diffraction (XRD), N2 adsorption–desorption, X-ray fluorescence (XRF), nuclear magnetic resonance (NMR), Fourier transform infrared (FT-IR) spectroscopy, elemental analysis, and acid–base titration were used to characterize the structure and acidity of the as-synthesized catalysts. Moreover, reusability performance of sulfonic acid-functionalized mesoporous SBA-15 was also studied herein.
2. Experimental
2.1. Catalyst preparation
2.2. Catalyst characterization
X-ray diffraction (XRD) was carried out using an X'Pert Pro MPD equipment produced by Panalytical with CuKα radiation. The scan range was set between 0.5° and 5° for small angle measurements. N2 adsorption–desorption experiments were carried out via a TriStar II instrument (micromeritics, USA) using nitrogen as the adsorption agent at −196 °C. The specific surface areas were calculated from the Brunauer–Emmett–Teller (BET) equation, and the total pore volumes were calculated by the BJH method. The BJH pore sizes were calculated from the desorption branch of the isotherms. The S, C, and H elemental analyses were carried out by a Thermo EA1112 Element Analyzer. X-ray fluorescence (XRF) was performed via a PANalytical Axios instrument. Fourier transform infrared spectra (FT-IR) were obtained using a Thermo Fisher Nicolet-6700 instrument. The 13C-NMR and 29Si-NMR spectra were obtained using a Bruker MSL 300 NMR spectrometer with the resonance frequencies of 75.5 MHz and 59.6 MHz, respectively. Thermogravimetric analysis was carried out under a high-purity N2 atmosphere at the heating rate of 10 °C min−1. The acid amounts of the catalysts were detected via a titration method as follows: 0.1 g of SO3H-SBA-15 catalyst was added to 20 ml of NaCl (2 M) aqueous solution; the mixture was stirred at room temperature for 12 h and then titrated with 0.05 M NaOH solution.32,332.3. Catalytic performance test
Experiments for the catalytic performance tests were carried out using a 100 ml stirred autoclave reactor. DMM, TOX, and SO3H-SBA-15 catalysts were quantitatively loaded into the reactor. The amounts of DMM and TOX were basically added according to the molar ratio from 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and the amount of SO3H-SBA-15 catalysts were 2 wt% of the total amount of the feedstock loading. The reaction temperature was set at 70–130 °C and controlled with ±1 °C of the set value via a modular controller; the reaction times were set from 20 to 120 min. The reaction pressure was fixed at 1 MPa and the agitation speed was set at 300 rpm.
2 and the amount of SO3H-SBA-15 catalysts were 2 wt% of the total amount of the feedstock loading. The reaction temperature was set at 70–130 °C and controlled with ±1 °C of the set value via a modular controller; the reaction times were set from 20 to 120 min. The reaction pressure was fixed at 1 MPa and the agitation speed was set at 300 rpm.
Synthetic products were analyzed by an Agilent 7820 gas chromatograph with an FID detector. An Agilent HP-5 capillary column (30 m × 0.32 mm × 0.25 μm) was used. Chromatographic conditions were set as follows: injection port temperature, 280 °C; detector temperature, 280 °C; oven temperature program: initially the temperature was maintained at 32 °C for 5 min, then increased to 45 °C at the rate of 3 °C min−1, and finally to 245 °C at the rate of 50 °C min−1 and maintained for 5 min. The internal standard method was adopted as a quantitative method and octane was used as the internal standard.
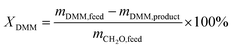
To estimate the conversion of feedstocks and the yield and selectivity of PODEn, the calculation formulas were employed as follows: DMM conversion:
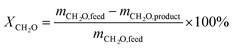
TOX conversion:
PODEn yield:
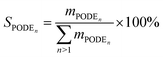
PODEn selectivity:
3. Results and discussion
3.1. Structural features of the catalysts
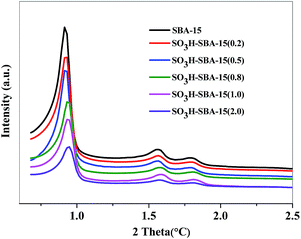
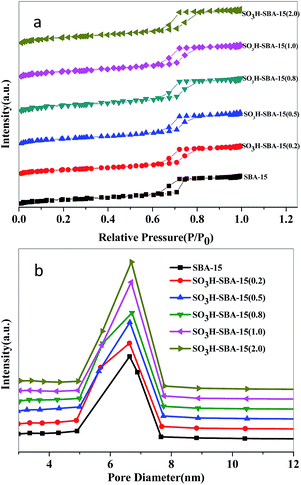
The small angle XRD patterns of SBA-15 and SO3H-SBA-15 with different sulfur loading are shown in Fig. 1. All the samples have three well-resolved diffraction peaks at about 0.92°, 1.55°, and 1.77°, corresponding to the (100), (110), and (200) reflections, respectively, which can be identified as typical two-dimensional hexagonal mesostructures (space group p6mm). XRD patterns for SO3H-SBA-15 catalysts did not change after the grafting process, evidencing that the two-dimensional hexagonal mesostructure was stable during the functionalization process.23The N2 adsorption–desorption isotherms and pore size distribution of SBA-15 and SO3H-SBA-15 are shown in Fig. 2. It can be seen that both the SBA-15 and SO3H-SBA-15 catalysts show type IV isotherm with H1 hysteresis capillary at 0.65 < P/P0 < 0.80 due to the presence of mesoporous structure and have narrow pore size distribution, which centralizes at about 6.5 nm.
 | ||
| Fig. 2 N2 adsorption–desorption isotherms (a) and pore size distribution (b) of SBA-15 and SO3H-SBA-15. | ||
The physical properties of SO3H-SBA-15 catalysts are shown in Table 1. The specific surface and pore volume and pore diameter of SBA-15 are 747 m2 g−1, 1.04 cm3 g−1, and 5.92 nm, respectively, and the surface area, pore volume, and pore sizes of the as-synthesized materials gradually decreased with the increase of sulfur loading, indicating that the grafting reactions not only proceeded on the surface of the catalyst, but also occurred inside of the pore and caused a certain degree of plugging phenomenon. The sulfur loadings of SO3H-SBA-15 catalysts, which are shown in Table 1, were determined by elemental analysis and further confirmed via XRF detection. It can be seen that the sulfur contents on the surface of SBA-15 supports gradually increased with the increase of silane concentration, and the actual measured sulfur contents are always lower than that of the feeding amount used for the grafting, which is consistent with a previous report.31 Acid amounts of SO3H-SBA-15 catalysts with different sulfur loading are shown in Table 1. The acid amounts are proportional to the sulfur content of the catalyst and gradually increased from 0.056 to 0.443 mmol g−1.
| Catalyst | Specific surface area m2 g−1 | Pore volume cm3 g−1 | Pore diameter nm | S contenta wt% | S contentb wt% | Amount of acid sites mmol g−1 |
|---|---|---|---|---|---|---|
| a S content detected from the elemental analysis.b Calculated by XRF. | ||||||
| SBA-15 | 747 | 1.04 | 5.92 | — | — | — |
| SO3H-SBA-15(0.2) | 658 | 1.02 | 5.89 | 0.18 | 0.16 | 0.056 |
| SO3H-SBA-15(0.5) | 639 | 0.98 | 5.86 | 0.42 | 0.43 | 0.132 |
| SO3H-SBA-15(0.8) | 625 | 0.95 | 5.85 | 0.53 | 0.54 | 0.166 |
| SO3H-SBA-15(1.0) | 618 | 0.92 | 5.81 | 0.84 | 0.89 | 0.262 |
| SO3H-SBA-15(2.0) | 609 | 0.92 | 5.79 | 1.42 | 1.45 | 0.443 |
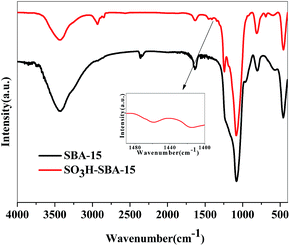
Infrared spectrum detection was used as a qualitative method to identify whether silane was successfully grafted on the silica surface.31,34 The strong and wide absorption band at 1093 cm−1 can be attributed to the Si–O–Si antisymmetric stretching vibration (Fig. 3). The bands at 809 cm−1 and 460 cm−1 are assigned to the Si–O stretching vibration and bending vibration, respectively. The peak at 958 cm−1 belongs to the bending vibration absorption peaks of the Si–OH bond. The attachment of silane on the silica surface was identified by the bands at 2940 cm−1 and 2854 cm−1, which are assigned to the asymmetric and symmetric stretching peaks of methylene, and the band at 1460 cm−1 can be attributed to the methylene symmetric bending vibration.
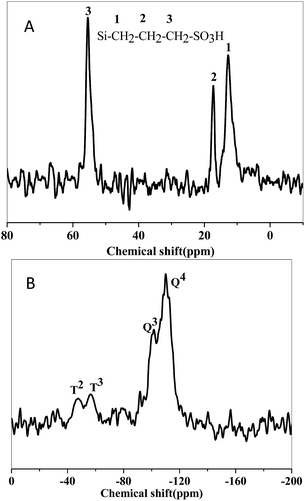
The incorporation of silane on the silica surface was further confirmed by 29Si MAS NMR and 13C-MAS NMR.31,35–37 The 13C MAS NMR spectra of SO3H-SBA-15(0.8) (Fig. 4(A)) exhibit the signals at 12.77, 17.52, and 55.39 ppm, which are assigned to the C1, C2, and C3 carbon peaks, respectively. No peak can be found at about 23 and 41 ppm, indicating that no disulphide was formed during the preparation. Fig. 4(B) shows the 29Si MAS NMR spectra of SO3H-SBA-15(0.8). The two distinct resonances at −112 ppm and −103 ppm refer to Q4 [Si(OSi)3] and Q3 [HOSi(OSi)3], respectively. The spectrum of SO3H-SBA-15(0.8) shows another two resonances at −67 ppm and −59 ppm, which illustrate that the Si–C bonds were successfully generated, and the organic functional groups were incorporated into the frameworks.
3.2. Catalytic performance
The catalytic performance of SO3H-SBA-15 catalysts with different sulfur loading for the synthesis of PODEn is presented in Fig. 5. There is a significant influence of the sulfur loading on the conversion rate of feedstock DMM and TOX and the selectivity and yield of main product PODE2–8. It can be seen from Fig. 5a that the conversion of DMM and TOX and the yield of PODE2–8 first increased and then decreased when the sulfur loading increased from 0.2 to 2 wt% and reached maximum values of 51.84, 95.57, and 61.86%, respectively, under the catalysis of SO3H-SBA-15(0.8). In addition, as shown in Fig. 5b, with the increase of sulfur loading, the selectivity of by-product methyl formate gradually increased and reached its highest value under the catalysis of SO3H-SBA-15(2.0), which could be attributed to the highest acid amount of SO3H-SBA-15(2.0), as illustrated in Table 1. Therefore, the SO3H-SBA-15(0.8) catalyst has the best catalytic performance for PODEn synthesis.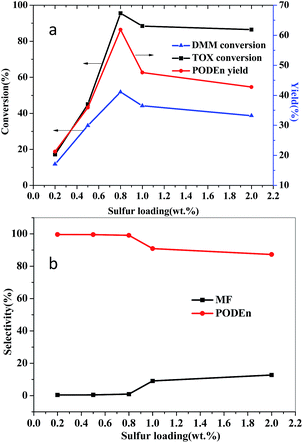 | ||
| Fig. 5 Catalytic activity of the catalysts with different sulfur loading. Reaction conditions: n(DMM)/n(TOX) = 1; catalyst loading: 2%; 100 °C; 60 min; 1 MPa. | ||
It is well known that the production process of PODEn can be roughly summarized as two steps: depolymerization of trioxymethylene into formaldehyde monomer and condensation reaction of formaldehyde monomer with DMM and PODEn−1. Moreover, the excessive formaldehyde monomer in the acidic environment will cause the generation of the by-product methyl formate in a Tischenko reaction.38 It was reported18 that the trioxymethylene dissociation process was a crucial step and depolymerization and transformation situation of trioxymethylene was related with the catalyst acidity, which directly affected the further condensation reaction and side reaction. Fig. 6 shows the reaction and depolymerization tendency of TOX in the presence of catalysts with different acid amounts, which can clearly explain the reaction rules. It can be seen that under the catalysis of SO3H-SBA-15(0.2) and SO3H-SBA-15(0.5) catalysts, which have relatively weak acidity, trioxymethylene was insufficiently depolymerized; therefore, the corresponding the concentration of trioxymethylene, which was converted to the main product PODE2–8 and byproduct MF, was relatively low. Under the catalysis of SO3H-SBA-15(0.8), which has moderate acidity, the undecomposed trioxymethylene in the product reached a minimum value and at the same time the depolymerized trioxymethylene, which was converted to PODEn, reached a maximum value. With the further increase of catalyst acidity, the depolymerized trioxymethylene, which was converted to the byproduct methyl formate, obviously increased, indicating that the depolymerization rate of trioxymethylene is far greater than its condensation rate with methylal in the presence of the catalyst with large amounts of acid, and accordingly, the excessive formaldehyde monomer generates methyl formate. Moreover, the trioxymethylene content in the product also increased under the catalysis of SO3H-SBA-15(1.0) and SO3H-SBA-15(2.0), illustrating that the excessive amount of acid will not only promote the occurrence of the side reaction, but also inhibits the depolymerization reaction of trioxymethylene, or accelerates the repolymerization of formaldehyde monomers into trioxymethylene. It can be concluded that the depolymerization of TOX requires a relatively moderate acid amount as an insufficient or excessive amount of acid will lead to incomplete depolymerization.
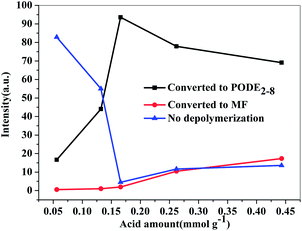 | ||
| Fig. 6 Conversion and depolymerization tendency of TOX in the presence of catalysts with different acid amounts. | ||
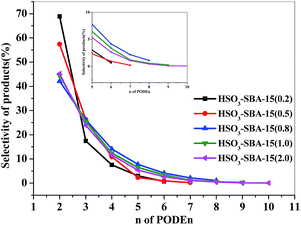
PODEn with the structure of CH3O–(CH2O)n–CH3 is a mixture of different degrees of polymerization, and with the increase of the degree of polymerization, the cetane number, which is one of the most important indexes for diesel oil, gradually improve.1 However, a high degree of polymerization (n > 8) will cause the increase of pour point and the cold filter plugging point of the mixture, which is not suitable to be used as a diesel fuel blending component and will need to be returned to the synthetic unit. Moreover, the PODE2 component does not meet the security criterion of the diesel fuel due to its low boiling point and low flash point; therefore, it cannot be used as a diesel blending component either. Thus, among PODEn products, relatively more PODE3–8 should be generated, and PODE2 and PODEn>8 components should be largely suppressed. The effects of sulfur loading on the product distribution and chain length are shown in Fig. 7. It can be clearly seen that the selectivity of PODEn with different polymerization and chain length obviously varied with the change of sulfur loading. With the increase of sulfur loading, the selectivity of PODE2–8 increased first and then decreased, and on the SO3H-SBA-15(0.8) catalyst, the selectivity of PODE3–8 reached the highest value among all the catalysts. In addition, the maximum chain length of PODEn obviously increased with the increase of sulfur loading. Under the catalysis of SO3H-SBA-15(0.2) catalyst, only PODE2–6 were generated and the product with the polymerization degree of n > 7 was not formed. On the SO3H-SBA-15(0.5) catalyst, PODE7 can be detected, and furthermore, PODE10 was generated under the catalysis of SO3H-SBA-15(2.0). Fortunately, only the desired components PODE3–8 were formed under the catalysis of SO3H-SBA-15(0.8). This result fully explains that the acidity of the catalysts also has a decisive effect on the product distribution and the chain length of the products. Thus, it is also an effective way to obtain the desired product distribution by adjusting the acidity of the catalyst.
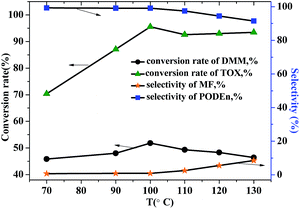
From a thermodynamic point of view, the ring opening depolymerization reaction of trioxymethylene is endothermic; thus, a relatively higher temperature is in favor of the depolymerization of trioxymethylene. However, the condensation reaction of formaldehyde and methylal is conversely a slightly exothermic reaction. Thus, it is very important to select an appropriate temperature to achieve the maximum efficiency of the reaction. The influence of temperature on the conversion rate of DMM and TOX and selectivity of PODEn and byproduct is shown in Fig. 8. With the increase of temperature, the conversion rate of TOX gradually increased, indicating that a higher temperature will accelerate the depolymerization process of TOX. A relatively low temperature will lead to the incomplete depolymerization of TOX. However, the selectivity of the byproduct MF also increased with the increase of temperature, especially after a temperature greater than 100 °C. This is probably due to the fact that the depolymerization rate of trioxymethylene is far greater than its condensation rate with methylal at high temperature; thus, the competitive reaction of the formation of the byproduct methyl formate is greatly promoted. Therefore, considering the feedstock conversion rate and product selectivity, 100 °C is the most appropriate temperature for the PODEn synthesis.
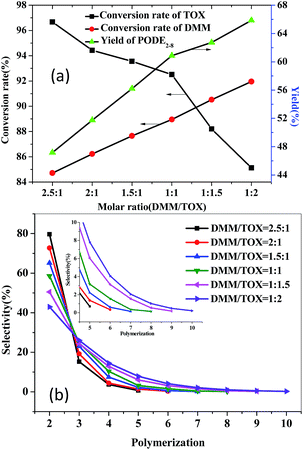
The molar ratio of feedstocks has a great influence on the conversion rate of the feedstocks and the yield of the products. It can be seen that increasing the amount of trioxymethylene is beneficial for enhancing the yield of PODE2–8 (Fig. 9a) and improving the conversion rate of DMM; however, the conversion rate of TOX was gradually reduced due to the limit of the equilibrium of the reaction. In addition, as shown in Fig. 9b, the product distribution and chain length obviously varied with the change of the molar ratio of DMM and TOX. With the increase of TOX proportion, the selectivity of PODE2 decreased, whereas the selectivity of PODE3–8 gradually increased, indicating that increasing the amount of TOX is in favour of promoting the reaction to generate products with a higher degree of polymerization. When the DMM/TOX molar ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, the product with the chain length of 10 was generated. With the increase of DMM proportion, the high degree of polymerization selectivity gradually decreased, and when the molar ratio of DMM/TOX reached 1
2, the product with the chain length of 10 was generated. With the increase of DMM proportion, the high degree of polymerization selectivity gradually decreased, and when the molar ratio of DMM/TOX reached 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, no product of n > 8 was formed. Therefore, to avoid the generation of products with a high degree of polymerization, a relatively large proportion of methylal was required. Although with the change of the DMM/TOX molar ratio from 2
1, no product of n > 8 was formed. Therefore, to avoid the generation of products with a high degree of polymerization, a relatively large proportion of methylal was required. Although with the change of the DMM/TOX molar ratio from 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, the yield of PODE2–8 gradually decreased, whereas the conversion rate of TOX significantly increased. Thus, a relatively large proportion of methylal will not only inhibit the generation of high degree of polymerization products, but also greatly improves the conversion rate of TOX. Therefore, to avoid the generation of a high degree of polymerization products, it is necessary to lose a certain yield of PODE2–8. Considering the yield of PODEn and conversion of TOX under the premise of no generation of PODEn in which n > 8, 1
2, the yield of PODE2–8 gradually decreased, whereas the conversion rate of TOX significantly increased. Thus, a relatively large proportion of methylal will not only inhibit the generation of high degree of polymerization products, but also greatly improves the conversion rate of TOX. Therefore, to avoid the generation of a high degree of polymerization products, it is necessary to lose a certain yield of PODE2–8. Considering the yield of PODEn and conversion of TOX under the premise of no generation of PODEn in which n > 8, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 is the optimum molar ratio of DMM and TOX in the reaction system.
1 is the optimum molar ratio of DMM and TOX in the reaction system.
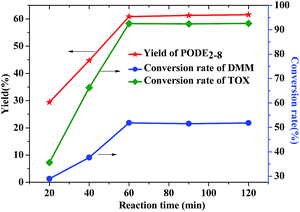
The yield of PODEn and the conversion rate of DMM and TOX all have a significant increase in the reaction for the first 60 minutes, which is shown in Fig. 10. Because the depolymerization process of TOX is relatively slow, a certain period of time is required to achieve the balance of trioxymethylene depolymerization. However, when the reaction time is prolonged to 90 min and 120 min, the conversion rate of feedstocks and the yield of the products are essentially flat compared with those at 60 min, which shows that the reaction system has basically reached chemical equilibrium and cannot be promoted by extending time. Thus, 60 min is the most appropriate time for PODEn synthesis.
The catalytic performance of SO3H-SBA-15(0.8) catalyst was further evaluated by comparing with the reference catalysts such as Amberlyst-15 sulfonic acid resin, ZSM-5 zeolite with Si/Al ratio of 200, and HY zeolite with the Si/Al ratio of 5.3. It can be seen from Fig. 11 that the conversion rate of TOX and the PODEn yield and selectivity under the catalysis of SO3H-SBA-15(0.8) are obviously higher than those of Amberlyst-15 sulfonic acid resin, and ZSM-5 and HY zeolite. One reason for the better performance of the SO3H-SBA-15(0.8) catalyst is attributed to the enhanced mesoporosity, which would overcome the diffusion limitation and promote the transformation of the reactants and products in the two-dimensional hexagonal mesostructure. Another more important reason is probably ascribed to the moderate acidity of the SO3H-SBA-15(0.8) catalyst. We know that the depolymerization of TOX requires a relatively moderate acid amount; insufficient or excessive amount of acid will promote the formation of the by-product methyl formate and lead to the incomplete depolymerization of TOX, thus affecting the conversion of TOX and yield of PODEn. There is a relatively high acid amount in the pores of Amberlyst-15 sulfonic acid resin, ZSM-5 zeolite, and HY zeolite, which leads to the far greater depolymerization rate of TOX than its condensation rate with methylal; therefore, a large number of methyl formate is generated and the depolymerization of TOX is suppressed.
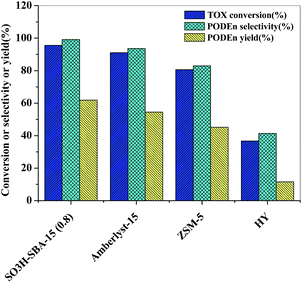 | ||
| Fig. 11 Comparison of SO3H-SBA-15(0.8), Amberlyst-15, ZSM-5 zeolite, and HY zeolite for PODEn synthesis. Reaction conditions: n(DMM)/n(TOX) = 1; catalyst loading: 2%; 100 °C; 1 MPa. | ||
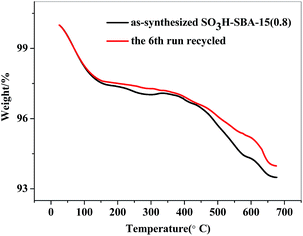
The catalytic stability of the as-synthesized catalyst, which is an important index for selecting the catalysts, was evaluated in the PODEn synthesis. SO3H-SBA-15(0.8) catalyst was used to evaluate the reusability. After completing a catalytic reaction, SO3H-SBA-15(0.8) was separated from the reaction system by filtration and was dried in vacuum at 60 °C for 12 h and then used for the next reaction. As illustrated in Fig. 12, the activity of the catalysts only slightly declined after being reused six times; the conversion rate of TOX decreased from 95.57% to 93.09%, and the yield of PODE2–8 also decreased from 61.86% to 59.13%. Moreover, the as-synthesized and the 6th run recycled SO3H-SBA-15(0.8) catalyst were characterized by elemental analysis and further verified by TGA (Fig. 13), showing that the sulfur content of the catalyst slightly reduced from 0.53% to 0.51%.
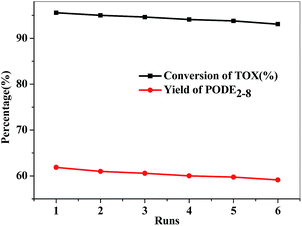 | ||
| Fig. 12 Recycling experiment of SO3H-SBA-15(0.8) catalyst. Reaction conditions: n(DMM)/n(TOX) = 1; catalyst loading: 2%; 100 °C; 1 MPa; 60 min. | ||
4. Conclusion
Herein, SO3H-SBA-15 catalysts with different sulfur loading were successfully prepared for the synthesis of PODEn from DMM and TOX. In the study of the effect of different sulfur loading (acid property) on the catalytic performance, it was found that a moderate amount of acid was required for PODEn synthesis, as proven by SO3H-SBA-15(0.8) catalyst, which showed the best catalytic performance, in that the conversion rate of DMM and TOX and the yield of PODE2–8 reached high values of 51.84, 95.57, and 61.86%, respectively. Inadequate or excessive amounts of acid will lead to the incomplete depolymerization of trioxymethylene. Moreover, the excessive amount of acid can cause the formation of a large number of by-product, methyl formate. In addition, the acidity of the catalysts also had a decisive effect on the product distribution and the chain length of the products. The optimum reaction conditions were investigated; through the comparison of reaction temperature, it can be concluded that a relatively low temperature ranging from 70 to 90 °C will lead to the incomplete depolymerization of trioxymethylene, and a higher temperature ranging from 110 to 130 °C will accelerate the depolymerization process of TOX and greatly promote the formation of the by-product methyl formate; thus, 100 °C was the most appropriate temperature. By adjusting the molar ratio of DMM and TOX, it was found that an increasing amount of TOX was in favor of improving the yield of PODEn and promoting the generation of the products with a higher degree of polymerization. Considering a higher product yield and reasonable product chain length, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was the optimum molar ratio of DMM and TOX, at which no PODEn>8 was generated. The reaction time analysis demonstrated that the reaction system had basically reached chemical equilibrium within 60 min and the reaction could not be promoted by further extending time. Moreover, according to the comparison of SO3H-SBA-15(0.8) catalyst and the reference catalysts, it can be concluded that the SO3H-SBA-15(0.8) catalyst had better catalytic activity than Amberlyst-15 sulfonic acid resin, and ZSM-5 and HY zeolite for PODEn synthesis. The activity of SO3H-SBA-15(0.8) catalyst just slightly declined after being reused six times, showing good application prospects.
1 was the optimum molar ratio of DMM and TOX, at which no PODEn>8 was generated. The reaction time analysis demonstrated that the reaction system had basically reached chemical equilibrium within 60 min and the reaction could not be promoted by further extending time. Moreover, according to the comparison of SO3H-SBA-15(0.8) catalyst and the reference catalysts, it can be concluded that the SO3H-SBA-15(0.8) catalyst had better catalytic activity than Amberlyst-15 sulfonic acid resin, and ZSM-5 and HY zeolite for PODEn synthesis. The activity of SO3H-SBA-15(0.8) catalyst just slightly declined after being reused six times, showing good application prospects.
Acknowledgements
This work was supported by the National Key Technology R&D Program (No. 2013BAB11B03).Notes and references
- J. Burger, M. Siegert, E. Ströfer and H. Hasse, Fuel, 2010, 89, 3315–3319 CrossRef CAS.
- Y. S. Lei, Q. Chen and Z. Shen, Acta Chim. Sin., 2009, 67, 767–772 CAS.
- H. Liu, Z. Wang, J. Wang, X. He, Y. Zheng, Q. Tang and J. Wang, Energy, 2015, 88, 793–800 CrossRef CAS.
- J. Liu, H. Wang, Y. Li, Z. Zheng, Z. Xue, H. Shang and M. Yao, Fuel, 2016, 177, 206–216 CrossRef CAS.
- Y. Li, L. Meng, K. Nithyanandan, T. H. Lee, Y. Lin, C.-f. F. Lee and S. Liao, Fuel, 2016, 184, 864–872 CrossRef CAS.
- L. Pellegrini, M. Marchionna, R. Patrini, C. Beatrice, N. Del Giacomo and C. Guido, Combustion Behaviour and Emission Performance of Neat and Blended Polyoxymethylene Dimethyl Ethers in a Light-Duty Diesel Engine, SAE Paper 2012-01-1053, 2012.
- L. Wang, W.-T. Wu, T. Chen, Q. Chen and M.-Y. He, Chem. Eng. Commun., 2014, 201, 709–717 CrossRef CAS.
- Y. Zheng, Q. Tang, T. Wang, Y. Liao and J. Wang, Chem. Eng. Technol., 2013, 36, 1951–1956 CrossRef CAS.
- J. Burger, E. Ströfer and H. Hasse, Ind. Eng. Chem. Res., 2012, 51, 12751–12761 CrossRef CAS.
- Q. Zhao, H. Wang, Z.-F. Qin, Z.-W. Wu, J.-B. Wu, W.-B. Fan and J.-G. Wang, J. Fuel Chem. Technol., 2011, 39, 918–923 CrossRef CAS.
- J. Wu, H. Zhu, Z. Wu, Z. Qin, L. Yan, B. Du, W. Fan and J. Wang, Green Chem., 2015, 17, 2353–2357 RSC.
- F. Wang, G. Zhu, Z. Li, F. Zhao, C. Xia and J. Chen, J. Mol. Catal. A: Chem., 2015, 408, 228–236 CrossRef.
- Q. Wu, M. Wang, Y. Hao, H. Li, Y. Zhao and Q. Jiao, Ind. Eng. Chem. Res., 2014, 53, 16254–16260 CrossRef CAS.
- Y. Wu, Z. Li and C. Xia, Ind. Eng. Chem. Res., 2016, 55, 1859–1865 CrossRef CAS.
- X. Fang, J. Chen, L. Ye, H. Lin and Y. Yuan, Sci. China: Chem., 2014, 58, 131–138 CrossRef.
- J. Zhang, D. Fang and D. Liu, Ind. Eng. Chem. Res., 2014, 53, 13589–13597 CrossRef CAS.
- R. Wang, Z. Wu, Z. Qin, C. Chen, H. Zhu, J. Wu, G. Chen, W. Fan and J. Wang, Chemcatchem, 2014, 6, 3080–3083 CrossRef.
- H. Li, H. Song, L. Chen and C. Xia, Appl. Catal., B, 2015, 165, 466–476 CrossRef CAS.
- E. Casas, B. Paredes, R. Van Grieken and J. M. Escola, Catal. Sci. Technol., 2013, 3, 2565–2570 CAS.
- E. Casas, R. van Grieken and J. M. Escola, Appl. Catal., A, 2012, 437–438, 44–52 CrossRef CAS.
- S.-Y. Chen, T. Yokoi, C.-Y. Tang, L.-Y. Jang, T. Tatsumi, J. C. C. Chan and S. Cheng, Green Chem., 2011, 13, 2920 RSC.
- J. Dhainaut, J.-P. Dacquin, A. F. Lee and K. Wilson, Green Chem., 2010, 12, 296–303 RSC.
- J. C. Manayil, C. V. M. Inocencio, A. F. Lee and K. Wilson, Green Chem., 2016, 18, 1387–1394 RSC.
- W. W. M. A. E. Somsook, J. Oleo Sci., 2013, 62, 435–442 CrossRef.
- I. Mbaraka, J. Catal., 2003, 219, 329–336 CrossRef CAS.
- I. Mbaraka and B. Shanks, J. Catal., 2005, 229, 365–373 CrossRef CAS.
- I. Kim, J. Kim and D. Lee, Appl. Catal., B, 2014, 148–149, 295–303 CrossRef CAS.
- D. Macina, Z. Piwowarska, K. Tarach, K. Góra-Marek, J. Ryczkowski and L. Chmielarz, Mater. Res. Bull., 2016, 74, 425–435 CrossRef CAS.
- Z. Xue, H. Shang, Z. Zhang, C. Xiong, C. Lu and G. An, Energy Fuels, 2017, 31, 279–286 CrossRef CAS.
- J. F. D. Zhao, Q. Huo, G. H. F. N. Melosh, B. F. Chmelka and G. D. Stucky, Science, 1998, 279, 548–552 CrossRef PubMed.
- S. Shylesh, S. Sharma, S. P. Mirajkar and A. P. Singh, J. Mol. Catal. A: Chem., 2004, 212, 219–228 CrossRef CAS.
- M. Breugst, R. Gree and K. N. Houk, J. Org. Chem., 2013, 78, 9892–9897 CrossRef CAS PubMed.
- J. S. Choi, D. J. Kim, S. H. Chang and W. S. Ahn, Appl. Catal., A, 2003, 254, 225–237 CrossRef CAS.
- J.-H. Won, H.-J. Lee, K.-S. Yoon, Y. T. Hong and S.-Y. Lee, Int. J. Hydrogen Energy, 2012, 37, 9202–9211 CrossRef CAS.
- M. Guan, W. Liu, Y. Shao, H. Huang and H. Zhang, Microporous Mesoporous Mater., 2009, 123, 193–201 CrossRef CAS.
- H. Jin, M. B. Ansari and S.-E. Park, Catal. Today, 2015, 245, 116–121 CrossRef CAS.
- B. Rác, P. Hegyes, P. Forgo and Á. Molnár, Appl. Catal., A, 2006, 299, 193–201 CrossRef.
- J. Burger, E. Ströfer and H. Hasse, Chem. Eng. Res. Des., 2013, 91, 2648–2662 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |