Open Access Article
Open Access ArticleComparative suppression of NLRP3 inflammasome activation with LPS-induced inflammation by blueberry extracts (Vaccinium spp.)
Huailing Wanga,
Xinbo Guo *ab,
Jie Liuc,
Tong Lib,
Xiong Fua and
Rui Hai Liu*b
*ab,
Jie Liuc,
Tong Lib,
Xiong Fua and
Rui Hai Liu*b
aSchool of Food Science and Engineering, South China University of Technology, Guangzhou 510641, China. E-mail: xbg720@gmail.com; Tel: +86-20-87113848
bDepartment of Food Science, Stocking Hall, Cornell University, Ithaca, New York 14853, USA. E-mail: rl23@cornell.edu; Tel: +1-607-255-6235
cInstitute of Traditional Chinese Medicine and Natural Products, College of Pharmacy, Jinan University, Guangzhou 510632, China
First published on 1st June 2017
Abstract
The aim of this study was to evaluate the anti-inflammation effects of blueberry extracts through NLRP3 inflammasome. The anti-inflammation activities of blueberry extracts were detected using lipopolysaccharide (LPS) activated the mononuclear macrophage (RAW264.7), and then detected the gene expression levels of NLRP3, Caspase-1, apoptosis-associated specked-like protein (ASC) and inflammatory factor including IL-1β, TNF-α, IL-6, and iNOS. Blueberry extracts could significantly inhibit the gene expression of IL-1β, TNF-α, IL-6, and iNOS, but the inhibitions were showed differently among the varieties of Blomidom (BD), North Country (NC) and JK-M7 (JK). The protein expressions of NLRP3 and Caspase-1 in the blueberry treated groups were significantly lower than the LPS treated group. Quercetin significantly inhibited LPS-induced ROS and inhibited the mRNA expression and protein levels of NLRP3 and Caspase-1 in RAW264.7 cells.
Introduction
In recent years there have been many epidemiological and clinical studies1 which have indicated that chronic inflammation is a significant risk factor in various human diseases, in particular cardiovascular diseases, diabetics and cancer.2,3 In terms of human immune-response, the innate immunity is the first defence against pathogens (such as bacteria, fungi, and viruses, etc.) discriminating “self” from “non-self”. The innate immune system utilizes an array of germline-encoded pattern-recognition receptors (PRRs) to detect invariant microbial attacks. These recognize situations of host danger promoting activation of the immune system and tissue repair in response to infection or injury. In such a situation the host secret several pro-inflammatory cytokines such as TNF-α, IL-1, IL-6, IL-8 and IL-12, and the anti-inflammatory cytokines such as IL-4, IL-10, IL-11 as well as transforming growth factor-β (TGF-β). The cytokines keep the balances in the body to play central roles in mediating and modulating inflammation.This activation is critical for pathogen clearance and the induction of an adaptive immune response.2 The central roles of the NLR family (including NLRP1, NLRP3, and NLRPC4) in the immune system has become increasingly noticed in recent years.4,5 One of the best characterized inflammasome is NLRP3, also known as CIAS1, CRYOPYRIN, NALP3 or PYPAF. This substance is encoded by the NLRP3 (NOD-like receptor family, pyrin domain) gene in humans. NLRP3-mediated inflammasome activation occurs in response to diverse molecular entities of bacteria,6 viruses,7,8 fungi,9 dying cells,6 crystal particles,10–13 and DNA.14 Normal activation of the NLRP3 inflammasome contributes to host defence. However, excessive activation of the NLRP3 signalling leads to the pathogenesis of a spectrum of auto-inflammatory diseases.15,16
The NLRP3 inflammasome is a multi-protein complex that could trigger the maturation of the pro-inflammatory cytokines IL-1β and IL-18.4,17 Upon activation, NLRs recruit adaptor ASC and effector Caspase-1 to form large cytoplasmic complexes, defined as the inflammasome.18 The encoded protein may play a role in the regulation of inflammation and apoptosis. Consistent with an essential role for NLRP3 inflammasome in antifungal immunity, Gross et al.9 showed that NLRP3-deficient mice were hyper-susceptible to C. albicans infection. Activation of the NLRP3 inflammasome in response to bacteria, viruses or to RNA/DNA was dependent upon liposomal maturation and reactive oxygen species production in human cells. It has been suggested that the NLRP3 inflammasome senses obesity-associated danger signals and contributes to obesity-induced inflammation and insulin resistance accumulation of inflammation markers in these tissues.19,20 Therefore, NLRP3 inflammasome signalling is regulated tightly to maintain immune balance.6,21
Reports22 had been suggested that the activated of the NLRP3 through 3 signals as the following: the ATP or nigericin could induce mitochondrial dysfunction, which contributes to NLRP3 inflammasome activation through release of mtDAMP, such as mtDNA and mtROS, or by inducing a borning mitochondrial movement. The second signal was that the activation of NLRP3 inflammasome which was related with the TLR receptors through the nuclear factor-kappa B (NF-κB) signal transduction pathway. The last signal was that the mitochondrial fusion protein 2 (Mfn2), antiviral immune signalling pathway junction protein (MAVS) and the mitochondrial membrane cardiolipin together recruit the NLRP3 and the NLRP3 protein, combine the ASC and active the procaspase-1 protein to assemble the NLRP3 inflammasome, which lead to the pro-inflammatory process through the production of IL-1β or IL-18. Many reports23,24 indicate that the microbial products, such as nigericin, maitotoxin, hemolysin, or bacterial RNA, induce the robust secretion of IL-1β from LPS-primed bone marrow-derived macrophages (BMDNs) in an NLRP3-dependent manner. To our knowledge, this was the first reports that the blueberry extracts and quercetin exhibited anti-inflammation activity through inhibiting the produce of the NLRP3 inflammasome.
Blueberries are cultivated widely throughout the world ranking behind strawberries in terms of total production25 and belong to the family of Ericaceae Vaccinium. Blueberries are popular in terms of taste and are rich in anthocyanin including (cyanidin chloride, petunidin chloride, peonidin chloride, delphinidin, Malvin), phenolics and flavonoids.26 These compounds have been studied in relation to the biological activity of blueberry extracts in mediating antitumor activity,27 antioxidant activity,28,29 and innate and or adaptive immune system.30 The increased consumption of fruits has been correlated with health benefits and linked with decreased incidence of several diseases, including metabolic syndrome,31 and diabetes.2,32
Blueberries have been shown to block the nuclear translocation of p65 mediated by NF-κB pathway.33 This is associated with inhibition of TNF-α-induced inflammatory responses,34 and potential stimulation of anti-inflammatory activity against interleukin-8 (IL-8) for inhibited of matrix metalloproteinase-1 (MMP-1) expression.35 The anthocyanins of blueberries have been shown to reduce the activation of NF-κB, induced by IL-1β in intestinal epithelial Caco-2 cells.36 These results support that blueberry bioactive compounds have potential bioactivities to act as dietary anti-inflammatory agents. Many researchers have reported that quercetin is a strong antioxidant in fruits.37,38 Though blueberry has been extensively consumed, the detailed molecular mechanism of actions is only being explored in recent years.
Previous studies suggested that NLRP3 inflammasome activity is negatively regulated by autophagy and positively regulated by reactive oxygen species (ROS) derived from an uncharacterized organelle. As the molecular mechanisms by which NLRP3 agonists induce mitochondrial ROS production and mitochondrial ROS activate the NLRP3 inflammasome are unknown, the relationship of the ROS and the NLRP3 need to be investigated. In this manuscript, the RAW264.7 cells were stimulated by LPS to produce NLRP3 inflammasome, and the expression of the mRNA and protein level in the stimulated cells was determined to evaluate the anti-inflammation activities of different blueberry varieties.
Results
Cytotoxicity assay of blueberry extracts
The cytotoxic effects of blueberry were tested on RAW264.7 cells using the method reported previously.39 The cytotoxic effects of quercetin, and blueberry extracts were tested on RAW264.7 cells line as shown in Fig. 1. The CC50 value of the standard was quercetin (241.0 ± 7.2 μmol L−1). The CC50 value of blueberry extracts ranged from 64.32 ± 3.44 to 162.5 ± 4.7 mg mL−1. The lowest CC50 values were found in varieties of BD (64.04 ± 5.91 mg mL−1), whilst the variety of NC was 90.33 ± 5.83 mg mL−1, and the varieties of JK had the highest values: 162.0 ± 9.5 mg mL−1. Previous reports40 have been suggested that the blueberry extracts (400 μg mL−1) had highly anti-inflammation effects in RAW264.7 macrophages.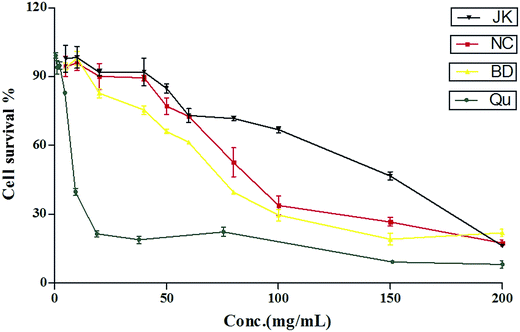 | ||
| Fig. 1 The cytotoxicity of the blueberry against mononuclear macrophage Raw264.7 cells (mean ± SD, n = 3). | ||
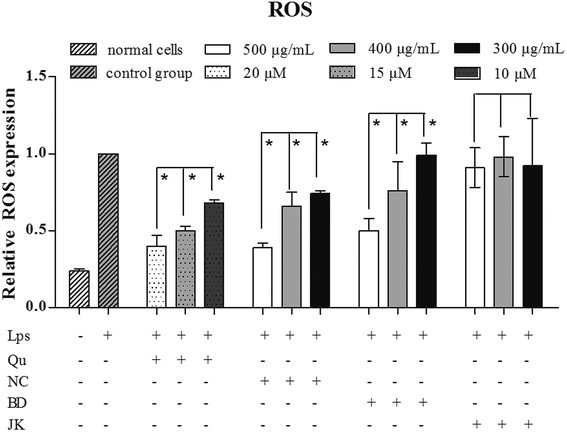
ROS detection of blueberry extracts
To investigate the oxidative damage effect of ROS, the RAW264.7 cells were stimulated with LPS. The blueberry extracts and quercetin dramatically inhibited cellular ROS expression (Fig. 2). The LPS-treated group was regarded as the negative group, and the relative expression of ROS expression was regarded as 1. The group without blueberry and no LPS regarded as control group, and the relative expression of the ROS was 0.240 ± 0.097. Quercetin was used as positive control. The relative expression of the ROS was ranged from 0.407 ± 0.007 to 0.681 ± 0.023 in a dose-dependent manner. The varieties of BD showed the lowest relative expression between 0.392 ± 0.031 and 0.743 ± 0.021 with a dose-dependent manner, whilst the JK variety emerged the highest relative expression between 0.911 ± 0.310 and 0.982 ± 0.130. The BD variety was 0.504 ± 0.082 to 0.993 ± 0.082.Based on the experimental results, the EC50 value of the relative expression of ROS was calculated. The value of the NC was 454 ± 12 μg mL−1, and the BD was 483 ± 19 μg mL−1. The EC50 values of JK variety cannot be calculated. The phytochemical compositions in blueberry have been reported42 that the varieties of the NC, BD and JK contained abundant of phenolics, flavonoids and several anthocyanin. Correlations among cyanidin, delphinidin, malvidin, petunidin, peonidin, p-coumaric, caffeic acid, ferulic acid, salicylic acid, quercetin, catechin, resveratrol, relative ROS expression in low concentration (ROS-L), relative ROS expression in middle concentration (ROS-M), relative ROS expression in high concentration (ROS-H) of blueberry were analysed. ROS-H values were highly positive correlation with ferulic acid (0.938), and negative correlation to catechin (−1.000) and petunidin value (−0.715). ROS-M values were highly correlation with ferulic acid (0.970), and negative correlation to catechin (−0.995) and p-coumaric value (−0.949). ROS-L were highly correlated to ferulic acid (0.726), resveratrol (0.904), caffeic acid (0.666) and quercetin (0.654) value, while low correlation with malvidin (−0.996). The results indicated that phenolics played the main roles for in ROS formation. ROS was considered as an oxidization molecule which played an important role in the oxidative process.41 In addition to its oxidization effects, ROS has been known as an immune-modulatory molecule, although the mechanisms underlying this immune-modulation are not well understood. In this manuscript, the inflammatory cytokine were detected.
Relative gene expression of inflammatory markers
LPS is a TLR4 specific agonist that has been used to be a strong inducer of inflammatory responses in macrophages. To investigate whether the production of the cytokine and NLRP3 inflammasome including ASC, NLRP3, IL-1β were influenced by the blueberry extracts, RAW264.7 cells were treated with the certain concentrations of the blueberry extracts and quercetin in the present of LPS. In this experiment, we determined the effect of quercetin and blueberry extracts on the relative expression of genes (MusASC, MusNLRP3, MusIL-1β, MusCaspase-1, MusIL-6, MusTNF-α, MusiNOS), and the results were presented in the Table 2.| Gene | Gene ID | Direction | Primer sequences (5′–3′) |
|---|---|---|---|
| MusGAPDH | 14433 | Forward | GTCATTGAGAGCAATGCCAG |
| Reverse | GTGTTCCTACCCCCAATGTG | ||
| MusNLRP3 | 216799 | Forward | GTGGAGATCCTAGGTTTCTCTG |
| Reverse | CAGGATCTCATTCTCTTGGATC | ||
| MusCaspase-1 | 12362 | Forward | GAGCTGATGTTGACCTCAGAG |
| Reverse | CTGTCAGAAGTCTTGTGCTCTG | ||
| MusIL-1β | 16176 | Forward | GAGCCTGTGTTTCCTCCTTG |
| Reverse | TCCAAGAAACCATCTGGCTAGG | ||
| MusASC | 66824 | Forward | CTCTGTATGGCAATGTGCTGAC |
| Reverse | GAACAAGTTCTTGCAGGTCAG | ||
| MusTNF-α | 21926 | Forward | GGGAGCAAAGGTTCAGTGAT |
| Reverse | CCTGGCCTCTCTACCTTGTT | ||
| MusIL-6 | 16193 | Forward | CTGACAATATGAATGTTGGG |
| Reverse | TCCAAGAAACCATCTGGCTAGG | ||
| MusiNOS | 18126 | Forward | AAGCAGCTGGCCAATGAG |
| Reverse | CCCCATAGGAAAAGACTGC |
| Qu | NC | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 10 (μM) | 15 (μM) | 20 (μM) | 0 | 300 (μg mL−1) | 400 (μg mL−1) | 500 (μg mL−1) | |
| MusNLRP3 | 0.091 ± 0.002 | 0.515 ± 0.016 | 0.244 ± 0.006 | 0.144 ± 0.014 | 0.091 ± 0.002 | 0.276 ± 0.018 | 0.184 ± 0.026 | 0.077 ± 0.096 |
| MusCaspase-1 | 0.523 ± 0.031 | 0.521 ± 0.018 | 0.395 ± 0.036 | 0.358 ± 0.079 | 0.523 ± 0.031 | 0.240 ± 0.058 | 0.180 ± 0.020 | 0.210 ± 0.004 |
| MusIL-1β | ND | 0.471 ± 0.081 | 0.250 ± 0.063 | 0.214 ± 0.046 | ND | 0.028 ± 0.001 | 0.008 ± 0.001 | 0.002 ± 0.0001 |
| MusASC | 0.568 ± 0.121 | 0.273 ± 0.014 | 0.180 ± 0.056 | 0.087 ± 0.033 | 0.568 ± 0.121 | 0.004 ± 0.001 | 0.005 ± 0.000 | 0.004 ± 0.0001 |
| MusTNF-α | 0.013 ± 0.001 | 0.462 ± 0.017 | 0.371 ± 0.029 | 0.321 ± 0.068 | 0.013 ± 0.001 | 0.682 ± 0.026 | 0.426 ± 0.011 | 0.287 ± 0.005 |
| MusIL-6 | ND | 0.731 ± 0.046 | 0.682 ± 0.035 | 0.348 ± 0.018 | ND | 0.178 ± 0.008 | 0.005 ± 0.001 | 0.012 ± 0.008 |
| MusiNOS | 0.002 ± 0.0001 | 0.664 ± 0.035 | 0.682 ± 0.013 | 0.751 ± 0.003 | 0.002 ± 0.000 | 0.068 ± 0.002 | 0.013 ± 0.001 | 0.011 ± 0.001 |
| BD | JK | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 300 (μg mL−1) | 400 (μg mL−1) | 500 (μg mL−1) | 0 | 300 (μg mL−1) | 400 (μg mL−1) | 500 (μg mL−1) | |
| MusNLRP3 | 0.091 ± 0.002 | 0.408 ± 0.067 | 0.365 ± 0.070 | 0.196 ± 0.039 | 0.091 ± 0.002 | 0.111 ± 0.009 | 0.054 ± 0.002 | 0.026 ± 0.001 |
| MusCaspase-1 | 0.523 ± 0.031 | 0.768 ± 0.014 | 0.802 ± 0.027 | 0.605 ± 0.035 | 0.523 ± 0.031 | 0.328 ± 0.032 | 0.238 ± 0.008 | 0.119 ± 0.004 |
| MusIL-1β | ND | 0.595 ± 0.081 | 0.334 ± 0.063 | 0.248 ± 0.046 | ND | 0.234 ± 0.013 | 0.157 ± 0.004 | 0.054 ± 0.029 |
| MusASC | 0.568 ± 0.121 | 0.272 ± 0.021 | 0.172 ± 0.033 | 0.158 ± 0.019 | 0.568 ± 0.121 | 0.001 ± 0.000 | 0.008 ± 0.006 | 0.001 ± 0.0001 |
| MusTNF-α | 0.013 ± 0.001 | 0.672 ± 0.021 | 0.510 ± 0.018 | 0.445 ± 0.033 | 0.013 ± 0.001 | 0.856 ± 0.122 | 0.940 ± 0.059 | 0.721 ± 0.030 |
| MusIL-6 | ND | 0.751 ± 0.003 | 0.774 ± 0.013 | 0.664 ± 0.035 | ND | 0.537 ± 0.047 | 0.497 ± 0.019 | 0.363 ± 0.015 |
| MusiNOS | 0.002 ± 0.0001 | 1.445 ± 0.046 | 1.157 ± 0.035 | 1.299 ± 0.018 | 0.002 ± 0.000 | 0.584 ± 0.021 | 0.536 ± 0.011 | 0.364 ± 0.021 |
The values of the LPS-induced treatment groups were expressed as 1. The relative expressions of MusNLRP3 were sharply decreased with the increasing concentrations of the blueberry extracts and the quercetin. In the quercetin treatment groups, the relative expressions of MusNLRP3 were between 0.515 ± 0.016 and 0.144 ± 0.014, the relative expressions of MusCaspase-1 were between 0.521 ± 0.018 and 0.358 ± 0.079, and the relative expressions of MusASC were 0.273 ± 0.014 to 0.087 ± 0.033. The relative expressions of the other Inflammatory factors were as following: MusIL-1β (0.471 ± 0.081 to 0.214 ± 0.046), MusTNF-α (0.462 ± 0.017 to 0.321 ± 0.068), MusIL-6 (0.731 ± 0.046 to 0.348 ± 0.018), MusiNOS (0.664 ± 0.035 to 0.751 ± 0.003). The NC variety highly inhibited the expression of the MusNLRP3, MusCaspase-1, MusIL-1β, MusASC, MusTNF-α, MusIL-6, and MusiNOS. Meanwhile the varieties of the blueberry extracts including BD, NC, and JK had high inhibitory effects to the inflammatory factors. In the NC variety, the relative expressions of MusNLRP3 were between 0.276 ± 0.018 and 0.077 ± 0.096, the relative expressions of MusCaspase-1 were between 0.240 ± 0.058 and 0.210 ± 0.004, and the relative expression of MusASC was 0.004 ± 0.001. The relative expressions of the other Inflammatory factors were as following: MusIL-1β (0.028 ± 0.001 to 0.002 ± 0.0001), MusTNF-α (0.682 ± 0.026 to 0.287 ± 0.005), MusIL-6 (0.178 ± 0.008 to 0.012 ± 0.008), and MusiNOS (0.068 ± 0.002 to 0.011 ± 0.001). The relative expression NLRP3 mRNA in BD was between 0.408 ± 0.067 and 0.196 ± 0.039, the relative expressions of MusCaspase-1 were between 0.768 ± 0.014 and 0.605 ± 0.035, and the relative expressions of MusASC were 0.272 ± 0.021 to 0.158 ± 0.019. But the relative expressions of MusiNOS had no inhibitory effects and the values were higher than the LPS-treated group. The relative of expressions of the other inflammatory factors were as following: MusIL-1β (0.595 ± 0.081 to 0.248 ± 0.046), MusTNF-α (0.672 ± 0.021 to 0.445 ± 0.033), MusIL-6 (0.774 ± 0.013 to 0.664 ± 0.035). In the JK variety, the relative expressions of MusNLRP3 were between 0.111 ± 0.009 and 0.026 ± 0.001, the relative expressions of MusCaspase-1 were between 0.328 ± 0.032 and 0.119 ± 0.004, and the relative expressions of MusASC was only 0.001 ± 0.0001. The relative expressions of the other inflammatory factors were as following: MusIL-1β (0.234 ± 0.013 to 0.054 ± 0.029), MusTNF-α (0.940 ± 0.059 to 0.721 ± 0.030), MusIL-6 (0.537 ± 0.047 to 0.363 ± 0.015), MusiNOS (0.584 ± 0.021 to 0.364 ± 0.021). In summary, the blueberry extracts and quercetin significantly inhibited the gene expressions of the MusNLRP3, which was important to the NLRP3 inflammasome, and other inflammatory factors.
Previous reports40 suggested that the phenolics from blueberry had significant anti-inflammatory activities which could suppress the gene expressions of MusIL-1β, MusIL-6, and MusIL-12p35 in LPS-induced RAW264.7 macrophages. The relative gene expression of MusNLRP3 was concentration-dependent, and the correlation values were: NC (0.998), BD (0.987), and JK (0.963). The highest inhibitory effects were observed in the MusIL-1β, and the correlation values were: NC (0.912), BD (0.922), JK (0.993). The correlation values of MusTNF-α were: NC (0.972), BD (0.943), and JK (0.373). The correlation values of MusIL-6 expression were: NC (0.718), BD (0.562), and JK (0.911). The correlation values of MusiNOS were as following: NC (0.776), BD (0.257), and JK (0.904). The correlation values of MusCaspase-1 were: NC (0.250), BD (0.599), and JK (0.994). The relative expression of MusASC did not exhibited concentration-dependent, and the correlation values were: NC (0.001), BD (0.841), JK (0.001).
According to the previously reports42 the correlations between phytochemical compositions including cyanidin, delphinidin, malvidin, petunidin, peonidin, p-coumaric, caffeic acid, ferulic acid, salicylic acid, quercetin, catechin, and resveratrol, and relative cell factor expressions in different concentrations were analyzed. MusNLRP3 was highly correlated with salicylic acid (0.822), quercetin (0.765), and caffeic acid (0.760). MusCaspase-1 was highly correlated with salicylic acid (0.838), quercetin (0.951), caffeic acid (0.953) and resveratrol (0.915). MusIL-1β was highly correlated with resveratrol (0.854). MusASC was highly correlated with salicylic acid (0.850), quercetin (0.954), caffeic acid (0.951) and resveratrol (0.902). MusIL-6 was highly correlated with resveratrol (0.985). MusiNOS was highly correlated with quercetin (0.886), caffeic acid (0.892) and resveratrol (0.985). MusTNF-α was correlated with phytochemical compositions, and the best correlation value was 0.749. The results indicated that phenolic acids (caffeic acid and salicylic acid) and flavonoids (quercetin) and resveratrol from blueberry played the main roles in cell factor expressions.
Phenolic compounds, for example anthocyanin, have been suggested to have an anti-inflammatory effect against LPS-induced changes in immune cells. Black rice extract39 could suppress of inflammatory responses via down-regulation of NF-κB and AP-1 signalling pathways. Red bean extracts43 were reported to inhibit LPS-induced inflammation and H2O2-induced oxidative stress in RAW264.7 cells. Johnson et al.37 reported that the anthocyanins and proanthocyanidins from blueberry–blackberry fermented beverages inhibit markers of inflammation in macrophage. Garcia-Diaz et al.40 reported that the anthocyanins of fermented berry beverages could inhibit inflammation related adiposity response in vitro. Beneficial effects of berries in the diet have also been attributed to their high phenolic contents.42 Research has shown that blueberries could reduce pro-inflammatory cytokine TNF-α, and IL-6 production in mouse macrophages by inhibiting NF-κB activation and the MAPK pathway.44
In summary, blueberry extracts and quercetin inhibited expressions of MusASC, MusCaspase-1, and MusNLRP3, which were the components of the NLRP3 inflammasome and the pro-inflammatory cytokines including TNF-a, IL-6 and iNOS.
Western blot analysis
To explore whether the blueberry extracts and the quercetin had an effect on LPS-induced activation of NLRP3 inflammasome, RAW264.7 cells were treated with blueberry extracts and quercetin in the presence or absence of LPS followed by Western blot analysis. Studying the activation of NLRP3 inflammasome, the expression of the Caspase-1 and the NLRP3 protein and the values of protein relative expression were presented in Fig. 3.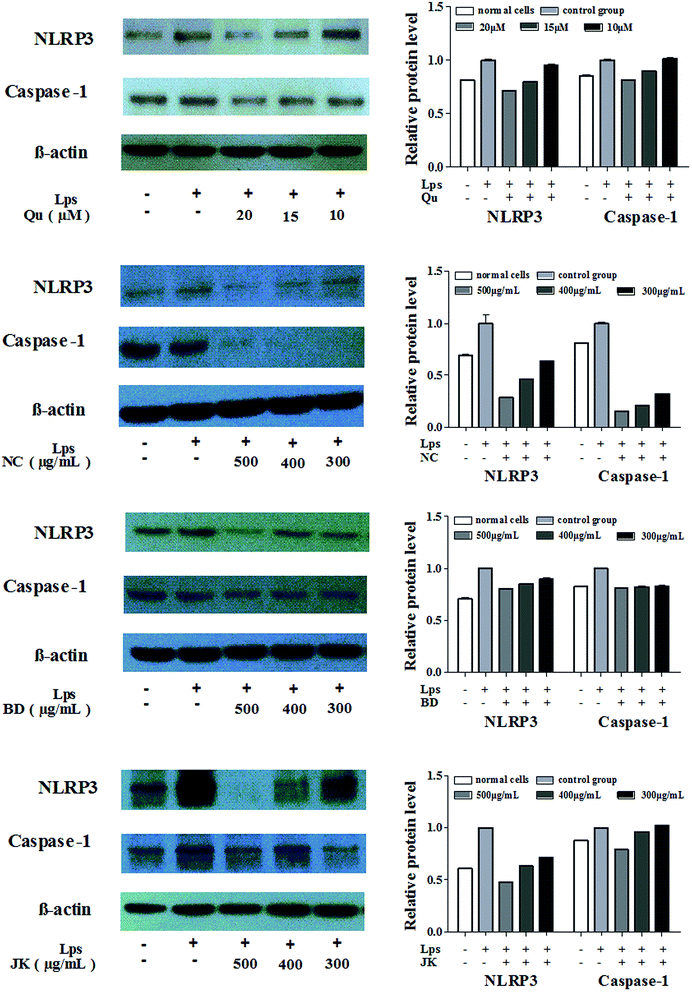 | ||
| Fig. 3 The relative expression level of NLRP3 and Caspase-1 protein in mononuclear macrophage Raw264.7 cells (mean ± SD, n = 3). | ||
The values of LPS-induced treatment groups were expressed as 1. The NLRP3 expressions of protein levels were sharply decreased with the increasing concentrations of the blueberry extracts and the quercetin. In the NC variety, the relative protein expressions of NLRP3 were between 0.637 ± 0.001 and 0.288 ± 0.001, the relative Caspase-1 protein expressions were between 0.321 ± 0.001 and 0.158 ± 0.001. The BD variety inhibited the expression of the NLRP3 (0.806 ± 0.006 to 0.898 ± 0.014) and Caspase-1 (0.832 ± 0.011 to 0.809 ± 0.008). Meanwhile the JK variety had low inhibition effects to the NLRP3 and Caspase-1. In the JK variety, the relative protein expressions of NLRP3 were between 0.715 ± 0.003 and 0.479 ± 0.001, and the relative protein Caspase-1 expressions were between 1.024 ± 0.006 and 0.788 ± 0.006. In summary, the blueberry extracts and quercetin could significantly inhibit the expression of the protein NLRP3 and Caspase-1 which were composed of the NLRP3 inflammasome, and other inflammatory factor.
The relative protein expression of NLRP3 was concentration-dependent with the highest inhibitory effects, and the correlation values were: NC (0.999), BD (0.999), and JK (0.970). The higher inhibitory effects were observed in the protein Caspase-1 with correlation values: NC (0.964), BD (0.987), and JK (0.930). The correlations between phytochemical composition and relative protein expression of the NLRP3 and Caspase-1 were analyzed. The relative expression of the NLRP3 values was highly correlated with resveratrol (0.843), quercetin (0.754), and caffeic acid (0.762). The relative protein expression of Caspase-1 values were highly correlated with ferulic acid (0.914), and negatively correlated to the anthocyanin (<−0.900). The results indicated that phenolic acids (caffeic acid, salicylic acid) and flavonoids (quercetin) and resveratrol from blueberries played the main roles in the regulation of protein expressions.
Correlation of gene expressions and ROS
In this study the inhibition of the ROS production and inflammatory cytokines were first detected in LPS-activated macrophages. Different varieties of blueberry extracts had different inhibitory effects. Our results suggest that the ROS may exert to suppress NLRP3 inflammasome activation, in turn inhibiting IL-1β secretion, Caspase-1 cleavage and ASC pyroptosome formation.Correlations among the expressions of MusNLRP3, MusIL-1β, MusASC, MusTNF-α, MusIL-6, MusiNOS and ROS by blueberry extracts were analyzed as shown in Table 3. MusASC were highly positively correlated with the expressions of MusNLRP3 and MusIL-1β. MusIL-6 was highly correlation with the expression of MusIL-1β, and MusASC. MusiNOS were highly correlated to the expression of MusIL-1β, MusCaspase-1 and MusIL-6. But the expression of the ROS was not strongly correlated to the expression of the mRNA and the highest correlation value was 0.304 with the MusTNF-α.
| Correlation | MusNLRP3 | MusCaspase-1 | MusIL-1β | MusASC | MusTNF-α | MusIL-6 | MusiNOS | ROS |
|---|---|---|---|---|---|---|---|---|
| MusNLRP3 | 1 | 0.726 | 0.746 | 0.841 | −0.173 | 0.553 | 0.456 | −0.716 |
| MusCaspase-1 | 1 | 0.844 | 0.838 | −0.124 | 0.791 | 0.891 | −0.174 | |
| MusIL-1β | 1 | 0.903 | 0.0905 | 0.855 | 0.828 | −0.297 | ||
| MusASC | 1 | −0.249 | 0.783 | 0.756 | −0.456 | |||
| MusTNF-α | 1 | 0.220 | 0.041 | 0.304 | ||||
| MusIL-6 | 1 | 0.860 | −0.016 | |||||
| MusiNOS | 1 | 0.102 | ||||||
| ROS | 1 |
Experimental
Chemicals and ingredients
Trizol Regents, LPS, quercetin, and dichlorofluorescin diacetate (DCFH-DA) were purchased from Sigma Aldrich (St. Louis, MO, USA). Mus macrophage cell RAW264.7 (ATCC TIB-71) was purchased from the American Tissue Culture Collection (ATCC, Manassas, VA, USA). Phosphate buffer solution (PBS), cell culture medium (DMEM), fetal bovine serum (FBS) were purchased from GIBCO (Life Technologies, Grand Island, NY). iTaq Universal SYBR Green Super-mix, NLRP3 and Caspase-1 antibody were purchased from Boshide Company (Wuhan, China). The gene-specific primers were synthesized from Sangon Biotech Co. Ltd. (Shanghai, China).Blueberries samples and standards preparation
Three varieties of fresh blueberries including: Blomidom (BD), North Country (NC) and JK-M7 (JK) were achieved from Jikang Company in Jilin province, China in 2015. Samples were prepared using the methods as reported previously.42 Quercetin (Qu) was used as standard for analysis.RAW264.7 macrophages cells cytotoxicity
The cytotoxicity activity effects of blueberry were detected with the methods as previously described39 using RAW264.7 cells plated in 96 well plates with a concentration of 1 × 105 cells per well and incubated with blueberry extracts for 24 h at 37 °C. At the end of treatment, the medium that control and blueberry-treated group were discard and added methylene blue solution which was used to stain the cells in order to determine the survival rate of cells.ROS detection
LPS and the blueberry extracts were used to induce the RAW264.7 cells. After incubated 12 h, intracellular ROS was measured with the ROS-specific fluorescent probe DCFH-DA (20 mM; Molecular Probes) using the method described previously in our laboratory.28 RAW264.7 cells were loaded for 30 min with 20 mM DCFH-DA, and washed twice with PBS, then read the fluorescent values using a multi-mode microplate reader (Filter Max F5, Molecular Devices, USA) with 485 nm excitation and 538 nm emission. The level of fluorescence was determined by Fluorescent Ascent FL plate-reader (37 °C).45Quantitative real-time RT-PCR analysis of gene expression
Total RNA were isolated from the control and treated cells using Trizol Regents. The quantity of total RNA was determined by a spectrophotometer using the absorbance at A260/A280 nm. cDNA was generated using the Prime Script TM RT reagent Kit (Takara, Japan). Quantitative real-time PCR were carried out using SYBR Green Super-mix for real-time PCR in triplicates and was performed using the Bio-Rad MiniOption™ Real Time PCR Detection System (Bio-Rad, Hercules, CA, USA). The primer sequences for RT-qPCR as Table 1. The thermal cycle conditions were optimized as 10 s at 95 °C followed by 40 cycles of amplification (5 s at 95 °C and 30 s at 60 °C). The relative expression of each gene was calculated using the comparative threshold cycle method normalized to MusGAPDH (reference gene). Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. The results were reported as mean ± SD (n = 3).Western-blot for NLRP3 inflammasome containing NLRP3, Caspase-1
Protein extraction for Western blot was conducted using the method reported previously.46 Cells were rinsed twice in ice-cold PBS, and then added cell lysis buffer containing 1 mM PMSF pyrolysis 5 min, harvested and kept on ice for 30 min in with constant agitation. Insoluble cell debris was discarded following centrifugation for 15 min at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm at 4 °C. The protein samples were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) on 10% gels and subsequently transferred to polyvinylidene (PVDF) membranes (Millipore).46 Immunoblotting was performed for NLRP3, Caspase-1 with β-actin expression as an internal control.
000 rpm at 4 °C. The protein samples were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) on 10% gels and subsequently transferred to polyvinylidene (PVDF) membranes (Millipore).46 Immunoblotting was performed for NLRP3, Caspase-1 with β-actin expression as an internal control.
Statistical analysis
The evaluation of statistical significance of observed differences was performed by one-way analysis of variance (one-way ANOVA), using SPSS software 21.0 (SPSS Inc., Chicago, IL, USA), statistical significance was set at p < 0.05. The graphs were used with the Graph Pad INSTAT software (GraphPad software, San Diego, CA, USA).Conclusions
Blueberry had been reported to have significant antioxidant activity using the method of the CAA, PSC, and ORAC. In this paper using mononuclear macrophage (RAW264.7) to detect the effects of the blueberry extracts on anti-inflammation activities. The mononuclear macrophage was treated with the blueberry extracts and quercetin after LPS stimulation, and the expressions of inflammatory factors and cytokines were detected. Blueberry extracts significantly inhibited genes expression of MusIL-1β, MusNLRP3, MusCaspase-1, MusASC, MusTNF-α, MusIL-6, and MusiNOS. MusNLRP3 and MusCaspase-1 were also detected from gene and protein levels and the blueberry treated groups were significantly lower than the LPS treated group. Meanwhile, quercetin significantly inhibited LPS-induced ROS in RAW264.7 cells. Furthermore, quercetin and blueberry extracts inhibited the protein expression of NLRP3 and Caspase-1. In conclusion, we used LPS stimulated RAW264.7 cells to produce ROS in mitochondria, and then induced mitochondrial dysfunction to further stimulate the NLRP3 inflammasome including the gene expressions of MusIL-1β, MusNLRP3, MusCaspase-1, MusASC, MusTNF-α, MusIL-6, and MusiNOS and protein expressions of NLRP3 and Caspase-1. Our results suggest that bioactive compounds of blueberries may provide health benefits with anti-inflammatory potentials.Acknowledgements
Authors are greatly thankful to National Natural Science Foundation of China (31501765), Science and Technology Planning Project of Guangdong-China (2016B020233004), Natural Science Foundation of Guangdong-China (2016A030312001) and the Leading Talents Program in Guangdong Province for financial support, valuable suggestions and guidelines.References
- X. Liu, L. Fang, T. B. Guo, H. Mei and J. Z. Zhang, Trends Immunol., 2013, 34, 120–128 CrossRef PubMed.
- P. Xi and R. H. Liu, Mol. Nutr. Food Res., 2016, 60, 1819–1836 CAS.
- X. Jiang, T. Li and R. H. Liu, J. Agric. Food Chem., 2016, 64, 1806–1816 CrossRef CAS PubMed.
- L. Franchi, T. Eigenbrod, R. Muñoz-Planillo and G. Nuñez, Nat. Immunol., 2009, 10, 241–247 CrossRef CAS PubMed.
- F. Martinon, A. Mayor and J. Tschopp, Annu. Rev. Immunol., 2009, 27, 229–265 CrossRef CAS PubMed.
- S. Mariathasan, K. Newton, D. M. Monack, D. Vucic, D. M. French, W. P. Lee, M. Roose-Girma, S. Erickson and V. M. Dixit, Nature, 2004, 430, 213–218 CrossRef CAS PubMed.
- I. C. Allen, M. A. Scull, C. B. Moore, E. K. Holl, E. McElvania-TeKippe, D. J. Taxman, E. H. Guthrie, R. J. Pickles and J. P.-Y. Ting, Immunity, 2009, 30, 556–565 CrossRef CAS PubMed.
- P. G. Thomas, P. Dash, J. R. Aldridge, A. H. Ellebedy, C. Reynolds, A. J. Funk, W. J. Martin, M. Lamkanfi, R. J. Webby and K. L. Boyd, Immunity, 2009, 30, 566–575 CrossRef CAS PubMed.
- O. Gross, H. Poeck, M. Bscheider, C. Dostert, N. Hannesschläger, S. Endres, G. Hartmann, A. Tardivel, E. Schweighoffer and V. Tybulewicz, Nature, 2009, 459, 433–436 CrossRef CAS PubMed.
- S. L. Cassel, S. C. Eisenbarth, S. S. Iyer, J. J. Sadler, O. R. Colegio, L. A. Tephly, A. B. Carter, P. B. Rothman, R. A. Flavell and F. S. Sutterwala, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 9035–9040 CrossRef CAS PubMed.
- F. Martinon, V. Pétrilli, A. Mayor, A. Tardivel and J. Tschopp, Nature, 2006, 440, 237–241 CrossRef CAS PubMed.
- C. Dostert, V. Pétrilli, R. Van Bruggen, C. Steele, B. T. Mossman and J. Tschopp, Science, 2008, 320, 674–677 CrossRef CAS PubMed.
- V. Hornung, F. Bauernfeind, A. Halle, E. O. Samstad, H. Kono, K. L. Rock, K. A. Fitzgerald and E. Latz, Nat. Immunol., 2008, 9, 847–856 CrossRef CAS PubMed.
- D. A. Muruve, V. Pétrilli, A. K. Zaiss, L. R. White, S. A. Clark, P. J. Ross, R. J. Parks and J. Tschopp, Nature, 2008, 452, 103–107 CrossRef CAS PubMed.
- I. Aksentijevich, C. D Putnam, E. F. Remmers, J. L. Mueller, J. Le, R. D. Kolodner, Z. Moak, M. Chuang, F. Austin and R. Goldbach-Mansky, Arthritis Rheum., 2007, 56, 1273–1285 CrossRef CAS PubMed.
- K. Shinkai, T. McCalmont and K. Leslie, Clin. Exp. Dermatol., 2008, 33, 1–9 CAS.
- K. Schroder and J. Tschopp, Cell, 2010, 140, 821–832 CrossRef CAS PubMed.
- F. Martinon, K. Burns and J. Tschopp, Mol. Cell, 2002, 10, 417–426 CrossRef CAS PubMed.
- S. D. Brydges, J. L. Mueller, M. D. McGeough, C. A. Pena, A. Misaghi, C. Gandhi, C. D. Putnam, D. L. Boyle, G. S. Firestein and A. A. Horner, Immunity, 2009, 30, 875–887 CrossRef CAS PubMed.
- G. Meng, F. Zhang, I. Fuss, A. Kitani and W. Strober, Immunity, 2009, 30, 860–874 CrossRef CAS PubMed.
- S. Mariathasan, D. S. Weiss, K. Newton, J. McBride, K. O'Rourke, M. Roose-Girma, W. P. Lee, Y. Weinrauch, D. M. Monack and V. M. Dixit, Nature, 2006, 440, 228–232 CrossRef CAS PubMed.
- C. Xie, J. Kang, M. E. Ferguson, S. Nagarajan, T. M. Badger and X. Wu, Mol. Nutr. Food Res., 2011, 55, 1587–1591 CAS.
- A. Cheng, H. Yan, C. Han, W. Wang, Y. Tian and X. Chen, Int. J. Biol. Macromol., 2014, 69, 382–387 CrossRef CAS PubMed.
- T. Misawa, T. Saitoh, T. Kozaki, S. Park, M. Takahama and S. Akira, Int. Immunol., 2015, 27, 425–434 CrossRef CAS PubMed.
- J. Kang, K. M. Thakali, G. S. Jensen and X. Wu, Plant Foods Hum. Nutr., 2015, 70, 56–62 CrossRef CAS PubMed.
- K. L. Wolfe and R. H. Liu, J. Agric. Food Chem., 2008, 56, 8404–8411 CrossRef CAS PubMed.
- X. Sun, N. Liu, Z. Wu, Y. Feng and X. Meng, Molecules, 2015, 20, 3841–3853 CrossRef CAS PubMed.
- K. L. Wolfe, X. Kang, X. He, M. Dong, Q. Zhang and R. H. Liu, J. Agric. Food Chem., 2008, 56, 8418–8426 CrossRef CAS PubMed.
- Z. Diaconeasa, L. Leopold, D. Rugină, H. Ayvaz and C. Socaciu, Int. J. Mol. Sci., 2015, 16, 2352–2365 CrossRef CAS PubMed.
- A. Ben Lagha, S. Dudonne, Y. Desjardins and D. Grenier, J. Agric. Food Chem., 2015, 63, 6999–7008 CrossRef CAS PubMed.
- M. Okamoto, W. Liu, Y. Luo, A. Tanaka, X. Cai, D. A. Norris, C. A. Dinarello and M. Fujita, J. Biol. Chem., 2010, 285, 6477–6488 CrossRef CAS PubMed.
- P. Limtrakul, S. Yodkeeree, P. Pitchakarn and W. Punfa, Asian Pac. J. Cancer Prev., 2015, 16, 4277–4283 CrossRef PubMed.
- W. Y. Huang, Y. M. Liu, J. Wang, X. N. Wang and C. Y. Li, Molecules, 2014, 19, 12827–12841 CrossRef PubMed.
- W.-Y. Huang, J. Wang, Y.-M. Liu, Q.-S. Zheng and C.-Y. Li, Eur. J. Pharmacol., 2014, 723, 67–72 CrossRef CAS PubMed.
- G. Flores, K. Dastmalchi, A. J. Dabo, K. Whalen, P. Pedraza-Penalosa, R. F. Foronjy, J. M. D'Armiento and E. J. Kennelly, Food Chem., 2012, 131, 119–125 CrossRef CAS PubMed.
- V. Taverniti, D. Fracassetti, C. Del Bo, C. Lanti, M. Minuzzo, D. Klimis-Zacas, P. Riso and S. Guglielmetti, J. Agric. Food Chem., 2014, 62, 8346–8351 CrossRef CAS PubMed.
- M. H. Johnson, E. G. de Mejia, J. Fan, M. A. Lila and G. G. Yousef, Mol. Nutr. Food Res., 2013, 57, 1182–1197 CAS.
- J. Boyer, D. Brown and R. H. Liu, J. Agric. Food Chem., 2004, 52, 7172–7179 CrossRef CAS PubMed.
- D. L. Felice, J. Sun and R. H. Liu, J. Funct. Foods, 2009, 1, 109–118 CrossRef.
- D. F. Garcia-Diaz, M. H. Johnson and E. G. de Mejia, J. Med. Food, 2015, 18, 489–496 CrossRef CAS PubMed.
- R. H. Liu, J. Food Sci., 2013, 78, A18–A25 CrossRef CAS PubMed.
- H. Wang, X. Guo, X. Hu, T. Li, X. Fu and R. H. Liu, Food Chem., 2017, 217, 773–781 CrossRef CAS PubMed.
- W. W. Chao, Y. C. Chung, I. P. Shih, H. Y. Wang, S. T. Chou and C. K. Hsu, J. Med. Food, 2015, 18, 724–730 CrossRef CAS PubMed.
- T. Tsuda, Mol. Nutr. Food Res., 2012, 56, 159–170 CAS.
- K. L. Wolfe and R. H. Liu, J. Agric. Food Chem., 2007, 55, 8896–8907 CrossRef CAS PubMed.
- R. H. Liu, J. Jacob and B. Tennant, BioTechniques, 1997, 22, 594–595 CAS.
| This journal is © The Royal Society of Chemistry 2017 |