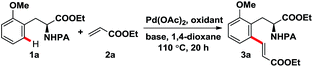Open Access Article
Open Access ArticlePalladium(II)-catalyzed ortho-C–H olefination of phenylalanine and phenylethylamine derivatives directed by removable picolinamide group†
Fei Zhao *a,
Xiuwen Jiaa,
Jingwei Zhaoa,
Chaoli Feia,
Liyang Liua,
Guannan Liub,
Dongping Wanga and
Fei Chena
*a,
Xiuwen Jiaa,
Jingwei Zhaoa,
Chaoli Feia,
Liyang Liua,
Guannan Liub,
Dongping Wanga and
Fei Chena
aAntibiotics Research and Re-evaluation Key Laboratory of Sichuan Province, Sichuan Industrial Institute of Antibiotics, Chengdu University, 168 Hua Guan Road, Chengdu 610052, P. R. China. E-mail: zhaofei@cdu.edu.cn
bCollege of Life Sciences, China Jiliang University, Hangzhou 310018, Zhejiang, P. R. China
First published on 10th May 2017
Abstract
Palladium-catalyzed ortho-C–H olefination of phenylalanine and phenylethylamine derivatives assisted by a removable picolinamide group has been achieved. This protocol shows broad substrate scope, functional tolerance and moderate to high yields, thus affording a practical approach for the direct modification of phenylalanine and phenylethylamine derivatives.
Introduction
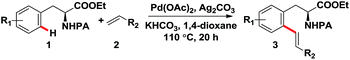
Transition-metal-catalyzed direct C–H functionalization has captured wide attention because of its high efficiency in constructing carbon–carbon and carbon–heteroatom bonds,1,2 and great progress has been made in C–H activation reactions with the assistance of various directing groups which can enhance the reactivity and achieve site selectivity in recent decades.3,4 In particular, C–H functionalization of nitrogen-containing compounds, such as amino acid derivatives, is especially important and attractive considering their prevalence in natural products and therapeutic agents. Moreover, functionalized amino acid derivatives have also been widely used as building blocks in medicinal chemistry for their broad biological activities.5 Therefore, the synthesis of these structures is of great importance. Compared with the conventional de novo synthesis strategy, the direct functionalization of C–H bonds of various readily available amino acid derivatives generates a much more straightforward and convenient access to functionalized amino acid derivatives, providing a potential protocol for the direct modification of amino acid derivatives. Despite the remarkable achievements made in the C–H functionalization of amino acid derivatives in recent years,6 reports on the direct olefination of phenylalanine derivatives at the ortho-position are still rare, and only one kind of directing group, namely sulfonyl amide, was employed successfully to achieve the ortho-C–H olefination of phenylalanine derivatives (Scheme 1a).7 Although pioneering works reported by Yu's group in 2008 realized the ortho-alkenylation of phenylalanine derivatives assisted by a trifluoro-sulfonamide group, this reaction suffered from the disadvantages of moderate yields, long reaction time and limited examples.7a Very recently, the ortho-olefination of phenylalanine derivatives was also developed using N-methyl-N-(2-pyridyl)sulfonyl as the directing group by Carretero's group, but only limited to electron-deficient alkenes.7b It is noteworthy that both reported methods employed sulfonyl amide as the directing group. In the present work, we report the palladium-catalyzed ortho-olefination of phenylalanine derivatives directed by removable picolinamide group with various electron-deficient and electron-rich alkenes in moderate to high yields (Scheme 1b), thus generating a practical strategy for the direct modification of phenylalanine derivatives to produce olefinated phenylalanine derivatives as potential building blocks for drug discovery. Remarkably, our approach was also compatible with phenylethylamine derivatives, which further broadens the substrate scope and highlights the synthetic utility of this methodology. Our method provides an alternative strategy and additional complementarity to the preexisting methods for the ortho-olefination of phenylalanine and phenylethylamine derivatives.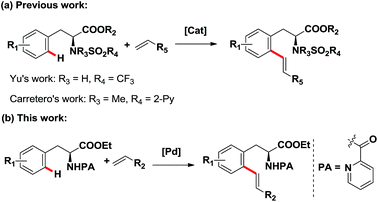 | ||
| Scheme 1 ortho-C–H Olefination of phenylalanine derivatives directed by sulfonyl amide and picolinamide groups. | ||
Results and discussion
Initial screening experiments were performed employing 1a and 2a as the model substrates to optimize the reaction conditions (Table 1). We tested the reaction of 1a and 2a with Pd(OAc)2 as catalyst in 1,4-dioxane at 110 °C for 20 h under an atmosphere of air at first, and the desired product 3a was obtained only in 4% yield with the starting materials recovered (entry 1). Pleasingly, 3a was achieved with a moderate yield (42%) when oxygen was employed as the oxidant (entry 2). It is worth noting that the addition of KHCO3 as base can significantly improve the yield in the presence of oxidant (entry 3), this might be because KHCO3 can accelerate the deprotonation process to promote this reaction. Subsequently, several common oxidants such as CuCl2, Cu(OAc)2, PhI(OAc)2, K2S2O8, DDQ and BQ were screened, however, none of them gave better yield than oxygen (entries 4–9). A further screening of the oxidants revealed that employment of silver salts such as AgNO3, AgSO3CF3, AgOAc, AgCO2CF3, Ag2O and Ag2CO3 generally can afford 3a in moderate to high yields (entries 10–15), and four of them (AgOAc, AgCO2CF3, Ag2O and Ag2CO3) gave higher yield than oxygen (entries 12–15). Among the silver salts screened, Ag2CO3 was found to be the most efficient oxidant for this transformation with 89% yield (entry 15). In addition, an exploration of the bases discovered that KHCO3 was the most suitable base for this reaction (entries 15–19). Besides, control experiments showed that Pd(OAc)2 played a crucial and indispensable role in this ortho-C–H olefination reaction (entry 20). In this way, the optimal reaction conditions were identified using the catalytic system of Pd(OAc)2/Ag2CO3/KHCO3 in 1,4-dioxane at 110 °C for 20 h.| Entry | Catalyst | Oxidant | Base | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.3 mmol), 2a (0.6 mmol, 2 equiv.), Pd(OAc)2 (0.03 mmol, 10 mol%), oxidant (0.6 mmol, 2 equiv.), and base (0.6 mmol, 2 equiv.) in 1,4-dioxane (2.0 ml) at 110 °C for 20 h in a 25 ml sealed tube.b Isolated yield.c No reaction. | ||||
| 1 | Pd(OAc)2 | — | — | 4 |
| 2 | Pd(OAc)2 | 1 atm O2 | — | 42 |
| 3 | Pd(OAc)2 | 1 atm O2 | KHCO3 | 68 |
| 4 | Pd(OAc)2 | CuCl2 | KHCO3 | 7 |
| 5 | Pd(OAc)2 | Cu(OAc)2 | KHCO3 | 36 |
| 6 | Pd(OAc)2 | PhI(OAc)2 | KHCO3 | Trace |
| 7 | Pd(OAc)2 | K2S2O8 | KHCO3 | 32 |
| 8 | Pd(OAc)2 | DDQ | KHCO3 | Trace |
| 9 | Pd(OAc)2 | BQ | KHCO3 | 67 |
| 10 | Pd(OAc)2 | AgNO3 | KHCO3 | 41 |
| 11 | Pd(OAc)2 | AgSO3CF3 | KHCO3 | 54 |
| 12 | Pd(OAc)2 | AgOAc | KHCO3 | 72 |
| 13 | Pd(OAc)2 | AgCO2CF3 | KHCO3 | 81 |
| 14 | Pd(OAc)2 | Ag2O | KHCO3 | 83 |
| 15 | Pd(OAc)2 | Ag2CO3 | KHCO3 | 89 |
| 16 | Pd(OAc)2 | Ag2CO3 | K3PO4 | 78 |
| 17 | Pd(OAc)2 | Ag2CO3 | NaHCO3 | 80 |
| 18 | Pd(OAc)2 | Ag2CO3 | Na2CO3 | 82 |
| 19 | Pd(OAc)2 | Ag2CO3 | K2CO3 | 76 |
| 20 | — | Ag2CO3 | KHCO3 | NRc |
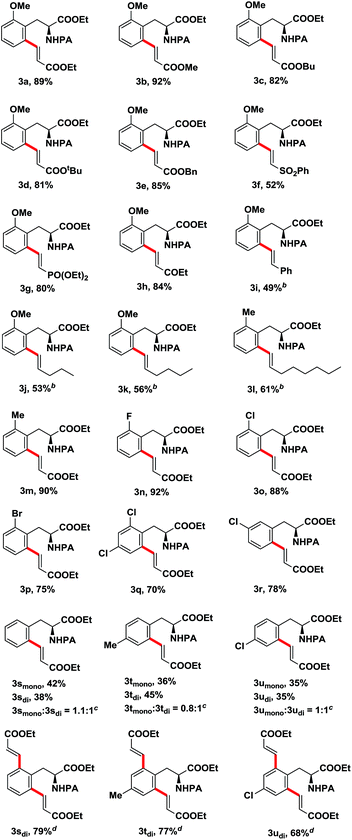
After determining the optimal reaction conditions, we then examined the general applicability of the process. In general, the olefination process tolerated a variety of phenylalanine derivatives with various substituents at R1 and alkenes with electron-donating or electron-withdrawing groups at R2, and furnished the desired products in moderate to high yields (Table 2). The reactions of substrate 1a bearing a methoxy group in the ortho-position with different acrylates afforded the alkenylated products in high yields (3a–3e). Except for acrylates, other activated alkenes, such as vinyl sulfone (3f), vinyl phosphate (3g) and vinyl ketone (3h), could undergo this transformation with moderate to high yields. Notably, unactivated alkenes such as styrene (3i), 1-pentene (3j), 1-hexene (3k) and 1-octene (3l) were also suitable for this reaction, furnishing the corresponding products in moderate yields. Subsequently, the scope of phenylalanine derivatives was explored. Substrates carrying a substituent (Me, F, Cl, Br) in the ortho-position reacted smoothly and produced the corresponding products in good to high yields (3m–3q). When meta-substituted substrate was employed, the C–H olefination reaction took place at the less sterically hindered position with good yield (3r). The reactions of unsubstituted or para-substituted phenylalanine derivatives afforded a separable mixture of mono- and diolefination products, and exclusive diolefination products were obtained in good yields by increasing the amounts of catalyst, alkene, oxidant and base (3s–3u), this may attribute to the transformation of monoolefination products which were produced in the reaction process into diolefination products when excessive reagents were used.
| a Reaction conditions: 1 (0.3 mmol), 2 (0.6 mmol, 2 equiv.), Pd(OAc)2 (0.03 mmol, 10 mol%), Ag2CO3 (0.6 mmol, 2 equiv.), and KHCO3 (0.6 mmol, 2 equiv.) in 1,4-dioxane (2.0 ml) at 110 °C for 20 h in a 25 ml sealed tube; isolated yields.b 2 (1.2 mmol, 4 equiv.), 48 h.c The ratio of mono- and diolefination products was determined by 1HNMR.d 2a (0.9 mmol, 3 equiv.), Pd(OAc)2 (0.045 mmol, 15 mol%), Ag2CO3 (0.9 mmol, 3 equiv.), and KHCO3 (0.9 mmol, 3 equiv.). |
|---|
 |
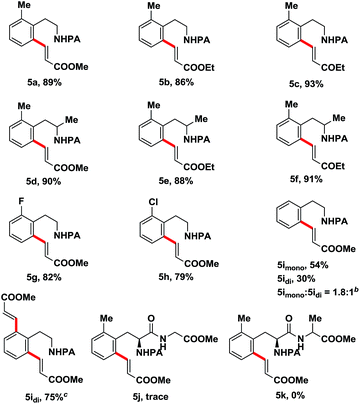
To further broaden the substrate scope of this process, phenylethylamine derivatives were tested as the reaction substrates. To our delight, this approach was also compatible with phenylethylamines (Table 3). The reactions of diverse substituted phenylethylamine derivatives with various alkenes proceeded smoothly under the optimal reaction conditions, affording the desired products in moderate to high yields (5a–5i). We also made our efforts to achieve the ortho-C–H olefination of peptides (5j–5k), albeit with unsuccessful results, this may attribute to the additional coordination of amide of the peptide bond with Pd(OAc)2 which inactivated the catalyst.
| a Reaction conditions: 4 (0.3 mmol), 2 (0.6 mmol, 2 equiv.), Pd(OAc)2 (0.03 mmol, 10 mol%), Ag2CO3 (0.6 mmol, 2 equiv.), and KHCO3 (0.6 mmol, 2 equiv.) in 1,4-dioxane (2.0 ml) at 110 °C for 20 h in a 25 ml sealed tube; isolated yields.b The ratio of mono- and diolefination products was determined by 1HNMR.c Methyl acrylate (0.9 mmol, 3 equiv.), Pd(OAc)2 (0.045 mmol, 15 mol%), Ag2CO3 (0.9 mmol, 3 equiv.), and KHCO3 (0.9 mmol, 3 equiv.). |
|---|
 |
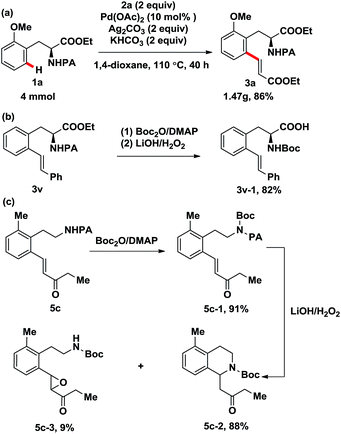
To further illustrate the practicality of this methodology, the C–H olefination reaction with 1a was carried out on a gram scale under optimal conditions. Impressively, the desired product 3a was achieved in 86% yield (Scheme 2a). In addition, the picolinamide directing group can be easily removed using the general and mature protocol reported by Chen (Scheme 2b).8 Besides, removal of the directing group of 5c and subsequent intramolecular cyclization gave the tetrahydroisoquinoine 5c-2 in 80% yield over two steps, interestingly, epoxide 5c-3 was obtained as the byproduct in extremely low yield (Scheme 2c). These aspects further highlight the advantages and potential application of this approach.
The plausible mechanism of this ortho-C–H olefination reaction was outlined in Scheme 3. Coordination of amides A to Pd(OAc)2 generates Pd(II) complex B, subsequent C–H bond activation at the ortho-position forms the Ar–Pd metastable intermediate C. Then, olefin coordination and 1,2-migratory insertion take place sequentially followed by β-H elimination, affording the olefination products F and a Pd(0) species. The reduced Pd(0) species was oxidized to Pd(II) oxidation state by Ag2CO3 to regenerate the Pd(II) catalyst. KHCO3 may play as a base which can accelerate the deprotonation process to promote this reaction.
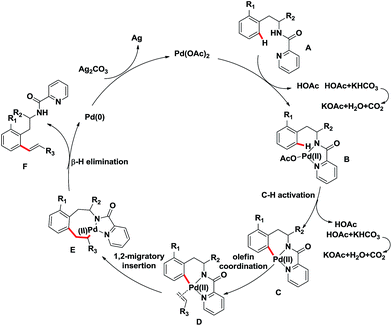 | ||
| Scheme 3 Proposed reaction mechanism of this ortho-C–H olefination of phenylalanine and phenylethylamine derivatives. | ||
Conclusions
In conclusion, we have developed an efficient and practical approach for the ortho-C–H olefination of phenylalanine and phenylethylamine derivatives with the assistance of removable picolinamide group. The broad substrate scope, functional tolerance, and moderate to high yields demonstrate the great potential of this method for the direct modification of phenylalanine and phenylethylamine derivatives. Other synthetic applications of this method are currently under investigation in our laboratory.Experimental section
General information
The reagents were purchased from commercial suppliers and used without further purification. Analytical thin-layer chromatography (TLC) was performed on HSGF 254 (0.15–0.2 mm thickness), visualized by irradiation with UV light (254 nm). Column chromatography was performed using silica gel FCP 200–300. Melting points were measured with a micro melting point apparatus. Nuclear magnetic resonance spectra were recorded on a Brucker AMX-400 or AMX-500 MHz instrument (TMS as IS). Chemical shifts were reported in parts per million (ppm, δ) downfield from tetramethylsilane. Proton coupling patterns were described as singlet (s), doublet (d), triplet (t), quartet (q), multiplet (m), and broad (br). Low- and high-resolution mass spectra (LRMS and HRMS) were measured on a spectrometer.Preparation and characterization data of compound 3
(S,E)-Ethyl-3-(2-(3-ethoxy-3-oxo-2-(picolinamido)propyl)-3-methoxyphenyl)acrylate (3a). White solid (113.9 mg, yield 89%), mp 125–126 °C. 1H NMR (400 MHz, CDCl3) δ 8.83 (d, J = 7.6 Hz, 1H), 8.53 (d, J = 4.7 Hz, 1H), 8.12–8.01 (m, 2H), 7.82–7.73 (m, 1H), 7.43–7.34 (m, 1H), 7.24–7.18 (m, 1H), 7.18–7.12 (m, 1H), 6.87 (d, J = 8.1 Hz, 1H), 6.30 (d, J = 15.7 Hz, 1H), 4.88–4.75 (m, 1H), 4.30–4.13 (m, 4H), 3.91 (s, 3H), 3.50–3.33 (m, 2H), 1.34 (t, J = 7.1 Hz, 3H), 1.27 (t, J = 7.0 Hz, 3H); 13C NMR (126 MHz, CDCl3) δ 171.57, 166.60, 164.36, 157.95, 149.57, 148.11, 141.42, 137.17, 135.56, 128.33, 126.23, 124.95, 122.28, 121.54, 119.31, 111.29, 61.47, 60.60, 55.75, 53.49, 27.97, 14.41, 14.19; LRMS (ESI): 427 [M + H]+; HRMS (ESI) calcd for C23H27N2O6 [M + H]+ 427.1869, found: 427.1866.
(S,E)-Methyl-3-(2-(3-ethoxy-3-oxo-2-(picolinamido)propyl)-3-methoxyphenyl)acrylate (3b). White solid (113.8 mg, yield 92%), mp 82–83 °C. 1H NMR (400 MHz, CDCl3) δ 8.79 (d, J = 7.5 Hz, 1H), 8.49 (d, J = 4.4 Hz, 1H), 8.10–7.97 (m, 2H), 7.77–7.69 (m, 1H), 7.38–7.31 (m, 1H), 7.20–7.14 (m, 1H), 7.13–7.06 (m, 1H), 6.84 (d, J = 8.0 Hz, 1H), 6.27 (d, J = 15.7 Hz, 1H), 4.86–4.72 (m, 1H), 4.23–4.08 (m, 2H), 3.87 (s, 3H), 3.76 (s, 3H), 3.46–3.30 (m, 2H), 1.24 (t, J = 6.9 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 171.47, 166.94, 164.28, 157.89, 149.44, 148.06, 141.66, 137.11, 135.38, 128.29, 126.19, 124.91, 122.19, 120.94, 119.19, 111.32, 61.40, 55.69, 53.40, 51.70, 27.91, 14.08; LRMS (ESI): 413 [M + H]+; HRMS (ESI) calcd for C22H25N2O6 [M + H]+ 413.1713, found: 413.1709.
(S,E)-Butyl-3-(2-(3-ethoxy-3-oxo-2-(picolinamido)propyl)-3-methoxyphenyl)acrylate (3c). Pale yellow oil (111.8 mg, yield 82%). 1H NMR (400 MHz, CDCl3) δ 8.81 (d, J = 7.6 Hz, 1H), 8.50 (d, J = 4.4 Hz, 1H), 8.11–7.98 (m, 2H), 7.79–7.68 (m, 1H), 7.41–7.30 (m, 1H), 7.20–7.08 (m, 2H), 6.84 (d, J = 7.9 Hz, 1H), 6.28 (d, J = 15.7 Hz, 1H), 4.84–4.73 (m, 1H), 4.23–4.09 (m, 4H), 3.88 (s, 3H), 3.47–3.30 (m, 2H), 1.71–1.62 (m, 2H), 1.47–1.37 (m, 2H), 1.25 (t, J = 7.0 Hz, 3H), 0.94 (t, J = 7.4 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 171.52, 166.66, 164.31, 157.91, 149.50, 148.07, 141.36, 137.13, 135.50, 128.28, 126.19, 124.88, 122.22, 121.47, 119.25, 111.25, 64.49, 61.42, 55.71, 53.43, 30.80, 27.92, 19.25, 14.14, 13.81; LRMS (ESI): 455 [M + H]+; HRMS (ESI) calcd for C25H31N2O6 [M + H]+ 455.2182, found: 455.2180.
(S,E)-tert-Butyl-3-(2-(3-ethoxy-3-oxo-2-(picolinamido)propyl)-3-methoxyphenyl)acrylate (3d). White solid (110.4 mg, yield 81%), mp 80–81 °C. 1H NMR (400 MHz, CDCl3) δ 8.85 (d, J = 7.3 Hz, 1H), 8.53 (d, J = 4.2 Hz, 1H), 8.07 (d, J = 7.7 Hz, 1H), 7.96 (d, J = 15.6 Hz, 1H), 7.83–7.72 (m, 1H), 7.43–7.32 (m, 1H), 7.22–7.10 (m, 2H), 6.85 (d, J = 8.0 Hz, 1H), 6.24 (d, J = 15.7 Hz, 1H), 4.87–4.74 (m, 1H), 4.27–4.12 (m, 2H), 3.91 (s, 3H), 3.50–3.30 (m, 2H), 1.54 (s, 9H), 1.26 (t, J = 7.0 Hz, 3H); 13C NMR (126 MHz, CDCl3) δ 171.61, 165.93, 164.40, 157.91, 149.60, 148.11, 140.29, 137.17, 135.70, 128.25, 126.21, 124.78, 123.41, 122.28, 119.31, 111.06, 80.58, 61.45, 55.73, 53.53, 28.28, 27.87, 14.21; LRMS (ESI): 455 [M + H]+; HRMS (ESI) calcd for C25H31N2O6 [M + H]+ 455.2182, found: 455.2178.
(S,E)-Benzyl-3-(2-(3-ethoxy-3-oxo-2-(picolinamido)propyl)-3-methoxyphenyl)acrylate (3e). Pale yellow oil (124.6 mg, yield 85%). 1H NMR (400 MHz, CDCl3) δ 8.72 (d, J = 7.6 Hz, 1H), 8.37 (d, J = 4.2 Hz, 1H), 8.03 (d, J = 15.7 Hz, 1H), 7.95 (d, J = 7.8 Hz, 1H), 7.67–7.59 (m, 1H), 7.35–7.30 (m, 2H), 7.29–7.21 (m, 4H), 7.12–7.06 (m, 1H), 7.05–7.01 (m, 1H), 6.76 (d, J = 7.8 Hz, 1H), 6.24 (d, J = 15.7 Hz, 1H), 5.15 (s, 2H), 4.80–4.68 (m, 1H), 4.13–3.97 (m, 2H), 3.79 (s, 3H), 3.38–3.24 (m, 2H), 1.12 (t, J = 7.1 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 171.51, 166.38, 164.31, 157.95, 149.50, 148.09, 142.04, 137.13, 136.15, 135.41, 128.64, 128.33, 128.27, 126.20, 125.02, 122.24, 121.03, 119.28, 111.40, 66.38, 61.44, 55.73, 53.40, 28.01, 14.14; LRMS (ESI): 511 [M + Na]+; HRMS (ESI) calcd for C28H28N2O6Na [M + Na]+ 511.1845, found: 511.1840.
(S,E)-Ethyl-3-(2-methoxy-6-(2-(phenylsulfonyl)vinyl)phenyl)-2-(picolinamido)propanoate (3f). Pale yellow oil (75.7 mg, yield 52%). 1H NMR (400 MHz, CDCl3) δ 8.84 (d, J = 7.5 Hz, 1H), 8.58–8.51 (m, 1H), 8.13–8.03 (m, 2H), 8.03–7.95 (m, 2H), 7.80–7.75 (m, 1H), 7.62–7.52 (m, 3H), 7.39 (ddd, J = 7.5, 4.8, 1.2 Hz, 1H), 7.23–7.14 (m, 1H), 7.04 (d, J = 7.9 Hz, 1H), 6.89 (d, J = 8.2 Hz, 1H), 6.78 (d, J = 15.1 Hz, 1H), 4.80–4.68 (m, 1H), 4.27–4.14 (m, 2H), 3.91 (s, 3H), 3.46 (dd, J = 13.9, 9.6 Hz, 1H), 3.35 (dd, J = 14.0, 5.2 Hz, 1H), 1.27 (t, J = 6.7 Hz, 3H); 13C NMR (126 MHz, CDCl3) δ 171.47, 164.42, 158.11, 149.44, 148.22, 140.65, 139.73, 137.26, 133.51, 133.29, 130.44, 129.47, 128.56, 127.95, 126.34, 125.74, 122.30, 119.57, 112.21, 61.68, 55.87, 53.61, 28.25, 14.24; LRMS (ESI): 495 [M + H]+; HRMS (ESI) calcd for C26H27N2O6S [M + H]+ 495.1590, found: 495.1592.
(S,E)-Ethyl-3-(2-(2-(diethoxyphosphoryl)vinyl)-6-methoxyphenyl)-2-(picolinamido)propanoate (3g). Pale yellow oil (117.7 mg, yield 80%). 1H NMR (400 MHz, CDCl3) δ 8.87 (d, J = 7.4 Hz, 1H), 8.58–8.49 (m, 1H), 8.08–8.01 (m, 1H), 7.88–7.73 (m, 2H), 7.37 (ddd, J = 7.6, 4.8, 1.2 Hz, 1H), 7.24–7.15 (m, 1H), 7.11 (d, J = 7.3 Hz, 1H), 6.85 (d, J = 7.8 Hz, 1H), 6.19 (dd, J = 18.7, 17.2 Hz, 1H), 4.82–4.67 (m, 1H), 4.24–4.08 (m, 6H), 3.91 (s, 3H), 3.43 (dd, J = 13.9, 10.0 Hz, 1H), 3.30 (dd, J = 13.9, 5.1 Hz, 1H), 1.36 (t, J = 7.1 Hz, 3H), 1.35 (t, J = 7.1 Hz, 3H), 1.23 (t, J = 7.1 Hz, 3H); 13C NMR (126 MHz, CDCl3) δ 171.55, 164.35, 157.81, 149.39, 148.08, 145.20 (d, JC–P = 6.8 Hz), 137.15, 135.98 (d, JC–P = 20.2 Hz), 128.28, 126.22, 124.39, 122.16, 119.00, 117.90 (d, JC–P = 189.7 Hz), 111.21, 62.03 (d, JC–P = 6.6 Hz), 61.98 (d, JC–P = 6.3 Hz), 61.40, 55.70, 53.42, 27.79, 16.46, 16.41, 14.10; LRMS (ESI): 491 [M + H]+; HRMS (ESI) calcd for C24H32N2O7P [M + H]+ 491.1947, found: 491.1941.
(S,E)-Ethyl-3-(2-methoxy-6-(3-oxopent-1-en-1-yl)phenyl)-2-(picolinamido)propanoate (3h). White solid (103.4 mg, yield 84%), mp 81–82 °C. 1H NMR (400 MHz, CDCl3) δ 8.83 (d, J = 7.3 Hz, 1H), 8.57–8.50 (m, 1H), 8.12–8.06 (m, 1H), 7.96 (d, J = 16.1 Hz, 1H), 7.84–7.76 (m, 1H), 7.41 (ddd, J = 7.6, 4.8, 1.2 Hz, 1H), 7.25–7.15 (m, 2H), 6.92–6.86 (m, 1H), 6.55 (d, J = 16.1 Hz, 1H), 4.85–4.72 (m, 1H), 4.26–4.11 (m, 2H), 3.91 (s, 3H), 3.48 (dd, J = 14.0, 9.0 Hz, 1H), 3.37 (dd, J = 14.0, 5.4 Hz, 1H), 2.77 (q, J = 7.3 Hz, 2H), 1.24 (t, J = 7.2 Hz, 3H), 1.17 (t, J = 7.3 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 201.24, 171.64, 164.38, 158.04, 149.53, 148.18, 139.34, 137.33, 135.75, 129.76, 128.43, 126.38, 125.17, 122.36, 119.23, 111.43, 61.59, 55.81, 53.66, 33.08, 28.16, 14.23, 8.36; LRMS (ESI): 433 [M + Na]+; HRMS (ESI) calcd for C23H26N2O5Na [M + Na]+ 433.1739, found: 433.1738.
(S,E)-Ethyl-3-(2-methoxy-6-styrylphenyl)-2-(picolinamido)propanoate (3i). White solid (62.5 mg, yield 49%), mp 107–108 °C. 1H NMR (400 MHz, CDCl3) δ 8.95 (d, J = 6.7 Hz, 1H), 8.49 (d, J = 4.7 Hz, 1H), 8.06 (d, J = 7.8 Hz, 1H), 7.78–7.71 (m, 1H), 7.59–7.48 (m, 3H), 7.41–7.33 (m, 3H), 7.29–7.20 (m, 3H), 6.98 (d, J = 16.0 Hz, 1H), 6.80 (d, J = 6.7 Hz, 1H), 4.86–4.74 (m, 1H), 4.28–4.12 (m, 2H), 3.93 (s, 3H), 3.50 (dd, J = 14.0, 9.5 Hz, 1H), 3.38 (dd, J = 14.0, 4.9 Hz, 1H), 1.28 (t, J = 7.2 Hz, 3H); 13C NMR (126 MHz, CDCl3) δ 172.01, 164.60, 157.85, 149.67, 148.12, 138.43, 137.50, 137.15, 131.80, 128.78, 128.11, 127.91, 126.87, 126.21, 125.54, 123.35, 122.32, 118.59, 109.17, 61.49, 55.70, 53.95, 28.04, 14.22; LRMS (ESI): 453 [M + Na]+; HRMS (ESI) calcd for C26H26N2O4Na [M + Na]+ 453.1790, found: 453.1786.
(S,E)-Ethyl-3-(2-methoxy-6-(pent-1-en-1-yl)phenyl)-2-(picolinamido)propanoate (3j). Pale yellow oil (63.3 mg, yield 53%). 1H NMR (400 MHz, CDCl3) δ 8.95 (d, J = 6.7 Hz, 1H), 8.54 (d, J = 4.4 Hz, 1H), 8.09 (d, J = 7.8 Hz, 1H), 7.83–7.73 (m, 1H), 7.39 (dd, J = 6.6, 4.8 Hz, 1H), 7.18–7.09 (m, 1H), 7.05 (d, J = 7.6 Hz, 1H), 6.78–6.64 (m, 2H), 6.17–6.04 (m, 1H), 4.79–4.65 (m, 1H), 4.27–4.14 (m, 2H), 3.92 (s, 3H), 3.40 (dd, J = 13.8, 10.1 Hz, 1H), 3.24 (dd, J = 13.8, 4.8 Hz, 1H), 2.30–2.10 (m, 2H), 1.55–1.46 (m, 2H), 1.27 (t, J = 7.2 Hz, 3H), 0.97 (t, J = 7.4 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 172.17, 164.65, 157.66, 149.78, 148.13, 139.25, 137.20, 134.57, 127.90, 126.90, 126.22, 122.40, 122.34, 119.03, 108.44, 61.36, 55.64, 53.82, 35.50, 27.85, 22.50, 14.25, 13.92; LRMS (ESI): 397 [M + H]+; HRMS (ESI) calcd for C23H29N2O4 [M + H]+ 397.2127, found: 397.2119.
(S,E)-Ethyl-3-(2-(hex-1-en-1-yl)-6-methoxyphenyl)-2-(picolinamido)propanoate (3k). Pale yellow oil (69.2 mg, yield 56%). 1H NMR (400 MHz, CDCl3) δ 8.94 (d, J = 6.7 Hz, 1H), 8.58–8.47 (m, 1H), 8.09 (d, J = 7.8 Hz, 1H), 7.82–7.72 (m, 1H), 7.38 (ddd, J = 7.6, 4.8, 1.2 Hz, 1H), 7.18–7.09 (m, 1H), 7.04 (d, J = 7.5 Hz, 1H), 6.77–6.64 (m, 2H), 6.18–6.02 (m, 1H), 4.77–4.67 (m, 1H), 4.26–4.15 (m, 2H), 3.92 (s, 3H), 3.40 (dd, J = 13.8, 10.2 Hz, 1H), 3.24 (dd, J = 13.8, 4.8 Hz, 1H), 2.29–2.16 (m, 2H), 1.49–1.35 (m, 4H), 1.26 (t, J = 7.2 Hz, 3H), 0.93 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 172.17, 164.64, 157.67, 149.82, 148.13, 139.27, 137.19, 134.79, 127.89, 126.71, 126.20, 122.40, 122.34, 119.03, 108.43, 61.34, 55.64, 53.81, 33.12, 31.47, 27.86, 22.46, 14.27, 14.11; LRMS (ESI): 411 [M + H]+; HRMS (ESI) calcd for C24H31N2O4 [M + H]+ 411.2284, found: 411.2276.
(S,E)-Ethyl-3-(2-methyl-6-(oct-1-en-1-yl)phenyl)-2-(picolinamido)propanoate (3l). Pale yellow oil (77.3 mg, yield 61%). 1H NMR (400 MHz, CDCl3) δ 8.63–8.50 (m, 2H), 8.11 (d, J = 7.8 Hz, 1H), 7.85–7.75 (m, 1H), 7.41 (ddd, J = 7.6, 4.8, 1.2 Hz, 1H), 7.24 (d, J = 7.4 Hz, 1H), 7.11–6.99 (m, 2H), 6.75 (d, J = 15.5 Hz, 1H), 6.11–5.97 (m, 1H), 4.99–4.84 (m, 1H), 4.20–3.99 (m, 2H), 3.30 (d, J = 7.9 Hz, 2H), 2.42 (s, 3H), 2.29–2.16 (m, 2H), 1.52–1.42 (m, 2H), 1.41–1.29 (m, 6H), 1.13 (t, J = 7.1 Hz, 3H), 0.89 (t, J = 6.9 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 172.24, 164.12, 149.54, 148.28, 138.57, 137.32, 137.09, 134.46, 132.09, 129.33, 127.86, 127.04, 126.37, 124.63, 122.35, 61.55, 52.68, 33.47, 32.47, 31.90, 29.44, 29.13, 22.79, 20.26, 14.25, 14.02; LRMS (ESI): 423 [M + H]+; HRMS (ESI) calcd for C26H35N2O3 [M + H]+ 423.2648, found: 423.2638.
(S,E)-Ethyl-3-(2-(3-ethoxy-3-oxo-2-(picolinamido)propyl)-3-methylphenyl)acrylate (3m). White solid (110.8 mg, yield 90%), mp 61–62 °C. 1H NMR (400 MHz, CDCl3) δ 8.57–8.47 (m, 2H), 8.08 (d, J = 15.7 Hz, 1H), 8.04 (d, J = 7.8 Hz, 1H), 7.80–7.71 (m, 1H), 7.37 (ddd, J = 7.6, 4.8, 1.2 Hz, 1H), 7.32 (d, J = 6.9 Hz, 1H), 7.19–7.08 (m, 2H), 6.20 (d, J = 15.6 Hz, 1H), 4.95–4.84 (m, 1H), 4.23 (q, J = 7.1 Hz, 2H), 4.16–4.05 (m, 2H), 3.43–3.28 (m, 2H), 2.44 (s, 3H), 1.31 (t, J = 7.1 Hz, 3H), 1.14 (t, J = 7.1 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 171.39, 166.59, 163.85, 149.13, 148.17, 142.46, 137.77, 137.16, 134.76, 134.46, 132.19, 127.25, 126.27, 124.88, 122.12, 120.84, 61.59, 60.41, 52.66, 32.36, 20.06, 14.31, 13.91; LRMS (ESI): 411 [M + H]+; HRMS (ESI) calcd for C23H27N2O5 [M + H]+ 411.1920, found: 411.1915.
(S,E)-Ethyl-3-(2-(3-ethoxy-3-oxo-2-(picolinamido)propyl)-3-fluorophenyl)acrylate (3n). Pale yellow oil (114.4 mg, yield 92%). 1H NMR (400 MHz, CDCl3) δ 8.58–8.43 (m, 2H), 8.11–8.03 (m, 1H), 7.98 (d, J = 15.7 Hz, 1H), 7.82–7.73 (m, 1H), 7.41–7.35 (m, 1H), 7.32 (d, J = 7.9 Hz, 1H), 7.24–7.18 (m, 1H), 7.07–6.98 (m, 1H), 6.25 (d, J = 15.7 Hz, 1H), 5.04–4.89 (m, 1H), 4.23–4.12 (m, 4H), 3.49–3.31 (m, 2H), 1.30 (t, J = 7.1 Hz, 3H), 1.22 (t, J = 7.1 Hz, 3H); 13C NMR (126 MHz, CDCl3) δ 171.06, 166.25, 164.05, 161.82 (d, JC–F = 245.3 Hz), 149.25, 148.29, 140.37 (d, JC–F = 3.1 Hz), 137.17, 136.64 (d, JC–F = 4.0 Hz), 128.76 (d, JC–F = 9.2 Hz), 126.33, 123.42 (d, JC–F = 16.0 Hz), 122.63 (d, JC–F = 2.9 Hz), 122.27, 121.95, 116.36 (d, JC–F = 23.4 Hz), 61.82, 60.65, 52.59, 28.22, 14.34, 14.05; LRMS (ESI): 415 [M + H]+; HRMS (ESI) calcd for C22H24FN2O5 [M + H]+ 415.1669, found: 415.1659.
(S,E)-Ethyl-3-(3-chloro-2-(3-ethoxy-3-oxo-2-(picolinamido)propyl)phenyl)acrylate (3o). Pale yellow oil (113.7 mg, yield 88%). 1H NMR (400 MHz, CDCl3) δ 8.57 (d, J = 8.6 Hz, 1H), 8.54–8.49 (m, 1H), 8.11–8.01 (m, 2H), 7.80–7.72 (m, 1H), 7.42–7.33 (m, 3H), 7.20–7.12 (m, 1H), 6.21 (d, J = 15.7 Hz, 1H), 5.06–4.96 (m, 1H), 4.26–4.12 (m, 4H), 3.55 (dd, J = 14.1, 6.4 Hz, 1H), 3.47 (dd, J = 14.1, 8.8 Hz, 1H), 1.32 (t, J = 7.1 Hz, 3H), 1.19 (t, J = 7.1 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 171.07, 166.20, 163.97, 149.15, 148.21, 141.20, 137.16, 136.71, 135.95, 133.88, 130.79, 128.40, 126.30, 125.65, 122.35, 122.20, 61.80, 60.66, 52.27, 32.69, 14.31, 14.00; LRMS (ESI): 433 ([M + H]+, (Cl37)), 431 ([M + H]+, (Cl35)); HRMS (ESI) calcd for C22H24ClN2O5 [M + H]+ 431.1374, found: 431.1371.
(S,E)-Ethyl-3-(3-bromo-2-(3-ethoxy-3-oxo-2-(picolinamido)propyl)phenyl)acrylate (3p). Pale yellow oil (107.0 mg, yield 75%). 1H NMR (400 MHz, CDCl3) δ 8.59 (d, J = 8.7 Hz, 1H), 8.52 (d, J = 4.7 Hz, 1H), 8.11–8.00 (m, 2H), 7.80–7.72 (m, 1H), 7.55 (dd, J = 7.9, 0.9 Hz, 1H), 7.43–7.35 (m, 2H), 7.12–7.04 (m, 1H), 6.19 (d, J = 15.6 Hz, 1H), 5.07–4.98 (m, 1H), 4.28–4.20 (m, 2H), 4.20–4.13 (m, 2H), 3.58 (dd, J = 14.1, 6.3 Hz, 1H), 3.49 (dd, J = 14.1, 9.1 Hz, 1H), 1.32 (t, J = 7.1 Hz, 3H), 1.19 (t, J = 7.1 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 171.10, 166.22, 164.03, 149.27, 148.27, 141.54, 137.20, 136.91, 135.52, 134.31, 128.78, 126.85, 126.43, 126.32, 122.55, 122.27, 61.86, 60.73, 52.38, 35.49, 14.38, 14.07; LRMS (ESI): 477 ([M + H]+, (Br81)), 475 ([M + H]+, (Br79)); HRMS (ESI) calcd for C22H24BrN2O5 [M + H]+ 475.0869, found: 475.0862.
(S,E)-Ethyl-3-(3,5-dichloro-2-(3-ethoxy-3-oxo-2-(picolinamido)propyl)phenyl)acrylate (3q). Pale yellow oil (98.1 mg, yield 70%). 1H NMR (400 MHz, CDCl3) δ 8.61–8.51 (m, 2H), 8.06 (d, J = 7.8 Hz, 1H), 8.00 (d, J = 15.7 Hz, 1H), 7.84–7.75 (m, 1H), 7.44–7.35 (m, 3H), 6.22 (d, J = 15.6 Hz, 1H), 5.06–4.96 (m, 1H), 4.29–4.16 (m, 4H), 3.52 (dd, J = 14.1, 6.2 Hz, 1H), 3.43 (dd, J = 14.1, 8.8 Hz, 1H), 1.34 (t, J = 7.1 Hz, 3H), 1.23 (t, J = 7.1 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 170.98, 165.93, 164.07, 149.17, 148.33, 140.12, 137.95, 137.33, 136.72, 133.73, 132.61, 130.38, 126.45, 125.84, 123.58, 122.37, 62.05, 60.93, 52.14, 32.60, 14.39, 14.15; LRMS(ESI): 465 [M + H]+; HRMS (ESI) calcd for C22H23Cl2N2O5 [M + H]+ 465.0984, found: 465.0981.
(S,E)-Ethyl-3-(4-chloro-2-(3-ethoxy-3-oxo-2-(picolinamido)propyl)phenyl)acrylate (3r). White solid (100.8 mg, yield 78%), mp 84–85 °C. 1H NMR (400 MHz, CDCl3) δ 8.52–8.48 (m, 1H), 8.46 (d, J = 8.4 Hz, 1H), 8.11–8.06 (m, 1H), 7.90 (d, J = 15.8 Hz, 1H), 7.82–7.74 (m, 1H), 7.49–7.42 (m, 1H), 7.38 (ddd, J = 7.6, 4.8, 1.2 Hz, 1H), 7.23–7.16 (m, 2H), 6.22 (d, J = 15.7 Hz, 1H), 5.05–4.93 (m, 1H), 4.21–4.07 (m, 4H), 3.37 (dd, J = 14.1, 6.2 Hz, 1H), 3.28 (dd, J = 14.1, 6.5 Hz, 1H), 1.26 (t, J = 7.1 Hz, 3H), 1.21 (t, J = 7.1 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 170.75, 166.22, 163.93, 149.05, 148.24, 140.18, 137.65, 137.16, 135.63, 132.67, 130.95, 128.16, 127.87, 126.35, 122.22, 120.78, 61.80, 60.48, 53.26, 35.23, 14.23, 14.04; LRMS (ESI): 433 ([M + H]+, (Cl37)), 431 ([M + H]+, (Cl35)); HRMS (ESI) calcd for C22H24ClN2O5 [M + H]+ 431.1374, found: 431.1369.
(S,E)-Ethyl-3-(2-(3-ethoxy-3-oxo-2-(picolinamido)propyl)phenyl)acrylate (3smono). White solid (50.0 mg, yield 42%), mp 69–70 °C. 1H NMR (400 MHz, CDCl3) δ 8.55–8.49 (m, 1H), 8.46 (d, J = 8.4 Hz, 1H), 8.15–8.08 (m, 1H), 8.01 (d, J = 15.7 Hz, 1H), 7.84–7.76 (m, 1H), 7.58–7.52 (m, 1H), 7.40 (ddd, J = 7.5, 4.8, 1.1 Hz, 1H), 7.29–7.22 (m, 3H), 6.28 (d, J = 15.7 Hz, 1H), 5.06–4.98 (m, 1H), 4.22–4.10 (m, 4H), 3.47–3.30 (m, 2H), 1.31 (t, J = 7.2 Hz, 3H), 1.21 (t, J = 7.1 Hz, 3H); 13C NMR (126 MHz, CDCl3) δ 171.20, 166.64, 164.02, 149.31, 148.32, 141.55, 137.24, 135.95, 134.26, 131.04, 130.05, 127.75, 127.02, 126.38, 122.34, 120.56, 61.73, 60.53, 53.54, 35.53, 14.38, 14.13; LRMS (ESI): 397 [M + H]+; HRMS (ESI) calcd for C22H25N2O5 [M + H]+ 397.1763, found: 397.1756.
(2E,2′E)-Diethyl-3,3′-(2-((S)-3-ethoxy-3-oxo-2-(picolinamido)propyl)-1,3-phenylene)diacrylate (3sdi). White solid (56.4 mg, yield 38%), mp 138–139 °C. 1H NMR (400 MHz, CDCl3) δ 8.55–8.50 (m, 1H), 8.47 (d, J = 8.7 Hz, 1H), 8.12 (d, J = 15.7 Hz, 2H), 8.07–8.00 (m, 1H), 7.82–7.74 (m, 1H), 7.53 (d, J = 7.8 Hz, 2H), 7.39 (ddd, J = 7.6, 4.8, 1.2 Hz, 1H), 7.31–7.26 (m, 1H), 6.26 (d, J = 15.6 Hz, 2H), 4.97–4.83 (m, 1H), 4.28–4.22 (m, 4H), 4.22–4.10 (m, 2H), 3.56 (dd, J = 14.5, 5.6 Hz, 1H), 3.43 (dd, J = 14.4, 8.7 Hz, 1H), 1.34 (t, J = 7.1 Hz, 6H), 1.23 (t, J = 7.1 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 171.00, 166.44, 163.98, 149.21, 148.28, 141.73, 137.21, 135.72, 135.32, 128.78, 127.97, 126.34, 122.30, 122.17, 61.95, 60.72, 53.43, 31.97, 14.45, 14.13; LRMS (ESI): 495 [M + H]+; HRMS (ESI) calcd for C27H31N2O7 [M + H]+ 495.2131, found: 495.2128.
(S,E)-Ethyl-3-(2-(3-ethoxy-3-oxo-2-(picolinamido)propyl)-5-methylphenyl)acrylate (3tmono). Pale yellow oil (44.3 mg, yield 36%). 1H NMR (400 MHz, CDCl3) δ 8.55–8.49 (m, 1H), 8.44 (d, J = 8.4 Hz, 1H), 8.14–8.08 (m, 1H), 7.97 (d, J = 15.8 Hz, 1H), 7.84–7.75 (m, 1H), 7.43–7.33 (m, 2H), 7.14–7.06 (m, 2H), 6.27 (d, J = 15.7 Hz, 1H), 5.06–4.93 (m, 1H), 4.22–4.08 (m, 4H), 3.40 (dd, J = 14.2, 6.0 Hz, 1H), 3.29 (dd, J = 14.2, 6.7 Hz, 1H), 2.31 (s, 3H), 1.29 (t, J = 7.1 Hz, 3H), 1.22 (t, J = 7.1 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 171.24, 166.68, 163.99, 149.36, 148.31, 141.70, 137.28, 137.21, 133.99, 132.96, 130.99, 130.95, 127.57, 126.33, 122.33, 120.20, 61.69, 60.46, 53.61, 35.04, 21.15, 14.38, 14.15; LRMS (ESI): 411 [M + H]+; HRMS (ESI) calcd for C23H27N2O5 [M + H]+ 411.1920, found: 411.1916.
(2E,2′E)-Diethyl-3,3′-(2-((S)-3-ethoxy-3-oxo-2-(picolinamido)propyl)-5-methyl-1,3-phenylene)diacrylate (3tdi). White solid (68.7 mg, yield 45%), mp 101–102 °C. 1H NMR (400 MHz, CDCl3) δ 8.53–8.47 (m, 1H), 8.43 (d, J = 8.6 Hz, 1H), 8.07 (d, J = 15.6 Hz, 2H), 8.03–7.99 (m, 1H), 7.81–7.71 (m, 1H), 7.40–7.30 (m, 3H), 6.23 (d, J = 15.6 Hz, 2H), 4.93–4.79 (m, 1H), 4.26–4.08 (m, 6H), 3.50 (dd, J = 14.5, 5.5 Hz, 1H), 3.37 (dd, J = 14.5, 8.6 Hz, 1H), 2.30 (s, 3H), 1.31 (t, J = 7.1 Hz, 6H), 1.23 (t, J = 7.1 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 170.95, 166.41, 163.90, 149.16, 148.19, 141.78, 137.36, 137.11, 135.42, 132.40, 129.48, 126.23, 122.21, 121.71, 61.82, 60.56, 53.47, 31.46, 21.09, 14.35, 14.04; LRMS (ESI): 509 [M + H]+; HRMS (ESI) calcd for C28H33N2O7 [M + H]+ 509.2288, found: 509.2281.
(S,E)-Ethyl-3-(5-chloro-2-(3-ethoxy-3-oxo-2-(picolinamido)propyl)phenyl)acrylate (3umono). Pale yellow oil (45.4 mg, yield 35%). 1H NMR (400 MHz, CDCl3) δ 8.58–8.50 (m, 1H), 8.45 (d, J = 8.4 Hz, 1H), 8.10 (d, J = 7.8 Hz, 1H), 7.91 (d, J = 15.8 Hz, 1H), 7.84–7.75 (m, 1H), 7.50 (d, J = 2.1 Hz, 1H), 7.40 (ddd, J = 7.6, 4.8, 1.2 Hz, 1H), 7.25–7.20 (m, 1H), 7.19–7.14 (m, 1H), 6.26 (d, J = 15.7 Hz, 1H), 5.05–4.95 (m, 1H), 4.25–4.07 (m, 4H), 3.39 (dd, J = 14.2, 6.0 Hz, 1H), 3.28 (dd, J = 14.2, 6.7 Hz, 1H), 1.29 (t, J = 7.1 Hz, 3H), 1.22 (t, J = 7.1 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 170.94, 166.20, 163.99, 149.14, 148.32, 140.20, 137.29, 135.91, 134.36, 133.58, 132.36, 129.84, 126.86, 126.45, 122.35, 121.72, 61.87, 60.67, 53.31, 34.96, 14.32, 14.14; LRMS (ESI): 433 ([M + H]+, (Cl37)), 431 ([M + H]+, (Cl35)); HRMS (ESI) calcd for C22H24ClN2O5 [M + H]+ 431.1374, found: 431.1365.
(2E,2′E)-Diethyl-3,3′-(5-chloro-2-((S)-3-ethoxy-3-oxo-2-(picolinamido)propyl)-1,3-phenylene)diacrylate (3udi). White solid (55.7 mg, yield 35%), mp 83–85 °C. 1H NMR (400 MHz, CDCl3) δ 8.52 (d, J = 4.2 Hz, 1H), 8.47 (d, J = 8.7 Hz, 1H), 8.09–7.97 (m, 3H), 7.82–7.76 (m, 1H), 7.53–7.44 (m, 2H), 7.40 (ddd, J = 7.5, 4.8, 1.1 Hz, 1H), 6.25 (d, J = 15.6 Hz, 2H), 4.94–4.82 (m, 1H), 4.30–4.12 (m, 6H), 3.51 (dd, J = 14.5, 5.5 Hz, 1H), 3.38 (dd, J = 14.5, 8.7 Hz, 1H), 1.34 (t, J = 7.1 Hz, 6H), 1.26 (t, J = 7.1 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 170.65, 165.93, 163.87, 148.96, 148.18, 140.34, 137.30, 137.17, 133.70, 133.67, 128.18, 126.31, 123.09, 122.22, 61.96, 60.73, 53.06, 31.60, 14.28, 14.01; LRMS (ESI): 531 ([M + H]+, (Cl37)), 529 ([M + H]+, (Cl35)); HRMS (ESI) calcd for C27H30ClN2O7 [M + H]+ 529.1742, found: 529.1744.
(S,E)-Ethyl-2-(picolinamido)-3-(2-styrylphenyl)propanoate (3v). Yellow oil (55.4 mg, 46%). 1H NMR (400 MHz, CDCl3) δ 8.58–8.47 (m, 1H), 8.46–8.37 (m, 1H), 8.00 (d, J = 7.8 Hz, 1H), 7.72–7.57 (m, 2H), 7.53–7.40 (m, 3H), 7.35–7.12 (m, 7H), 6.90 (d, J = 16.0 Hz, 1H), 5.11–4.93 (m, 1H), 4.24–4.02 (m, 2H), 3.52–3.29 (m, 2H), 1.16 (t, J = 7.1 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 171.55, 164.09, 149.28, 148.16, 137.47, 137.16, 137.11, 134.30, 130.96, 130.80, 128.68, 127.70, 127.68, 127.63, 126.77, 126.27, 126.06, 125.70, 122.29, 61.65, 53.79, 35.89, 14.13; LRMS (ESI): 401 [M + H]+; HRMS (ESI) calcd for C25H25N2O3 [M + H]+ 401.1865, found: 401.1860.
(E)-Methyl-3-(3-methyl-2-(2-(picolinamido)ethyl)phenyl)acrylate (5a). Pale yellow oil (86.6 mg, yield 89%). 1H NMR (400 MHz, CDCl3) δ 8.59–8.49 (m, 1H), 8.31–8.17 (m, 2H), 8.11 (d, J = 15.7 Hz, 1H), 7.90–7.82 (m, 1H), 7.46–7.38 (m, 2H), 7.23 (d, J = 7.1 Hz, 1H), 7.20–7.13 (m, 1H), 6.31 (d, J = 15.7 Hz, 1H), 3.77 (s, 3H), 3.63–3.55 (m, 2H), 3.18–3.09 (m, 2H), 2.46 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 167.17, 164.44, 149.80, 148.02, 142.76, 137.64, 137.24, 136.68, 134.04, 132.29, 126.83, 126.10, 124.79, 122.10, 120.06, 51.61, 39.40, 29.35, 19.94; LRMS (ESI): 325 [M + H]+; HRMS (ESI) calcd for C19H21N2O3 [M + H]+ 325.1552, found: 325.1548.
(E)-Ethyl-3-(3-methyl-2-(2-(picolinamido)ethyl)phenyl)acrylate (5b). Pale yellow oil (87.2 mg, yield 86%). 1H NMR (400 MHz, CDCl3) δ 8.59–8.49 (m, 1H), 8.26–8.16 (m, 2H), 8.10 (d, J = 15.7 Hz, 1H), 7.89–7.80 (m, 1H), 7.46–7.38 (m, 2H), 7.23 (d, J = 7.1 Hz, 1H), 7.19–7.14 (m, 1H), 6.31 (d, J = 15.7 Hz, 1H), 4.23 (q, J = 7.1 Hz, 2H), 3.63–3.54 (m, 2H), 3.18–3.09 (m, 2H), 2.46 (s, 3H), 1.33 (t, J = 7.1 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 166.92, 164.53, 149.92, 148.13, 142.62, 137.75, 137.37, 136.73, 134.25, 132.32, 126.93, 126.19, 124.92, 122.22, 120.68, 60.55, 39.50, 29.49, 20.06, 14.39; LRMS (ESI): 339 [M + H]+; HRMS (ESI) calcd for C20H23N2O3 [M + H]+ 339.1709, found: 339.1706.
(E)-N-(2-Methyl-6-(3-oxopent-1-en-1-yl)phenethyl)picolinamide (5c). White solid (89.5 mg, yield 93%), mp 86–87 °C. 1H NMR (400 MHz, CDCl3) δ 8.57–8.47 (m, 1H), 8.31–8.22 (m, 1H), 8.20 (d, J = 7.8 Hz, 1H), 8.04 (d, J = 16.0 Hz, 1H), 7.90–7.82 (m, 1H), 7.47–7.40 (m, 2H), 7.23 (d, J = 7.1 Hz, 1H), 7.20–7.14 (m, 1H), 6.57 (d, J = 16.0 Hz, 1H), 3.63–3.55 (m, 2H), 3.19–3.11 (m, 2H), 2.72 (q, J = 7.3 Hz, 2H), 2.45 (s, 3H), 1.13 (t, J = 7.3 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 201.28, 164.66, 149.88, 148.19, 140.41, 137.68, 137.50, 137.01, 134.60, 132.43, 128.82, 127.02, 126.34, 124.92, 122.27, 39.56, 33.64, 29.51, 20.11, 8.32; LRMS (ESI): 323 [M + H]+; HRMS (ESI) calcd for C20H23N2O2 [M + H]+ 323.1760, found: 323.1762.
(E)-Methyl-3-(3-methyl-2-(2-(picolinamido)propyl)phenyl)acrylate (5d). Pale yellow oil (91.4 mg, yield 90%). 1H NMR (400 MHz, CDCl3) δ 8.57–8.48 (m, 1H), 8.19 (d, J = 15.7 Hz, 1H), 8.14 (d, J = 7.8 Hz, 1H), 8.04 (d, J = 8.3 Hz, 1H), 7.87–7.78 (m, 1H), 7.41 (ddd, J = 7.6, 4.8, 1.2 Hz, 1H), 7.37 (d, J = 7.6 Hz, 1H), 7.23–7.19 (m, 1H), 7.17–7.10 (m, 1H), 6.25 (d, J = 15.7 Hz, 1H), 4.45–4.31 (m, 1H), 3.81 (s, 3H), 3.21 (dd, J = 13.9, 6.6 Hz, 1H), 2.99 (dd, J = 13.9, 8.0 Hz, 1H), 2.49 (s, 3H), 1.25 (d, J = 6.7 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 167.47, 163.58, 149.98, 148.01, 143.81, 138.04, 137.46, 136.70, 134.64, 132.44, 126.89, 126.16, 124.95, 122.28, 119.93, 51.79, 46.35, 36.08, 20.42, 20.36; LRMS (ESI): 339 [M + H]+; HRMS (ESI) calcd for C20H23N2O3 [M + H]+ 339.1709, found: 339.1708.
(E)-Ethyl-3-(3-methyl-2-(2-(picolinamido)propyl)phenyl)acrylate (5e). Pale yellow oil (93.4 mg, yield 88%). 1H NMR (400 MHz, CDCl3) δ 8.57–8.49 (m, 1H), 8.18 (d, J = 15.7 Hz, 1H), 8.14 (d, J = 7.8 Hz, 1H), 8.05 (d, J = 8.3 Hz, 1H), 7.86–7.78 (m, 1H), 7.43–7.34 (m, 2H), 7.23–7.17 (m, 1H), 7.16–7.10 (m, 1H), 6.25 (d, J = 15.7 Hz, 1H), 4.44–4.32 (m, 1H), 4.31–4.23 (m, 2H), 3.21 (dd, J = 13.9, 6.6 Hz, 1H), 2.99 (dd, J = 13.9, 8.0 Hz, 1H), 2.49 (s, 3H), 1.35 (t, J = 7.1 Hz, 3H), 1.25 (d, J = 6.7 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 167.08, 163.55, 149.96, 147.99, 143.54, 138.00, 137.48, 136.66, 134.71, 132.37, 126.87, 126.16, 124.95, 122.29, 120.38, 60.57, 46.35, 36.08, 20.42, 20.37, 14.47; LRMS (ESI): 353 [M + H]+; HRMS (ESI) calcd for C21H25N2O3 [M + H]+ 353.1865, found: 353.1860.
(E)-N-(1-(2-Methyl-6-(3-oxopent-1-en-1-yl)phenyl)propan-2-yl)picolinamide (5f). White solid (91.6 mg, yield 91%), mp 75–76 °C. 1H NMR (400 MHz, CDCl3) δ 8.57–8.48 (m, 1H), 8.20–8.10 (m, 2H), 8.04 (d, J = 8.0 Hz, 1H), 7.88–7.79 (m, 1H), 7.45–7.39 (m, 2H), 7.24–7.18 (m, 1H), 7.18–7.11 (m, 1H), 6.56 (d, J = 15.9 Hz, 1H), 4.41–4.29 (m, 1H), 3.28 (dd, J = 13.9, 6.2 Hz, 1H), 2.96 (dd, J = 13.9, 8.4 Hz, 1H), 2.90–2.71 (m, 2H), 2.48 (s, 3H), 1.25 (d, J = 6.7 Hz, 3H), 1.18 (t, J = 7.3 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 201.49, 163.63, 149.96, 148.07, 141.14, 137.98, 137.51, 136.93, 134.81, 132.45, 128.59, 126.91, 126.25, 124.93, 122.28, 46.65, 35.97, 33.64, 20.64, 20.07, 8.39; LRMS (ESI): 337 [M + H]+; HRMS (ESI) calcd for C21H25N2O2 [M + H]+ 337.1916, found: 337.1914.
(E)-Methyl-3-(3-fluoro-2-(2-(picolinamido)ethyl)phenyl)acrylate (5g). White solid (80.8 mg, yield 82%), mp 77–79 °C. 1H NMR (400 MHz, CDCl3) δ 8.53–8.46 (m, 1H), 8.23–8.08 (m, 2H), 7.98 (d, J = 15.8 Hz, 1H), 7.87–7.79 (m, 1H), 7.40 (ddd, J = 7.6, 4.8, 1.1 Hz, 1H), 7.35 (d, J = 7.8 Hz, 1H), 7.26–7.20 (m, 1H), 7.13–7.02 (m, 1H), 6.30 (d, J = 15.8 Hz, 1H), 3.74 (s, 3H), 3.70–3.64 (m, 2H), 3.17–3.12 (m, 2H); 13C NMR (101 MHz, CDCl3) δ 166.83, 164.45, 161.72 (d, JC–F = 245.2 Hz), 149.86, 148.05, 140.90 (d, JC–F = 3.5 Hz), 137.41, 136.24 (d, JC–F = 4.5 Hz), 128.28 (d, JC–F = 9.1 Hz), 126.21, 126.02 (d, JC–F = 16.1 Hz), 122.60 (d, JC–F = 3.3 Hz), 122.34, 121.33, 116.68 (d, JC–F = 23.3 Hz), 51.85, 39.72, 25.44; LRMS (ESI): 329 [M + H]+; HRMS (ESI) calcd for C18H18FN2O3 [M + H]+ 329.1301, found: 329.1296.
(E)-Methyl-3-(3-chloro-2-(2-(picolinamido)ethyl)phenyl)acrylate (5h). White solid (81.6 mg, yield 79%), mp 93–95 °C. 1H NMR (400 MHz, CDCl3) δ 8.53–8.47 (m, 1H), 8.21–8.09 (m, 2H), 8.04 (d, J = 15.7 Hz, 1H), 7.87–7.80 (m, 1H), 7.47–7.37 (m, 3H), 7.24–7.16 (m, 1H), 6.26 (d, J = 15.7 Hz, 1H), 3.74 (s, 3H), 3.72–3.67 (m, 2H), 3.29 (t, J = 7.2 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 166.74, 164.52, 149.89, 148.09, 141.81, 137.36, 136.42, 136.16, 135.76, 131.10, 128.10, 126.21, 125.70, 122.31, 121.67, 51.86, 39.00, 29.99; LRMS (ESI): 347 ([M + H]+, (Cl37)), 345 ([M + H]+, (Cl35)); HRMS (ESI) calcd for C18H18ClN2O3 [M + H]+ 345.1006, found: 345.1003.
(E)-Methyl-3-(2-(2-(picolinamido)ethyl)phenyl)acrylate (5imono). Pale yellow oil (50.3 mg, yield 54%). 1H NMR (400 MHz, CDCl3) δ 8.54–8.45 (m, 1H), 8.23–8.09 (m, 2H), 8.04 (d, J = 15.8 Hz, 1H), 7.88–7.79 (m, 1H), 7.58 (d, J = 7.9 Hz, 1H), 7.41 (ddd, J = 7.6, 4.8, 1.2 Hz, 1H), 7.37–7.31 (m, 1H), 7.30–7.23 (m, 2H), 6.35 (d, J = 15.8 Hz, 1H), 3.77 (s, 3H), 3.72–3.65 (m, 2H), 3.10 (t, J = 7.3 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 167.28, 164.42, 149.88, 148.10, 141.93, 138.66, 137.45, 133.61, 130.65, 130.41, 127.33, 126.95, 126.25, 122.32, 119.94, 51.82, 40.69, 33.27; LRMS (ESI): 311 [M + H]+; HRMS (ESI) calcd for C18H19N2O3 [M + H]+ 311.1396, found: 311.1397.
(2E,2′E)-Dimethyl-3,3′-(2-(2-(picolinamido)ethyl)-1,3-phenylene)diacrylate (5idi). White solid (35.5 mg, yield 30%), mp 106–107 °C. 1H NMR (400 MHz, CDCl3) δ 8.50–8.44 (m, 1H), 8.16 (d, J = 7.8 Hz, 1H), 8.14–8.06 (m, 3H), 7.83–7.78 (m, 1H), 7.56 (d, J = 7.8 Hz, 2H), 7.39 (ddd, J = 7.6, 4.8, 1.2 Hz, 1H), 7.31–7.27 (m, 1H), 6.29 (d, J = 15.7 Hz, 2H), 3.76 (s, 6H), 3.63–3.57 (m, 2H), 3.28–3.20 (m, 2H); 13C NMR (101 MHz, CDCl3) δ 166.92, 164.52, 149.77, 148.06, 142.07, 137.60, 137.28, 135.22, 128.76, 127.54, 126.19, 122.28, 121.42, 51.85, 40.23, 28.93; LRMS (ESI): 395 [M + H]+; HRMS (ESI) calcd for C22H23N2O5 [M + H]+ 395.1607, found: 395.1601.
(S,E)-2-((tert-Butoxycarbonyl)amino)-3-(2-styrylphenyl)propanoic acid (3v-1). Yellow oil (41.8 mg, 82%). 1H NMR (400 MHz, DMSO) δ 12.61 (s, 1H), 7.71 (d, J = 7.7 Hz, 1H), 7.68–7.60 (m, 2H), 7.56 (d, J = 16.2 Hz, 1H), 7.42–7.36 (m, 2H), 7.32–7.20 (m, 5H), 7.17 (d, J = 16.3 Hz, 1H), 4.14–4.04 (m, 1H), 3.29 (dd, J = 14.1, 4.2 Hz, 1H), 2.96 (dd, J = 14.0, 10.0 Hz, 1H), 1.31 (s, 9H); 13C NMR (101 MHz, DMSO) δ 173.62, 155.53, 137.31, 136.01, 135.73, 130.77, 130.12, 128.67, 127.72, 127.34, 126.96, 126.64, 125.38, 125.29, 78.10, 54.92, 34.23, 28.13; LRMS (ESI): 390 [M + Na]+; HRMS (ESI) calcd for C22H25NO4Na [M + Na]+ 390.1681, found: 390.1675.
(E)-tert-Butyl-2-methyl-6-(3-oxopent-1-en-1-yl)phenethyl(picolinoyl)carbamate (5c-1). Pale yellow oil (115.4 mg, yield 91%). 1H NMR (400 MHz, CDCl3) δ 8.62–8.55 (m, 1H), 8.23 (d, J = 16.2 Hz, 1H), 7.86–7.79 (m, 1H), 7.68 (d, J = 7.8 Hz, 1H), 7.46 (d, J = 7.3 Hz, 1H), 7.41 (ddd, J = 7.6, 4.8, 1.2 Hz, 1H), 7.24–7.13 (m, 2H), 6.60 (d, J = 16.2 Hz, 1H), 3.99–3.88 (m, 2H), 3.26–3.15 (m, 2H), 2.96 (q, J = 7.3 Hz, 2H), 2.49 (s, 3H), 1.19 (t, J = 7.3 Hz, 3H), 1.15 (s, 9H); 13C NMR (101 MHz, CDCl3) δ 202.57, 171.55, 154.83, 153.06, 148.23, 140.97, 138.02, 137.13, 136.16, 134.78, 132.28, 129.85, 127.12, 125.39, 124.68, 122.89, 83.39, 45.14, 32.22, 28.57, 27.43, 19.96, 8.64; LRMS (ESI): 445 [M + Na]+; HRMS (ESI) calcd for C25H30N2O4Na [M + Na]+ 445.2103, found: 445.2099.
tert-Butyl-5-methyl-1-(2-oxobutyl)-3,4-dihydroisoquinoline-2(1H)-carboxylate (5c-2). White solid (76.3 mg, yield 88%), mp 104–106 °C. 1H NMR (400 MHz, CDCl3) δ 7.20–6.92 (m, 3H), 5.72–5.48 (m, 1H), 4.36–3.97 (m, 1H), 3.38–3.12 (m, 1H), 2.98–2.38 (m, 6H), 2.23 (s, 3H), 1.45 (s, 9H), 1.06 (t, J = 7.2 Hz, 3H); 13C NMR (126 MHz, CDCl3, rotamer peaks exist in the spectra) δ 209.02, 208.59, 154.58, 154.36, 137.13, 136.96, 136.82, 136.61, 132.94, 132.78, 128.33, 126.04, 124.87, 124.64, 80.42, 80.01, 51.90, 51.39, 50.00, 49.84, 38.06, 37.15, 36.96, 35.87, 28.47, 25.98, 25.77, 19.48, 7.76; LRMS (ESI): 340 [M + Na]+; HRMS (ESI) calcd for C19H27NO3Na [M + Na]+ 340.1889, found: 340.1884.
tert-Butyl-2-methyl-6-(3-propionyloxiran-2-yl)phenethylcarbamate (5c-3). White solid (8.3 mg, yield 9%), mp 90–91 °C. 1H NMR (400 MHz, CDCl3) δ 7.21–7.00 (m, 3H), 4.85–4.71 (m, 1H), 4.48–4.36 (m, 1H), 3.45–3.29 (m, 1H), 3.27–3.15 (m, 2H), 2.98–2.83 (m, 2H), 2.62 (q, J = 7.2 Hz, 2H), 2.37 (s, 3H), 1.42 (s, 9H), 1.12 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 207.49, 156.05, 137.00, 135.54, 134.69, 130.73, 126.97, 122.53, 79.50, 62.99, 56.29, 40.26, 30.63, 30.15, 28.50, 19.56, 7.04; LRMS (ESI): 356 [M + Na]+; HRMS (ESI) calcd for C19H27NO4Na [M + Na]+ 356.1838, found: 356.1832.
Acknowledgements
We gratefully acknowledge financial support from the National Natural Science Foundation of China (No. 21602022), the Open Project Program of Antibiotics Research and Re-evaluation Key Laboratory of Sichuan Province (No. ARRLKF15-01), Chengdu University New Faculty Start-up Funding (No. 2081915037) and Zhejiang Provincial Natural Science Foundation (No. LQ14B020004).Notes and references
- For recent reviews: (a) J. Wencel-Delord and F. Glorius, Nat. Chem., 2013, 5, 369–375 CrossRef CAS PubMed; (b) M.-L. Louillat and F. W. Patureau, Chem. Soc. Rev., 2014, 43, 901–910 RSC; (c) L. Ackermann, R. Vicente and A. R. Kapdi, Angew. Chem., Int. Ed., 2009, 48, 9792–9826 CrossRef CAS PubMed; (d) J. Wencel-Delord, T. Droge, F. Liu and F. Glorius, Chem. Soc. Rev., 2011, 40, 4740–4761 RSC; (e) O. Daugulis, H.-Q. Do and D. Shabashov, Acc. Chem. Res., 2009, 42, 1074–1086 CrossRef CAS PubMed; (f) D. A. Colby, R. G. Bergman and J. A. Ellman, Chem. Rev., 2010, 110, 624–655 CrossRef CAS PubMed; (g) G. Rouquet and N. Chatani, Angew. Chem., Int. Ed., 2013, 52, 11726–11743 CrossRef CAS PubMed; (h) K. Godula and D. Sames, Science, 2006, 312, 67–72 CrossRef CAS PubMed; (i) S. H. Cho, J. Y. Kim, J. Kwak and S. Chang, Chem. Soc. Rev., 2011, 40, 5068–5083 RSC; (j) B.-J. Li and Z.-J. Shi, Chem. Soc. Rev., 2012, 41, 5588–5598 RSC; (k) S. Messaoudi, J.-D. Brion and M. Alami, Eur. J. Org. Chem., 2010, 6495–6516 CrossRef CAS; (l) K. M. Engle, T.-S. Mei, M. Wasa and J.-Q. Yu, Acc. Chem. Res., 2012, 45, 788–802 CrossRef CAS PubMed; (m) L. F. McMurray and M. J. Gaunt, Chem. Soc. Rev., 2011, 40, 1885–1898 RSC; (n) F. Kakiuchi and S. Murai, Acc. Chem. Res., 2002, 35, 826–834 CrossRef CAS PubMed; (o) X. Chen, K. M. Engle, D.-H. Wang and J.-Q. Yu, Angew. Chem., Int. Ed., 2009, 48, 5094–5115 CrossRef CAS PubMed; (p) C.-L. Sun, B.-J. Li and Z.-J. Shi, Chem. Commun., 2010, 677–685 RSC; (q) J. Yamaguchi, A. D. Yamaguchi and K. Itami, Angew. Chem., Int. Ed., 2012, 51, 8960–9009 CrossRef CAS PubMed; (r) B. Li and P. H. Dixneuf, Chem. Soc. Rev., 2013, 42, 5744–5767 RSC; (s) D. Alberico, M. E. Scott and M. Lautens, Chem. Rev., 2007, 107, 174–238 CrossRef CAS PubMed; (t) T. W. Lyons and M. S. Sanford, Chem. Rev., 2010, 110, 1147–1169 CrossRef CAS PubMed; (u) I. V. Seregin and V. Gevorgyan, Chem. Soc. Rev., 2007, 36, 1173–1193 RSC; (v) Y.-Y. Ping, Q.-P. Ding and Y.-Y. Peng, ACS Catal., 2016, 6, 5989–6005 CrossRef CAS; (w) M. Gulías and J. L. Mascareñas, Angew. Chem., Int. Ed., 2016, 55, 11000–11019 CrossRef PubMed; (x) M. P. Drapeau and L. J. Gooßen, Chem.–Eur. J., 2016, 22, 18654–18677 CrossRef PubMed; (y) D.-H. Wei, X.-J. Zhu, J.-L. Niu and M.-P. Song, ChemCatChem, 2016, 8, 1242–1263 CrossRef CAS; (z) Z. Chen, B. Wang, J. Zhang, W. Yu, Z. Liu and Y. Zhang, Org. Chem. Front., 2015, 2, 1107–1295 RSC.
- For selected papers: (a) E. T. Nadres and O. Daugulis, J. Am. Chem. Soc., 2012, 134, 7–10 CrossRef CAS PubMed; (b) G. He, S.-Y. Zhang, W. A. Nack, Q. Li and G. Chen, Angew. Chem., Int. Ed., 2013, 52, 11124–11128 CrossRef CAS PubMed; (c) G. Shan, X.-L. Yang, Y. Zong and Y. Rao, Angew. Chem., Int. Ed., 2013, 52, 13606–13610 CrossRef CAS PubMed; (d) Q. Wang, J. Han, C. Wang, J.-Y. Zhang, Z.-B. Huang, D.-Q. Shi and Y.-S. Zhao, Chem. Sci., 2014, 5, 4962–4967 RSC; (e) A. Deb, S. Bag, R. Kancherla and D. Maiti, J. Am. Chem. Soc., 2014, 136, 13602–13605 CrossRef CAS PubMed; (f) H. Shiota, Y. Ano, Y. Aihara, Y. Fukumoto and N. Chatani, J. Am. Chem. Soc., 2011, 133, 14952–14955 CrossRef CAS PubMed; (g) Y.-J. Liu, H. Xu, W.-J. Kong, M. Shang, H.-X. Dai and J.-Q. Yu, Nature, 2014, 515, 389–393 CrossRef CAS PubMed; (h) W.-B. Yang, S.-Q. Ye, D. Fanning, T. Coon, Y. Schmidt, P. Krenitsky, D. Stamos and J.-Q. Yu, Angew. Chem., Int. Ed., 2015, 54, 2501–2504 CrossRef CAS PubMed; (i) S.-Y. Yan, Y.-J. Liu, B. Liu, Y.-H. Liu and B.-F. Shi, Chem. Commun., 2015, 51, 4069–4072 RSC; (j) Q. Zhang, X.-S. Yin, K. Chen, S.-Q. Zhang and B.-F. Shi, J. Am. Chem. Soc., 2015, 137, 8219–8226 CrossRef CAS PubMed; (k) D. Shabashov and O. Daugulis, J. Am. Chem. Soc., 2010, 132, 3965–3972 CrossRef CAS PubMed; (l) V. G. Zaitsev, D. Shabashov and O. Daugulis, J. Am. Chem. Soc., 2005, 127, 13154–13155 CrossRef CAS PubMed; (m) G. Li, L. Wan, G.-F. Zhang, D. Leow, J. Spangler and J.-Q. Yu, J. Am. Chem. Soc., 2015, 137, 4391–4397 CrossRef CAS PubMed; (n) L.-S. Zhang, G.-H. Chen, X. Wang, Q.-Y. Guo, X.-S. Zhang, F. Pan, K. Chen and Z.-J. Shi, Angew. Chem., Int. Ed., 2014, 53, 3899–3903 CrossRef CAS PubMed; (o) P. Becker, D. L. Priebbenow, R. Pirwerdjan and C. Bolm, Angew. Chem., Int. Ed., 2014, 53, 269–271 CrossRef CAS PubMed; (p) F. W. Patureau, T. Besset and F. Glorius, Angew. Chem., Int. Ed., 2011, 50, 1064–1067 CrossRef CAS PubMed; (q) K. M. Engle, D.-H. Wang and J.-Q. Yu, Angew. Chem., Int. Ed., 2010, 49, 6169–6173 CrossRef CAS PubMed; (r) M. Castaing, S. L. Wason, B. Estepa, J. F. Hooper and M. C. Willis, Angew. Chem., Int. Ed., 2013, 52, 13280–13283 CrossRef CAS PubMed; (s) L. Grigorjeva and O. Daugulis, Angew. Chem., Int. Ed., 2014, 53, 10209–10212 CrossRef CAS PubMed; (t) L. Yang, G.-Y. Zhang and H.-M. Huang, Adv. Synth. Catal., 2014, 356, 1509–1515 CrossRef CAS; (u) K. Parthasarathy and C. Bolm, Chem.–Eur. J., 2014, 20, 4896–4900 CrossRef CAS PubMed; (v) K. Yang, Y.-Q. Wang, X.-Y. Chen, A. A. Kadi, H.-K. Fun, H. Sun, Y. Zhang and H.-J. Lu, Chem. Commun., 2015, 51, 3582–3585 RSC; (w) C. Wang, C.-P. Chen, J.-Y. Zhang, J. Han, Q. Wang, K. Guo, P. Liu, M.-Y. Guan, Y.-M. Yao and Y.-S. Zhao, Angew. Chem., Int. Ed., 2014, 53, 9884–9888 CrossRef CAS PubMed; (x) X.-H. Ye and X.-D. Shi, Org. Lett., 2014, 16, 4448–4451 CrossRef CAS PubMed; (y) P. Liu, J. Han, Q. Wang, Z.-B. Huang, D.-Q. Shi, R.-S. Zeng and Y.-S. Zhao, RSC Adv., 2015, 5, 60646–60649 RSC; (z) G. Kumar and G. Sekar, RSC Adv., 2015, 5, 28292–28298 RSC.
- Recent reviews for directing group assisted C–H functionalization: (a) R.-Y. Zhu, M.-E. Farmer, Y.-Q. Chen and J.-Q. Yu, Angew. Chem., Int. Ed., 2016, 55, 10578–10599 CrossRef CAS PubMed; (b) D. A. Colby, A. S. Tsai, R. G. Bergman and J. A. Ellman, Acc. Chem. Res., 2011, 45, 814–825 CrossRef PubMed; (c) J. J. Mousseau and A. B. Charette, Acc. Chem. Res., 2013, 46, 412–424 CrossRef CAS PubMed; (d) R. Giri, B.-F. Shi, K. M. Engle, N. Maugel and J.-Q. Yu, Chem. Soc. Rev., 2009, 38, 3242–3272 RSC; (e) A. Ros, R. Fernandez and J. M. Lassaletta, Chem. Soc. Rev., 2014, 43, 3229–3243 RSC; (f) J.-D. Liu, G.-S. Chen and Z. Tan, Adv. Synth. Catal., 2016, 358, 1174–1194 CrossRef CAS; (g) C. S. Yeung and V. M. Dong, Chem. Rev., 2011, 111, 1215–1292 CrossRef CAS PubMed; (h) G. Rousseau and B. Breit, Angew. Chem., Int. Ed., 2011, 50, 2450–2494 CrossRef CAS PubMed; (i) L. Ackermann, Acc. Chem. Res., 2014, 47, 281–295 CrossRef CAS PubMed; (j) A. P. Pulis and D. J. Procter, Angew. Chem., Int. Ed., 2016, 55, 9842–9860 CrossRef CAS PubMed; (k) J. L. Roizen, M. E. Harvey and J. D. Bois, Acc. Chem. Res., 2012, 45, 911–922 CrossRef CAS PubMed; (l) L. Ackermann, Chem. Rev., 2011, 111, 1315–1345 CrossRef CAS PubMed; (m) C. Zheng and S.-L. You, RSC Adv., 2014, 4, 6173–6214 RSC; (n) G.-F. Zha, H.-L. Qin and E. A. B. Kantchev, RSC Adv., 2016, 6, 30875–30885 RSC; (o) T. S. Lobana, RSC Adv., 2015, 5, 37231–37274 RSC; (p) S. E. Kazzouli, J. Koubachi, N. E. Brahmia and G. Guillaumet, RSC Adv., 2015, 5, 15292–15327 RSC; (q) M. Zhang, Y. Zhang, X. Jie, H. Zhao, G. Li and W. Su, Org. Chem. Front., 2014, 1, 843–895 RSC; (r) G. Qiu and J. Wu, Org. Chem. Front., 2015, 2, 169–178 RSC.
- Selected papers for directing group assisted C–H functionalization: (a) Y.-Q. Yang, X.-D. Qiu, Y. Zhao, Y.-C. Mu and Z.-Z. Shi, J. Am. Chem. Soc., 2016, 138, 495–498 CrossRef CAS PubMed; (b) K. L. Hull, E. L. Lanni and M. S. Sanford, J. Am. Chem. Soc., 2006, 128, 14047–14049 CrossRef CAS PubMed; (c) L. V. Desai, K. L. Hull and M. S. Sanford, J. Am. Chem. Soc., 2004, 126, 9542–9543 CrossRef CAS PubMed; (d) M. Yu, Y.-J. Xie, C.-S. Xie and Y.-H. Zhang, Org. Lett., 2012, 14, 2164–2167 CrossRef CAS PubMed; (e) X. Chen, C. E. Goodhue and J.-Q. Yu, J. Am. Chem. Soc., 2006, 128, 12634–12635 CrossRef CAS PubMed; (f) Y.-J. Liu, Y.-H. Liu, Z.-Z. Zhang, S.-Y. Yan, K. Chen and B.-F. Shi, Angew. Chem., Int. Ed., 2016, 128, 14063–14066 CrossRef; (g) L. Ackermann, A. Althammer and R. Born, Angew. Chem., Int. Ed., 2006, 45, 2619–2622 CrossRef CAS PubMed; (h) C.-H. Huang, B. Chattopadhyay and V. Gevorgyan, J. Am. Chem. Soc., 2011, 133, 12406–12409 CrossRef CAS PubMed; (i) X.-B. Wan, Z.-X. Ma, B.-J. Li, K.-Y. Zhang, S.-K. Cao, S.-W. Zhang and Z.-J. Shi, J. Am. Chem. Soc., 2006, 128, 7416–7417 CrossRef CAS PubMed; (j) T. Kang, Y. Kim, D. Lee, Z. Wang and S. Chang, J. Am. Chem. Soc., 2014, 136, 4141–4144 CrossRef CAS PubMed; (k) M. Nishino, K. Hirano, T. Satoh and M. Miura, Angew. Chem., Int. Ed., 2013, 52, 4457–4461 CrossRef CAS PubMed; (l) O. Daugulis and V. G. Zaitsev, Angew. Chem., Int. Ed., 2005, 44, 4046–4048 CrossRef CAS PubMed; (m) A. R. Dick, K. L. Hull and M. S. Sanford, J. Am. Chem. Soc., 2004, 126, 2300–2301 CrossRef CAS PubMed; (n) D.-D. Li, T.-T. Yuan and G.-W. Wang, Chem. Commun., 2011, 47, 12789–12791 RSC; (o) C.-M. Wang, H. Chen, Z.-F. Wang, J.-A. Chen and Y. Huang, Angew. Chem., Int. Ed., 2012, 51, 7242–7245 CrossRef CAS PubMed; (p) M. Wasa, K. M. Engle and J.-Q. Yu, J. Am. Chem. Soc., 2009, 131, 9886–9887 CrossRef CAS PubMed; (q) Z. Ren, F. Mo and G. Dong, J. Am. Chem. Soc., 2012, 134, 16991–16994 CrossRef CAS PubMed; (r) M. Mewald, J. A. Schiffner and M. Oestreich, Angew. Chem., Int. Ed., 2012, 51, 1763–1765 CrossRef CAS PubMed; (s) H.-Y. Thu, W.-Y. Yu and C.-M. Che, J. Am. Chem. Soc., 2006, 128, 9048–9049 CrossRef CAS PubMed; (t) G.-X. Cai, Y. Fu, Y.-Z. Li, X.-B. Wan and Z.-J. Shi, J. Am. Chem. Soc., 2007, 129, 7666–7673 CrossRef CAS PubMed; (u) A. García-Rubia, R. G. Arrayás and J. C. Carretero, Angew. Chem., Int. Ed., 2009, 48, 6511–6515 CrossRef PubMed; (v) Z.-L. Fan, J.-B. Ni and A. Zhang, J. Am. Chem. Soc., 2016, 138, 8470–8475 CrossRef CAS PubMed; (w) Y. Ano, M. Tobisu and N. Chatani, J. Am. Chem. Soc., 2011, 133, 12984–12986 CrossRef CAS PubMed; (x) B. Liu, R. Li, W. Zhan, X. Wang, Z. Ge and R. Li, RSC Adv., 2016, 6, 48205–48211 RSC; (y) R. Sunke, V. Kumar, E. V. V. S. Ramarao, R. Bankala, K. V. L. Parsa and M. Pal, RSC Adv., 2015, 5, 70604–70608 RSC; (z) C. Geng, M. Jiang, L. Feng and P. Jiao, RSC Adv., 2016, 6, 56971–56976 RSC.
- (a) C. T. Walsh, R. V. O'Brien and C. Khosla, Angew. Chem., Int. Ed., 2013, 52, 7098–7124 CrossRef CAS PubMed; (b) H. B. Kroona, N. L. Peterson, J. F. Koerner and R. L. Johnson, J. Med. Chem., 1991, 34, 1692–1699 CrossRef CAS PubMed; (c) J. S. R. Kumar and A. Datta, Tetrahedron Lett., 1997, 38, 473–476 CrossRef CAS; (d) F. Schweizer, Angew. Chem., Int. Ed., 2002, 41, 230–253 CrossRef CAS; (e) S. Y. Kwak, J. K. Yang, J. H. Kim and Y. S. Lee, Biopolymers, 2013, 100, 584–591 CrossRef CAS PubMed; (f) E. Gershonov, R. Granoth, E. Tzehoval, Y. Gaoni and M. Fridkin, J. Med. Chem., 1996, 39, 4833–4843 CrossRef CAS PubMed; (g) Y. Wang, J. B. Gloer, J. A. Scott and D. Malloch, J. Nat. Prod., 1995, 58, 93–99 CrossRef CAS PubMed.
- For selected reviews and papers: (a) G. He, B. Wang, W. A. Nack and G. Chen, Acc. Chem. Res., 2016, 49, 635–645 CrossRef CAS PubMed; (b) M.-Y. Fan and D.-W. Ma, Angew. Chem., Int. Ed., 2013, 52, 12152–12155 CrossRef CAS PubMed; (c) B. D. Dangel, J. A. Johnson and D. Sames, J. Am. Chem. Soc., 2001, 123, 8149–8150 CrossRef CAS PubMed; (d) B. Wang, W. A. Nack, G. He, S.-Y. Zhang and G. Chen, Chem. Sci., 2014, 5, 3952–3957 RSC; (e) S. Aspin, A. S. Goutierre, P. Larini, R. Jazzar and O. Baudoin, Angew. Chem., Int. Ed., 2012, 51, 10808–10811 CrossRef CAS PubMed; (f) B. V. S. Reddy, L. R. Reddy and E. J. Corey, Org. Lett., 2006, 8, 3391–3394 CrossRef CAS PubMed; (g) S.-Y. Zhang, Q. Li, G. He, W. A. Nack and G. Chen, J. Am. Chem. Soc., 2013, 135, 12135–12141 CrossRef CAS PubMed; (h) J. He, S. Li, Y. Deng, H. Fu, B. N. Laforteza, J. E. Spangler, A. Homs and J.-Q. Yu, Science, 2014, 343, 1216–1220 CrossRef CAS PubMed; (i) Q. Zhang, K. Chen, W.-H. Rao, Y. Zhang, F.-J. Chen and B.-F. Shi, Angew. Chem., Int. Ed., 2013, 52, 13588–13592 CrossRef CAS PubMed; (j) G.-F. Zhang, X.-Q. Xie, J.-F. Zhu, S.-S. Li, C.-R. Ding and P. Ding, Org. Biomol. Chem., 2015, 13, 5444–5449 RSC; (k) L. D. Tran and O. Daugulis, Angew. Chem., Int. Ed., 2012, 51, 5188–5191 CrossRef CAS PubMed; (l) F. Gao, W. Zhu, D.-Y. Zhang, S.-J. Li, J. Wang and H. Liu, J. Org. Chem., 2016, 81, 9122–9130 CrossRef CAS PubMed; (m) R.-Y. Zhu, K. Tanaka, G.-C. Li, J. He, H.-Y. Fu, S.-H. Li and J.-Q. Yu, J. Am. Chem. Soc., 2015, 137, 7067–7070 CrossRef CAS PubMed; (n) N. Rodriguez, J. A. Romero-Revilla, M. A. Fernandez-Ibanez and J. C. Carretero, Chem. Sci., 2013, 4, 175–179 RSC; (o) K. Chen, F. Hu, S.-Q. Zhang and B.-F. Shi, Chem. Sci., 2013, 4, 3906–3911 RSC; (p) J. Kim, M. Sim, N. Kim and S. Hong, Chem. Sci., 2015, 6, 3611–3616 RSC; (q) F.-M. Meyer, S. Liras, A. Guzman-Perez, C. Perreault, J.-W. Bian and K. James, Org. Lett., 2010, 12, 3870–3873 CrossRef CAS PubMed.
- (a) J.-J. Li, T.-S. Mei and J.-Q. Yu, Angew. Chem., Int. Ed., 2008, 47, 6452–6455 CrossRef CAS PubMed; (b) A. García-Rubia, E. Laga, C. Cativiela, E. P. Urriolabeitia, R. Gómez-Arrayás and J. C. Carretero, J. Org. Chem., 2015, 80, 3321–3331 CrossRef PubMed.
- Y.-S. Zhao and G. Chen, Org. Lett., 2011, 13, 4850–4853 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and additional spectra. See DOI: 10.1039/c7ra02574b |
| This journal is © The Royal Society of Chemistry 2017 |