Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A combined therapy of rtPA-loaded thermoresponsive gels and ultrasound on hematoma in a rat model of intracerebral hemorrhage
Wei Suna,
Zhongxin Qiana,
Mingzhu Zhaoa,
Ming Shenb,
Yourong Duan *b and
Weidong Liu*a
*b and
Weidong Liu*a
aDepartment of Neurosurgery, Pu Nan Hospital, Shanghai 200125, China. E-mail: liuwd8b@sh163.net
bState Key Laboratory of Oncogenes and Related Genes, Shanghai Cancer Institute, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai 200032, China. E-mail: yrduan@shsci.org; Fax: +86-21-58893020
First published on 10th March 2017
Abstract
To develop and validate an effective method for the removal of residual intracerebral hematoma, we prepared a recombinant tissue-type plasminogen activator (rtPA)-loaded Pluronic F127 (NP-rtPA) delivery system, assess the use of ultrasound (US) to dissolve hematoma in vitro and in a rat model of intracerebral hemorrhage (ICH) in vivo, and evaluate the neurological response of the ICH rat model. There were five experimental groups: control, rtPA, rtPA + US, NP-rtPA and NP-rtPA + US, and the US treatment was at 1 MHz, 0.4 W. The hematoma dissolution in vitro was measured at 6, 24, and 72 hours. Then, to create a rat model of ICH in vivo, rtPA and NP-rtPA were injected into the hematoma cavity with ultrasound-controlled release of rtPA at scheduled times. The neurological behavior of rats was evaluated. NP-rtPA + US dissolved hematoma 40% at 6 hours and 60% at 72 hours compared with the control rtPA (dissolving 20% and 40%, respectively). There was an obvious difference at 0 and 1 day between the rtPA and Np-rtPA. The water content in brain tissue was found to be statistically different. Differences in the behavior of rats treated with rtPA + US and Np-rtPA + US were statistically significant at 21 and 28 days. Ultrasound can control the release of rtPA from rtPA-Pluronic F127, making it better at dissolving hematoma, this effect was better than that achieved with common rtPA. In addition, this treatment may reduce brain edema and provide an effective method for the removal of residual hematoma in ICH using minimally invasive surgery.
Introduction
Hypertensive intracerebral hemorrhage (HICH) is a common and serious disease. The results of a STICH study showed only 31% of the patients are functionally independent at 3 months. Only 38% of the patients survive the 1st year.1,2 As minimally invasive technology has entered into all fields of the clinical practice of neurosurgery, particularly neuronavigation and neuroendoscopy, minimally invasive methods are finding wider clinical applications.3,4 However, even with the use of the micro invasive method, the residual hematoma needs to be dissolved through catheter drainage after surgery for HICH.Recombinant tissue type plasminogen activator (recombinant human tissue-type plasminogen, rtPA) has been shown clinically to have better effects than the traditional urokinase treatment in dissolving hematoma. Because rtPA has a rapid onset and a short half-life, once the drugs are injected in the hematoma cavity, the effective time is short and cannot be sustained in the local cavity.5–7 The aim of this study was to prepare a rtPA nanometer particle controlled release drug delivery system (NP-rtPA), which was applied to HICH during minimally-invasive surgery, according to the observed postoperative residual blood clot in vitro, using a low frequency ultrasound to excite the release of rtPA as needed and exert a continuous influence on the residual blood clot to shorten the time for dissolution and absorption of the clot.
In order to extend the controlled release of the release of rtPA, we introduce the poly(ethylene oxide)-b-poly(propylene oxide)-b-poly(ethylene oxide) (PEO–PPO–PEO), which is a triblock copolymer (trade name Pluronics), is known as a family of materials according to the PEO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PPO ratio and the different relative molecular mass capable of forming a block copolymer. Temperature-sensitive polymer is a type of amphiphilic polymer.8,9 In a water solution, the relative movement of hydrophilic and hydrophobic chains in the molecule increased when the environment temperature changes.10 As a result, the hydrogen bonds with water molecules break down and the solution-gel/micelles transition is achieved. Some temperature-sensitive polymers have negative temperature-sensitivity, in which gelatinization occurs upon rising the temperature. In situ gel reservoir is set up when the solution is injected into the body. This characteristic has been used in pharmaceutics for sustained release formulation.11,12
PPO ratio and the different relative molecular mass capable of forming a block copolymer. Temperature-sensitive polymer is a type of amphiphilic polymer.8,9 In a water solution, the relative movement of hydrophilic and hydrophobic chains in the molecule increased when the environment temperature changes.10 As a result, the hydrogen bonds with water molecules break down and the solution-gel/micelles transition is achieved. Some temperature-sensitive polymers have negative temperature-sensitivity, in which gelatinization occurs upon rising the temperature. In situ gel reservoir is set up when the solution is injected into the body. This characteristic has been used in pharmaceutics for sustained release formulation.11,12
Pluronic®F127 (F127) is one of the widely studied temperature-sensitive polymers and has been approved by the Food and Drug Administration. It is commercially available and considered to be non-toxic.13 Drugs are first solubilized into F127 micelles and form F127 gel by adding an extra amount of F127. This formulation was studied as a regional drug delivery vehicle by different routes of administration such as intratumoral, subcutaneous, intraperitoneal and intramuscular.14,15 Like many other thermo-sensitive polymers, the F127 hydrogel (F127-Gel) is based on the ordered packing and release of F127 micelles during the process of gel erosion.16,17 Drugs encapsulated in the gel can be released by two approaches. First, drug molecules move through the water channels between the micelles in the gel. This release follows the Fick diffusion model. Second, drug molecules are released during gel erosion. These two release mechanisms always occur simultaneously.
Based on the characteristics of rtPA and Pluronic F127, the purpose of our study was to attempt to prepare a new carrier rtPA Pluronic F127 controlled release system, able to be activated through ultrasonic control, to determine the pharmacokinetics and pharmacodynamics of suitable concentrations of rtPA and Pluronic F127, to complete in vitro clot lysis assays and in vivo animal experiments after treatment to better remove residual hematoma and provide the basis for further minimally invasive procedures for HICH.
Materials and methods
Preparation of the nano rtPA sustained release drug delivery system and dissolution experiments of hematoma in vitro using this system
| Subgroup name | The meaning of the subgroups | |
|---|---|---|
| Control group | Control | Only hematoma, no drug and no ultrasound group |
| Experimental group | rtPA | Common rtPA group |
| rtPA-Us | Common rtPA + ultrasound group | |
| Np-rtPA | rtPA-Pluronic F127 drug delivery system | |
| Np-rtPA-Us | rtPA-Pluronic F127 drug delivery system + the ultrasound group |
In the group rtPA, the concentration of rtPA was 1 mg mL−1 and the volume injected was 1 mL. The prepared hematoma in groups with 5 cases per group were observed at room temperature and in a thermostatic water bath at 37 °C at different time points (6 h, 12 h, 24 h, 48 h, 72 h, 4 d, 7 d, and 14 d), which the time means the duration of rtPA incubation and the weight of hematoma was measured and denoted as w1. The dissolution rate of R% = w1/w0 × 100%.
Dosing of Pluronic F127 release rtPA and ultrasound-induced controlled release in vivo
| Subgroup name | The meaning of the subgroups | |
|---|---|---|
| Control group | Sham operation | Brain puncture without intracerebral hematoma of injection |
| Only hematoma | Intracerebral hematoma in 50 μL injection of autologous arterial blood | |
| Experiment group | Hematoma + F127 | Intracerebral hematoma in 50 μL injection of autologous arterial blood + Pluronic F127 |
| Hematoma + rtPA | Intracerebral hematoma in 50 μL injection of autologous arterial blood + rtPA | |
| Hematoma + Np-rtPA | Intracerebral hematoma in 50 μL injection of autologous arterial blood + rtPA Pluronic F127 | |
| Hematoma + Np-rtPA + Us | Intracerebral hematoma in 50 μL injection of autologous arterial blood + rtPA Pluronic F127 + ultrasound |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min. The concentration in the supernatant was measured using HPLC (Agilent 1200, VWD) with a Zorbax SB-C8 column (4.6 nm × 250 nm, 5 μm) set at a temperature of 40 centigrades and the detection wavelength was 280 nm. The mobile phase was a linear gradient from 70/30/0.1 (v/v/v) of water/acetonitrile/TFA to 50/50/0.1 (v/v/v) of water/acetonitrile/TFA in 80 minutes.
000 rpm for 10 min. The concentration in the supernatant was measured using HPLC (Agilent 1200, VWD) with a Zorbax SB-C8 column (4.6 nm × 250 nm, 5 μm) set at a temperature of 40 centigrades and the detection wavelength was 280 nm. The mobile phase was a linear gradient from 70/30/0.1 (v/v/v) of water/acetonitrile/TFA to 50/50/0.1 (v/v/v) of water/acetonitrile/TFA in 80 minutes.The water content of the brain tissue was also measured. The 7 day rats were divided into four groups: the first group was the normal rats (control group); the second group received only an intracerebral hematoma (positive control group); the third group was the common rtPA + ultrasound group; was the fourth group was the rtPA-Pluronic F127 + ultrasound group. Under anesthesia (40 mg kg−1 intraperitoneally administered pentobarbital), the brains of rats were removed and cut in coronal slice 3 mm thick, starting 4 mm from the frontal lobes. Brain sections were divided along the midline into two hemispheres, and each hemisphere was divided into cortex and medulla. The cerebellum was taken separately as a control. The wet weights of the brains were immediately measured. The samples were then dried in a gravity oven at 100 °C for 24 hours to obtain the dry weight, water contents were expressed as a percentage of wet weight; the formula for calculation was (WW − DW)/WW. The dehydrated samples were digested in 1 mL of 1 M nitric acid for 1 week, and the sodium content of this solution was measured with an automatic flame photometer. Ion content was expressed in milliequivalents per kilogram of dehydrated brain tissue (mEq per kg dry wt). The effects of rtPA + US and NP-rtPA + US treatment by ultrasound brain water content 7 days after ICH were showed by the bar graphs. There were five rats in each group. Measurements were made in contralateral basal ganglia of brains obtained from rats after ICH model was made. The calculation formula is as follows: (wet weight − dry weight)/wet.
Observation of the animal neurological function defects of rats were scored (mNSS, 0–18 scores, 0 means normal),19 and their behaviors were evaluated at 6 hours, 1 day, 3 days and 7 days. The mNSS scores and behavioral changes were described and the water content of the cerebral tissue were recorded.
The present study was reviewed and approved by the Ethics Committee of Shanghai Jiao Tong University (Shanghai, China), in compliance with NIH guidelines and signed informed consent was obtained from all patients.
Results
Characterization of nano rtPA and in vitro drug release
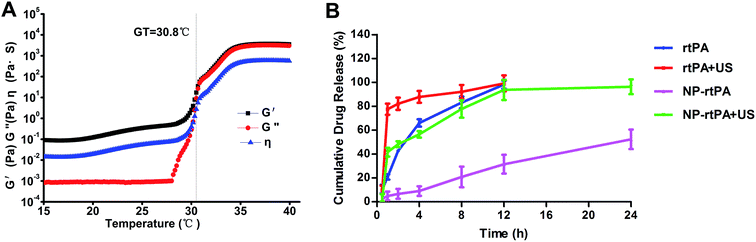
We used a rheological method to follow up gelatinization with temperature rise. The S curve of the gel elastic modulus G′ induced by temperature, and in a region below CAT which was very low (as shown in Fig. 1A). G′ raised sharply when the temperature went up to a certain level, which illustrated the transformation from solution to gel. This transition point is the gelation temperature Tgel = 30.8 °C, and the variation of G′ with the temperature tended to be flat in the gel.At the same time, the rtPA release characteristics were studied in the all samples. The results are displayed in Fig. 1B and NP-rtPA group showed slow release, which suggested that the corrosion behavior of the gel controlled drug release. With the corrosion of gel, the rtPA were released from the nanoparticles.
Monitoring clot lysis in vitro
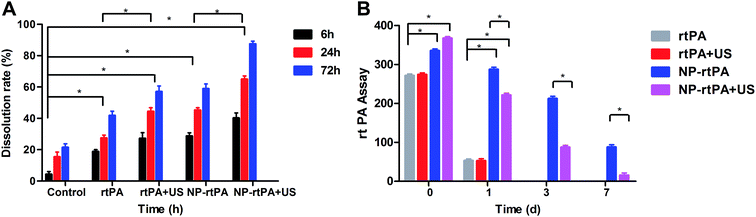
Hematoma at each time segment monitoring (6 h, 24 h and 72 h): in the control group and NP-rtPA group, the rate of hematoma dissolution showed no significant difference (P = 0.18 > 0.05). For the rtPA group and rtPA + US group, the hematoma dissolution rate was also not significantly different (P = 0.31 > 0.05). In the control group and NP-rtPA + US group, the difference in the rate of hematoma dissolution was statistically significant (P < 0.05). For the rtPA group and NP-rtPA + US group, the difference of the hematoma dissolution rate was statistically significant (P = 0.02 < 0.05).In the group NP-rtPA + US, hematoma was dissolved 40% at 6 h and greater than 60% at 72 h The rtPA group showed only 20% dissolution at 6 h and 40% at 72 h; if combined with ultrasound with the group rtPA, the dissolution effect was minimal. In the hematoma group without any drugs, the dissolution was only 20% at 72 h. The significance of differences between the experimental groups and statistics had shown in Fig. 2A.
The detection of the concentration of rtPA in vivo
In the normal rtPA group with or without ultrasound, ultrasound treatment had little effect (P > 0.05); however, the ultrasonic effect on the carrier rtPA-Pluronic F127 system was significant (P < 0.05). Because the average rtPA has a short half-life, the ordinary group rtPA at 3 d and 7 d that had been completely dissolved could not be detected. While the carrier rtPA-Pluronic F127 system only allows for slow release of drug under the ultrasonic control, the comparison in the application of ultrasound after 1 d, 3 d and 7 d showed a statistically significant difference (P < 0.05). The rtPA group and rtPA-Pluronic F127 group at 0 d and 1 d showed a statistically significant difference (P > 0.05), as shown in Fig. 2B.Determination of the brain tissue water content
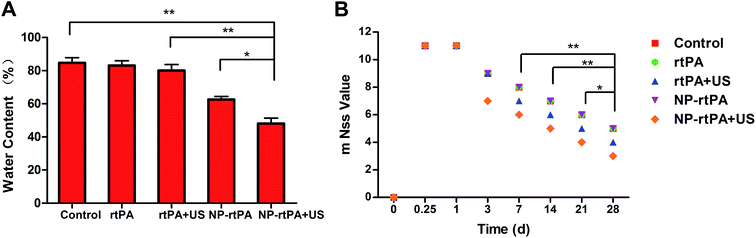
Comparison of the four groups demonstrated significantly different water content (P < 0.05), as shown in Fig. 3A.Determination of rats' neurological behavior
The rat's neurological function was measured by the modified neural function defect score (mNSS) in rats, as shown in Fig. 3B. The action of rats were determined using the modified neurological scores were mNSS (score 0–18, 0 normal, 18 as severe deficit). The control group (simple hematoma group), rtPA + rtPA-Pluronic F127 + ultrasound group and ultrasound group, showed visible defects in the short time after the operation (day 0, day 7). The three groups of behavior were not significantly different (P > 0.05). In contrast at day 14, day 21 and day 28, the two groups of data showed statistically significant differences (P < 0.05), as shown in Fig. 3B.Records of the experiments
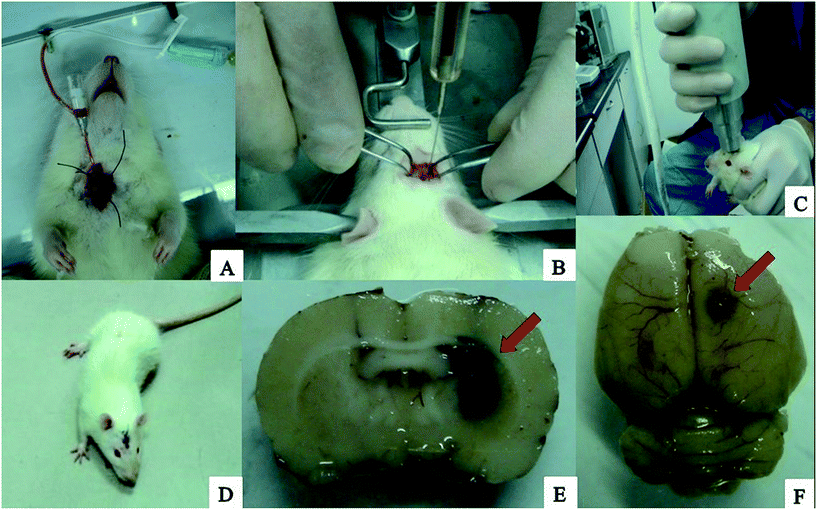
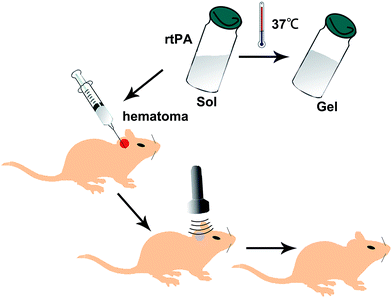
The processes of the preparation of loaded rtPA-Pluronic F127 controlled drug delivery system (Np-rtPA) in vitro and the intracerebral hemorrhage rat model in vivo were illustrated in Fig. 4 and 5, respectively. After 24 hours in the experiment series using rat ICH model, measurement of hematoma size (volume) was nearly 47.23 ± 2.11 μL by the pathological measurement (in Fig. 5E).Discussion
The ideal rtPA controlled release system such as the rtPA-Pluronic F127 (F127) system meet the following requirements. (1) Normal dissolved after the formation of a self-assembled encapsulated drug sol, a few tens of nanometers in diameter; (2) this type of material has the characteristics of temperature sensitivity going from a sol state at normal temperature into a gel state at body temperature; (3) ultrasound can trigger micelles release containing drug. The conventional treatment using nanometer drug can greatly increase the drug particle surface area, so that the contact area with the host increases, thus improving the efficacy, and reducing the dosage and side effects. F127 is one type of preferred nano material. The nano material used in the delivery of antineoplastic drugs (doxorubicin) has been widely used in the rt-PA study but there had been no related applications and reports. F127 is non-toxic, non-stimulating, non-immunogenic, and is a safe drug carrier. The American Food and Drug Administration (FDA) have approved its use in humans.20,21The drug carrier system formed by Pluronic F127 can be induced by ultrasound to release the enclosed drug. When the ultrasound is applied, self-assembled structures are distorted, which allows for the release of drugs. When the ultrasound is turned off, the self-assembled structures reassembled, which traps the drugs.22,23 Self-assembly into micelles involves mainly the hydrophobic PPO block as the kernel and the PEO block for the outside shell. PEO–PPO–PEO block copolymer is sensitive to temperature and the solution is mainly in the form of micelle. Below the critical micelle concentration (CMC), the copolymer molecules in monomeric form dispersed in solution whereas above the critical micelle concentration, the polymerization of monomers to form micelles dominants. The transition point temperature at which the drug containing F127 showed a sol state under the body temperatures, and a gel state at body temperature is conducive to the protection of drugs and the controlled release of drugs through the application of ultrasound frequency and power to the preparation of the drug delivery system. The encapsulation of the F127 release of drugs, can achieve the clinically required concentration. The features of the ultrasonic application are that it is non-invasive and can penetrate the body tissues through focused ultrasound energy to achieve fine control (Fig. 6).
The rtPA loaded sustained-release preparation for this experiment administration system meets the clinical preparation requirements: first, this drug carrier system is a liquid at room temperature, can be conveniently stored and used in the minimally invasive treatment by intraoperative injection in the intracranial hematoma. Once into the host body, this preparation is warmed to the body temperature on the surface of solid hematoma, without the drainage discharge in vitro. While the operation is performed according to the results of the clinical head CT examination, residual hematoma treated by ultrasonic technology allows for the timely release of rtPA. Lysis of the hematoma can be accelerated. If the postoperative volume of hematoma is greater than the 25% initial hematoma, the ultrasound is triggered to release rtPA. If the ultrasound is not induced, the degradation of F127 is slow, but within the host body fluid and cerebrospinal fluid, and F127 will gradually be dissolved in the body water 24 hours later. Because of the formation at the nanometer level, this dissolution is a very slow process to meet the clinical safety needs.24–26
Nanotechnology has been widely used in biology and medicine. In particular, the development of nano drugs has broad prospects for the development of new drugs. The conventional treatment using nanometer drugs can greatly increase the drug particle surface area, such that the contact area with the host increases. This process improves the efficacy and can reduce the dosage and the side effects.27,28 According to the basic and clinical studies, Pluronic F-127 can satisfy the clinical requirements, be used to encapsulate rtPA, form nanoparticles, and establish rtPA as a controlled release of drug delivery system by ultrasound irradiation.
Pluronic F127 is a triblock PEO–PPO–PEO copolymer, which is in a sol state in liquid water at room temperature but as the temperature rises to the critical micelle temperature, this copolymer converts to a non-flowing gel. The gel properties of this type of temperature sensitive copolymer can be used as an excellent encapsulated drug carrier. The preparation of sustained-release hydrogels by mechanical stirring with good uniformity and high entrapment efficiency can be used for the preparation of intravenous or intramuscular injection or oral administration or local sustained-release hydrogels for drug. Not only can this process be used to remove residual hematoma after HICH, it can also be used for in situ injection in the treatment of tumor. Gathered at the site of the tumor, encapsulated drugs in the extracellular space can slowly release drugs into tumor cells. At present, only foreign applications of ultrasound triggered micelles to release encapsulated anticancer drugs have been reported. There have been no direct reports and domestic related reports.
The brain tissue water content of each group showed significant differences. The effect of rtPA-Pluronic F127 to reduce the load in the ultrasound groups on cerebral edema is best, because the ultrasound treatment can accelerate the release of the drug loaded system rtPA. Once rtPA is released, lysis of the hematoma occurs rapidly. Ultrasound can also stimulate rtPA release gradually, fully integrated with the hematoma, thus dissolving the effect. The experimental group and the control group showed significant differences in brain edema, for the reason that rtPA itself is a short-acting fibrinolytic agent with a short half-life. The control group after injection of rtPA, the rtPA may immediately degrade and disappear. Because of the drug loading system with self degradation, there can be a slow and sustained release of rtPA with good effect on edema. At the same time as mentioned earlier, the ultrasound stimulated release system after release of the rtPA. If the rtPA was not fully applied to the hematoma, then the next administration of Pluronic F127 can improve the utilization rate of the rtPA and the efficacy in dissolving hematoma.
Rat neurological functional defects were determined using modified neurological scores mNSS. The neural function defect showed the better neurological behavior in the rtPA-Pluronic F127-US group than in the other groups in our study. Although the performance in the rtPA-Pluronic F127-US group showed the same neurological scores as with the normal rtPA group, there were obvious differences for the long-term recovery of nerve function.
The ultrasound is widely applied as an imaging modality, resulting from its real time applications, low cost, simplicity, and safety. More recently, studies revealed that ultrasound can facilitate local drug and gene delivery and the encapsulated drug release could be triggered and controlled by ultrasound. The experiments based on ultrasound stimulated rtPA release system in the model of hypertensive intracerebral hemorrhage in rats, confirm that the delivery system can release in vivo under ultrasound control the dissolving effect on brain hematoma better than the use of rtPA alone. This study also investigated the efficacy of ultrasound for clinical application including the rtPA time, the dose and the ultrasonic method, and even laid the foundation for the clinical use of drugs. Pluronic F127 can be used to encapsulate rtPA, prepared by the rtPA loaded sustained-release drug delivery system, in combination with the ultrasound technology for drug delivery system to release rtPA. The in vivo experiments confirmed that the greater dissolving effect on hematoma compared to common rtPA for minimally invasive surgical treatment of HICH and for other related fields has great potential.
This study also has some defects: firstly, the clinical implication of this study might be limited as there is no current evidence that early surgical intervention is definitely better than conservative treatment (STICH and STICH II trials). Especially, in these two clinical trials, the indication for surgical intervention were hematoma near to the cerebral cortex (within 1 cm from the surface) while the hematoma generated in this model were deep seated hematomas. Secondly, for the patients with intracerebral hemorrhage, the lysed clot is drained in the conventional method. But the fixed drainage tube has difficulty in animal, and the animal awake unusually active, so that there has been very inaccurate to record the drainage. To the volumes of hematoma, we have also considered using large animal, the amount of bleeding can be close to the brain, but the clot lysis was made according to the dose effect relationship, and the reference of making animal model of cerebral hemorrhage, many studies have showed that compared with the large animal, making rat intracerebral haemorrhage model relatively stable, nerve defect function more obvious, the prognosis for relatively significant difference. Based on the above reasons, we consider the choice of small volumes of intracerebral hemorrhage model in rats. But if we can take the appropriate way, we perhaps make stable large animal model of cerebral hemorrhage, can be considered large volumes of hematoma animal model in future study. Thirdly, moreover, the limitation of the animal model itself as it does not directly simulates hypertensive ICH should be considered. In the clinical context, rapid lysis of hematoma does not mean better outcome as there is a high possibility of rebleeding when early evacuation of hematoma is done. Therefore, there is a high chance that improvement of the neurological scale in this study would not be demonstrated in the real-world practice.
Conclusions
The combined application of rtPA-Pluronic F127 with ultrasound not only can accelerate the dissolution of hematoma in vitro and can control the release of rtPA from the rtPA-Pluronic F127 system in vivo, which can promote the lysis of blood clots, but also can reduce brain edema and dissolve intracranial hematoma better than rtPA alone.Conflict of interest
The authors have declared no conflict of interest.Acknowledgements
This work was supported by the health system outstanding young medical talents cultivation of Shanghai Pudong New District (PWRq2013-13) and funded by the Pudong New district science and technology commission (PKJ2012-Y58) in china. This work was supported by the National Natural Science Foundation of China (81472841). We would like to thank the reviewers for their valuable comments and suggestions.References
- A. D. Mendelow, B. A. Gregson, H. M. Fernandes, G. D. Murray and G. M. Teasdale, et al., Lancet, 2005, 365, 387–397 CrossRef.
- A. D. Mendelow, B. A. Gregson, E. N. Rowan, G. D. Murray and A. Gholkar, et al., Lancet, 2013, 382, 397–408 CrossRef.
- A. Bakshi, A. Bakshi and A. K. Banerji, Neurosurg. Focus, 2004, 16, e9 Search PubMed.
- J. A. Zurasky, V. Aiyagari, A. R. Zazulia, A. Shackelford and M. N. Diringer, Neurology, 2005, 64, 725–727 CrossRef CAS PubMed.
- P. C. Hsieh, D. Y. Cho, W. Y. Lee and J. T. Chen, Surg. Neurol., 2005, 64, 147–153 CrossRef PubMed.
- M. D. King, D. J. McCracken, F. M. Wade, S. E. Meiler and C. J. Alleyne, et al., J. Neurosurg., 2011, 115, 116–123 CrossRef CAS PubMed.
- J. M. Montes, J. H. Wong, P. B. Fayad and I. A. Awad, Stroke, 2000, 31, 834–840 CrossRef CAS PubMed.
- D. Rassoul, K. Elham, A. Fatemeh and E. H. Mohammad, Drug Delivery, 2006, 13, 345–350 CrossRef PubMed.
- H. Gupta, S. Jain, R. Mathur, P. Mishra, A. K. Mishra and T. Velpandian, Drug Delivery, 2009, 14, 507–515 CrossRef PubMed.
- Z. G. Gao, H. D. Fain and N. Rapoport, J. Controlled Release, 2005, 102, 203–222 CrossRef CAS PubMed.
- G. A. Husseini, G. D. Myrup, W. G. Pitt, D. A. Christensen and N. Y. Rapoport, J. Controlled Release, 2000, 69, 43–52 CrossRef CAS PubMed.
- N. Rapoport, Int. J. Pharm., 2004, 277, 155–162 CrossRef CAS PubMed.
- X. Zheng, X. Wang, M. Gou, J. Zhang and K. Men, et al., Drug Delivery, 2010, 17, 138–144 CrossRef CAS PubMed.
- A. Marin, M. Muniruzzaman and N. Rapoport, J. Controlled Release, 2001, 75, 69–81 CrossRef CAS PubMed.
- J. L. Nelson, B. L. Roeder, J. C. Carmen, F. Roloff and W. G. Pitt, Cancer Res., 2002, 62, 7280–7283 CAS.
- Z. Gao, H. D. Fain and N. Rapoport, Mol. Pharm., 2004, 1, 317–330 CrossRef CAS.
- S. D. Desai and J. Blanchard, Drug Delivery, 2000, 7, 201–207 CrossRef CAS PubMed.
- G. Y. Yang, A. L. Betz and T. L. Chenevert, et al., J. Neurosurg., 1994, 81, 93–102 CrossRef CAS PubMed.
- M. Bakhtiary, M. Marzban, M. Mehdizadeh, M. T. Joghataei and S. Khoei, et al., Iran. Biomed. J., 2010, 14, 142–149 Search PubMed.
- A. J. De Graaf, P. D. S. I. Azevedo, E. H. Pieters, D. T. Rijkers and C. F. van Nostrum, et al., J. Controlled Release, 2012, 162, 582–590 CrossRef CAS PubMed.
- Y. Liu, W. L. Lu, J. C. Wang, X. Zhang and H. Zhang, et al., J. Controlled Release, 2007, 117, 387–395 CrossRef CAS PubMed.
- R. Bhardwaj and J. Blanchard, J. Pharm. Sci., 1996, 85, 915–919 CrossRef CAS PubMed.
- G. Dumortier, J. L. Grossiord, F. Agnely and J. C. Chaumeil, Pharm. Res., 2006, 23, 2709–2728 CrossRef CAS PubMed.
- D. M. Patel, S. P. Patel and C. N. Patel, Int. J. Pharm. Invest., 2014, 4, 174–182 CrossRef PubMed.
- B. Srividya, R. M. Cardoza and P. D. Amin, J. Controlled Release, 2001, 73, 205–211 CrossRef CAS PubMed.
- P. L. Wang and T. P. Johnston, Int. J. Pharm., 1995, 113, 73–81 CrossRef CAS.
- B. Albertini, M. D. Sabatino, C. Melegari and N. Passerini, J. Microencapsulation, 2015, 32, 181–192 CrossRef CAS PubMed.
- K. P. Dehghan, E. Saadat, F. Ravar, H. Akbari and F. Dorkoosh, Pharm. Dev. Technol., 2014, 1–9 Search PubMed.
| This journal is © The Royal Society of Chemistry 2017 |