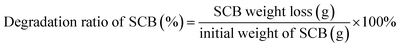Open Access Article
Open Access ArticleEnhanced saccharification of cellulose and sugarcane bagasse by Clostridium thermocellum cultures with Triton X-100 and β-glucosidase/Cellic®CTec2 supplementation
Xiao-Su Qua,
Bin-Bin Hua and
Ming-Jun Zhu *ab
*ab
aSchool of Bioscience and Bioengineering, South China University of Technology, Guangzhou Higher Education Mega Center, Panyu, Guangzhou 510006, People's Republic of China. E-mail: mjzhu@scut.edu.cn; Fax: +86 20 39380601; Tel: +86 20 39380623
bSchool of Life and Geographical Sciences, Kashi University, 29 Xueyuan Road, Kashi 844006, Xinjiang Uygur Autonomous Region, People's Republic of China
First published on 18th April 2017
Abstract
Clostridium thermocellum is the most efficient cellulose-degrading bacterium known for producing cellulosome, which was used to directly degrade sugarcane bagasse (SCB) with limited glucose accumulation. However, the glucose production was significantly enhanced with 0.25% Triton X-100 and 30 U g−1 of β-glucosidase supplementation. A glucose accumulation of 21.33 ± 0.35 g L−1 and substrate degradation of 61.91 ± 0.77% from 60 g L−1 sodium hydroxide-pretreated SCB were reached, with a 609.41% and 75.53% increase over the control (3.01 ± 0.26 g L−1, 35.27 ± 2.91%), respectively. More interestingly, a synergism between C. thermocellum cultures and Cellic®CTec2 (30 CBU per g, 1.2 FPU per g substrate) in the enzymolysis of Avicel was observed and the glucose concentration and saccharification ratio of Avicel reached 56.90 ± 1.20 g L−1 and 94.8% at 60 g L−1 Avicel. Moreover, a highest glucose concentration of 83.17 g L−1 was achieved at 100 g L−1 Avicel. The present work provides an effective and promising process for industrial saccharification of cellulose.
1. Introduction
Lignocellulosic biomass obtained from agricultural and industrial residues is an abundant, inexpensive and renewable resource of sugars.1 Cellulose and hemicelluloses are the major constituents of plant biomass. When subjected to enzymatic hydrolysis, these polysaccharide are transformed into glucose and other fermentable carbohydrates, which might further be converted to liquid fuels and many other useful chemicals.2 According to contribution of renewable energy and biofuels in the total energy consumed in the world in 2011, sugarcane consists of 34.0% of the all feedstock used for ethanol production.3 Thus, a reasonable way to handle the residue of the sugar industry, sugarcane bagasse (SCB), has become a serious problem.The “biochemical” route transforms sugar polymers present in SCB to monomeric sugars, which then are fermented using microorganisms to produce fuels and chemicals. Clostridium thermocellum is an anaerobic, thermophilic and spore-forming bacterium, and is famous for its multienzyme complex, the cellulosome. The cellulosome is composed of a primary scaffoldin subunit that can integrate up to nine enzymes into the complex. The scaffoldin subunit contains a single CBM together with numerous cohesion modules and binds strongly to a dockerin module borne by each cellulosomal enzyme.4 The cellulosome has been reported more effectively than fungal free enzyme in degrading cellulose.5 Moreover, using multi-enzyme complexes, C. thermocellum can saccharify lignocellulose during cultivation, which would greatly reduce operating costs.6 Fermentation products generated by C. thermocellum are mostly ethanol (low-tolerant generally) and acetic acid and the production of all products besides hydrogen are generally limited. However, the previous research in our laboratory found that the addition of nonionic surfactant (Triton X-100) in C. thermocellum cultures resulted in an accumulation in reducing sugars and showed little negative effect on hydrogen production.7,8 And the possible reasons of enhancing saccharification by C. thermocellum cultures with Triton X-100 addition was also studied before. Triton X-100 showed the detrimental effect on growth and metabolism of C. thermocellum, resulting in less transportation and consumption of reducing sugar. On the other hand, Triton X-100 had positive effect on enzyme activity and concentration in the supernatant, indicating that cellulases or cellulosome might be released from cell surface.8 However, when a new thermophilic bacterium was used, more glucose was released and a certain amount of cellobiose was detected. In order to optimize the cellulolytic abilities of cellulosomes, it is necessary to eliminate the inhibition of cellobiose.9 Thus, there are still many potential aspects that need to be studied to improve the accumulation of reducing sugar including glucose and xylose. Additionally, most common commercially available cellulases are produced by Trichoderma and Aspergillus species and limited researches7,10,11 focus on improving glucose production by C. thermocellum cultures. The proteins secreted by cellulolytic fungi are above 20 g L−1, but costs are high due to long fermentation time and high power demand for broth aeration.2,12 So, a study on combination of cellulosome and fungal cellulase can be performed to reduce the utilization of high-cost commercial cellulase.
Herein, we bring up a promising strategy for the converting SCB and Avicel into glucose and increasing the degradation of substrate further by C. thermocellum cultures with nonionic surfactant Triton X-100 and β-glucosidase supplementation. Moreover, an efficient synergism is found in enzymatic hydrolysis of Avicel, resulting in reducing the cost of utilization of commercial cellulase.
2. Materials and methods
2.1 Substrates, surfactant and enzymes
SCB was provided by the Guangzhou Sugarcane Industry Research Institute (Guangdong province, China). Alkali pretreatment was performed as described previously in Cheng et al.7 Briefly, the SCB was soaked in 3% (w/v) NaOH solution with a liquid to solid ratio of 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/w) and incubated for 3 h in a water bath at 80 °C. Afterwards, the pretreated SCB was washed into neutral with distilled water and then dried for 48 h at 60 °C. Finally, the dried SCB was milled and sieved through a 100 mesh screen. The chemical compositions of the pretreated SCB were determined according to the methods of the National Renewable Energy Laboratory.13 The main chemical components of the SCB were 59.89 ± 0.16% cellulose, 17.99 ± 0.15% hemicellulose, 8.30 ± 1.31% Klason lignin, 2.68 ± 0.08% acid soluble lignin and other components. Microcrystalline cellulose Avicel PH 105 was obtained from FMC Corporation (Philadelphia, PA, USA).
1 (v/w) and incubated for 3 h in a water bath at 80 °C. Afterwards, the pretreated SCB was washed into neutral with distilled water and then dried for 48 h at 60 °C. Finally, the dried SCB was milled and sieved through a 100 mesh screen. The chemical compositions of the pretreated SCB were determined according to the methods of the National Renewable Energy Laboratory.13 The main chemical components of the SCB were 59.89 ± 0.16% cellulose, 17.99 ± 0.15% hemicellulose, 8.30 ± 1.31% Klason lignin, 2.68 ± 0.08% acid soluble lignin and other components. Microcrystalline cellulose Avicel PH 105 was obtained from FMC Corporation (Philadelphia, PA, USA).
Non-ionic surfactant, Triton X-100 was purchased from Sigma (St. Louis, MO, USA) and the concentration used in this study was 0.25% (w/v).
Two β-glucosidases were BglA10,14 cloned from C. thermocellum ATCC 27405 and Cellic®CTec2 provided kindly by Novozymes Corporation. Specifically, DNA encoding the BglA was amplified from C. thermocellum strain ATCC 27405 genomic DNA by PCR using 5′-CAGTCCATGGCAAAGATAAC-3′ for sense prime and 5′-CGAGCTCGAAACCGTTGTTTTTGATTAC-3′ for antisense primer (NcoI and SacI sites in italic, respectively). The PCR amplified BglA gene was digested by NcoI/SacI and ligated into pET30a resulting in the final vector p30a-βGDs. Expression of the proteins was achieved by adding isopropyl-β-D-thiogalactopyranoside (1 mM final concentration) to mid-exponential phase cultures of E. coli BL21 (DE3) harboring target plasmids with incubation for a further 3 h at 37 °C. The harvested cell pellets were resuspended in lysis buffer (50 mM potassium phosphate, pH 6.0) and ultrasounded in a 50 ml plastic centrifuge tube with an ultrasonic cell disintegrator (SCIENTZ-IID, Ningbo Scientz Biotechnology Co., Ltd, Zhejiang Province, China) of 300 W ultrasonic power, 5 s interval time, 4 s ultrasonic time and 25 min total working time. The samples were kept in an ice bath during the ultrasonic process to prevent overheating15 and then centrifuged for 15 min at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm and collected the supernatant as crude enzyme for use. The activity of crude enzyme was 25 CBU per ml. Cellic®CTec2 had an activity of 110 FPU per ml and 2743 CBU per ml measured according to International Union of Pure and Applied Chemistry standard assay.16
000 rpm and collected the supernatant as crude enzyme for use. The activity of crude enzyme was 25 CBU per ml. Cellic®CTec2 had an activity of 110 FPU per ml and 2743 CBU per ml measured according to International Union of Pure and Applied Chemistry standard assay.16
2.2 Microorganism and inoculum
0.8 g SCB was added into 50 ml serum bottles and supplemented with 18 ml DSMZ 122 media containing the following (per liter): 1.30 g of (NH4)2SO4, 2.60 g of MgCl2·6H2O, 1.43 g of KH2PO4, 5.50 g of K2HPO4, 0.13 g CaCl2·2H2O, 6.00 g of Na2-β-glycerol phosphate·4H2O, 0.25 g of L-glutathione reduced, 4.50 g of Yeast extract, 1.10 mg of FeSO4·7H2O and 1.00 mg of Na-resazurin. The bottles were crimp-sealed, purged with N2 three times and sterilized by autoclaving at 115 °C for 20 min. For 10% (v/v) inoculation, the bottles were then injected with 2 ml exponential phase cultures grown on 10 g L−1 Avicel PH 105 in DSMZ 122 media. The bottles were incubated in a shaking incubator (C24KC refrigerated incubator shaker, Edison, New Jersey, United States) with temperature controlled at 55 °C and rotation speed set at 150 rpm.2.3 Optimization of Triton X-100 addition time and substrate concentration
To study the addition time of Triton X-100 in C. thermocellum cultures, experiment with various addition times (0, 6, 12, 18, 24 and 48 h) were conducted and the pH of C. thermocellum cultures was measured. Bottles without Triton X-100 addition were used as the control. Similarly, in order to achieve more glucose accumulation, a series of substrate concentration (40, 60, 80 and 100 g L−1) were considered at the optimal addition time.The concentration of metabolites at endpoint of fermentation was measured by HPLC17 using culture supernatant centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min. The residual pellets were collected and dried at 60 °C until a constant weight and then the degradation ratio of SCB was calculated.
000 rpm for 10 min. The residual pellets were collected and dried at 60 °C until a constant weight and then the degradation ratio of SCB was calculated.
2.4 Saccharification using C. thermocellum cultures with Triton X-100 and BglA or Cellic®CTec2 supplementation
At optimal addition time of Triton X-100 and substrate concentration, 30 CBU per g substrate of BglA or Cellic®CTec2 (containing 1.2 FPU per g substrate) was supplemented directly into C. thermocellum cultures by single-use syringe and incubator for another 120 h at 55 °C and 150 rpm. Meantime, Avicel containing equal glucan content as the SCB at optimal concentration was also considered to study the synergism between C. thermocellum cultures and Cellic®CTec2. Several controls were set and detailed addition volume of each component was shown in Table 1.| Group | SCB/Avicel (%, w/v) | pH 4.8 sodium citrate buffer (ml) | Inocula (%, v/v) | Triton X-100 (%, w/v) | β-Glucosidase (CBU per g) |
|---|---|---|---|---|---|
| +TX | 6.0/3.6 | — | 10 | 0.25 | — |
| +CTec2 + TX | 6.0/3.6 | — | 10 | 0.25 | 30 |
| Enzymolysis | 6.0/3.6 | 19.92 | — | — | 30 |
| enz + TX | 6.0/3.6 | 19.52 | — | 0.25 | 30 |
3. Results and discussion
3.1 Effect of Triton X-100 addition on saccharification of SCB by C. thermocellum cultures
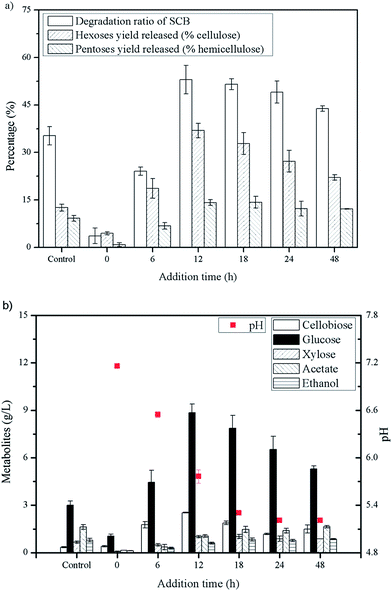
As described in previous study, addition of Triton X-100 in C. thermocellum cultures was primarily used to promote release and detachment of cellulosome from cell and also had a negative influence on the growth and metabolism of cell.8 Thus, a suitable time for Triton X-100 addition was important and studied first. As can be seen in Fig. 1(b), the addition time of Triton X-100 showed great influence on glucose accumulation and all tests exhibited a certain degree of increase at different addition time of fermentation except for that at 0 h, which means Triton X-100 addition and inoculum were operated at the same time. As the addition time was prolonged, the glucose accumulation increased rapidly and reached a plateau at 12 h, and with a further extension of the time, the glucose production decreased gradually. With Triton X-100 addition at 12 h, the glucose concentration increased significantly from 3.01 g L−1 of the control to 8.84 g L−1, resulting in an increase of 193.69% over the control. Our previous study indicated that Triton X-100 showed the inhibitive or detrimental effect on growth and metabolism of C. thermocellum, resulting in less transportation and consumption of reducing sugar. However, Triton X-100 had positive effect on enzyme activity and concentration in the supernatant and saccharification still continued, leading to accumulation of sugars.8 In order to eliminate the discrepancy of growth among different batches of the experiment, the addition time of Triton X-100 was associated with the pH of the cultures. Exactly, when the pH of the culture reached 5.5–6.0, which meant C. thermocellum was during late exponential phase, the optimal time for Triton X-100 addition was determined. PH of broth as an indicator reflected metabolite production (formate, lactate and acetate) of strain. With a high pH, the cell was during lag to early log phase and limited cellulosomes were produced, accompanied with a low degradation of SCB (Fig. 1(a)). However, when the pH of the culture dropped to a constant value and the cell was during stationary phase like 18, 24 and 48 h, the degradation ratios of SCB were all approximately 50% while the glucose concentration showed a distinct decrease. This might be due to part of the input cellulose was utilized in maintaining cellular homeostasis, such as for energy and coproduct production (Fig. 1(b)).
3.2 Enhancement of saccharification by C. thermocellum cultures with BglA supplementation
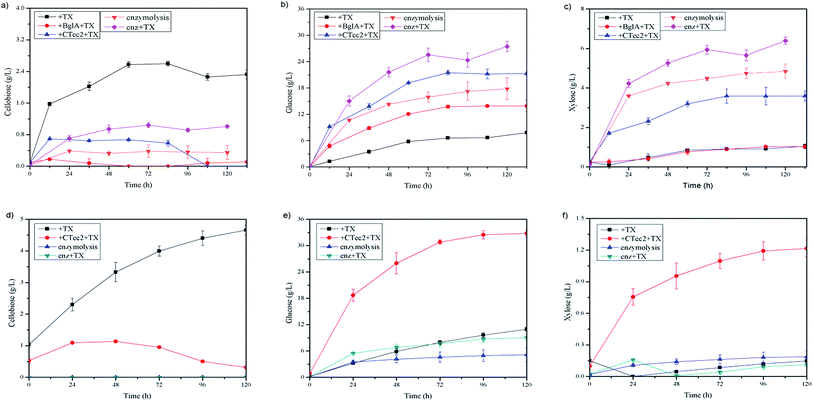
As shown in Fig. 2(b), about 3 g L−1 cellobiose was produced in the cultures and in order to relieve cellobiose inhibition and enhance the degradation of cellulose further, a certain amount of β-glucosidase was needed due to the shortage of this category in cellulosome.9,10In this study, we tested a thermostable β-glucosidase BglA, which was cloned from C. thermocellum ATCC 27405. As can be seen in Fig. 3, when 30 U g−1 of BglA was supplemented with Triton X-100 simultaneously, no cellobiose was detected anymore in the cultures and the glucose concentration increased significantly from 7.58 to 16.09 g L−1 (an increase of 112.27%). Meantime, the degradation ratio of SCB and hexoses yield released had a 34.88% and 112.43% increase respectively.
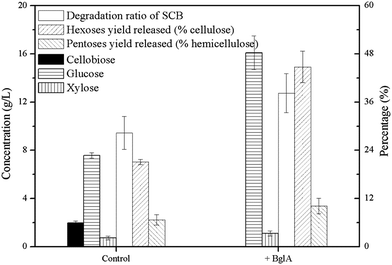 | ||
| Fig. 3 Enzymatic hydrolysis of 6% SCB by C. thermocellum cultures with Triton X-100 and 30 CBU per g of BglA supplementation. | ||
3.3 Synergistic saccharification of cellulose between C. thermocellum cultures and Cellic®CTec2
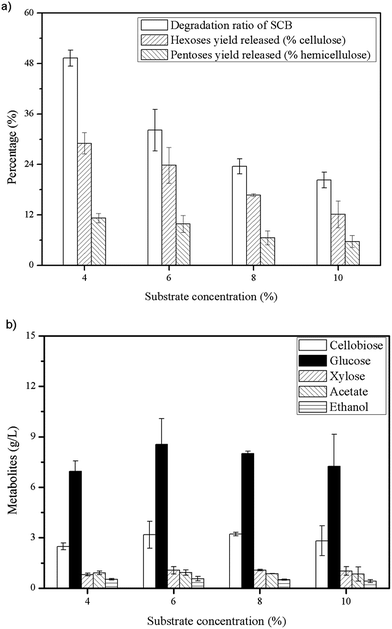
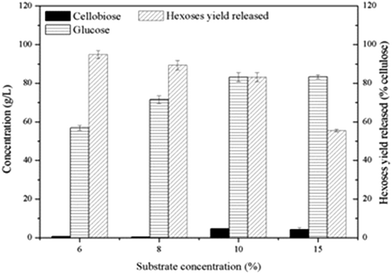
With a high level of β-glucosidase in commercial cellulase of Cellic®CTec2, a study about synergism between C. thermocellum cultures and Cellic®CTec2 in SCB or Avicel degradation was conducted. The C. thermocellum cultures with Triton X-100 added alone was assayed as noted above at their optimal conditions. The Cellic®CTec2 (the same dosage as in C. thermocellum cultures) with or without Triton X-100 added alone was also assayed at their optimal conditions as described in Table 1 in the methods section. Interestingly, the results showed that the combination of C. thermocellum cultures and Cellic®CTec2 exhibited the synergism on Avicel rather than SCB tested. The final glucose concentration of three groups, namely C. thermocellum culture with Triton X-100 addition, Cellic®CTec2 with Triton X-100 addition and combination of both were 10.95, 9.03 and 32.78 g L−1, respectively (Fig. 4(e)). Moreover, the initial (24 h) enzymatic hydrolysis rate were 0.134, 0.229 and 0.799 g L−1 h−1, respectively. A ‘1 + 1 > 2’ result was reflected between C. thermocellum culture and Cellic®CTec2. However, the synergistic effect was not observed in SCB and the performance of hydrolysis by Cellic®CTec2 (30 CBU per g, 1.2 FPU per g) with Triton X-100 added was superior to the combination of the C. thermocellum cultures, Triton X-100 and Cellic®CTec2 (Fig. 4(b)). Furthermore, we noted that in the case of pretreated biomass, fungal cellulases (Cellic®CTec2) are superior to cellulosome in enzymatic hydrolysis. While on high purity cellulose like Avicel, cellulosome showed better performance than that of fungal cellulases (Cellic®CTec2). The difference may be due to the different physical mechanism of cellulose deconstruction by fungal cellulases and cellulosomes.24High cellulose loading is preferred for industrial processes due to the benefits of lower capital cost and higher production of desirable products.10 Thus, to confirm potential of this synergistic system in cellulose saccharification, a series of substrate concentration (60, 80, 100 and 150 g L−1) was investigated and the saccharification time was extended to 240 h. As shown in Fig. 5, high hexoses yield released of 94.8% and 89.4% were obtained in cultures using 60 and 80 g L−1 cellulose and 83.17 g L−1 glucose was achieved at a cellulose concentration of 100 g L−1. More cellobiose was produced again with higher substrate loading and as a result, negative impact on further enzymatic hydrolysis was shown. Thus, in the future, a more efficient enzyme of β-glucosidase can be explored to match well with the C. thermocellum cultures and Triton X-100.
4. Conclusion
This study established an efficient saccharification system by C. thermocellum cultures with Triton X-100 and β-glucosidase supplementation in lignocellulose degradation. Not only increasing biodegradation of SCB but also more glucose accumulation was achieved. Moreover, the synergistic effect on pure cellulose saccharification between C. thermocellum cultures, Triton X-100 and Cellic®CTec2 was observed, leading to greatly increasing saccharification rate with decreasing commercial cellulase utilization. The present study suggested that the C. thermocellum cultures could be used for enzymatic hydrolysis directly without further separation and purification.Acknowledgements
The authors gratefully acknowledge the financial support of the National Natural Science Foundation of China [grant no. 51478190], Guangdong Provincial Natural Science Foundation Key Project [grant no. 2014A030311014] and Guangzhou Science and Technology Program [grant no. 201510010288 & 2014Y2-00047].References
- T. Hasunuma, F. Okazaki, N. Okai, K. Y. Hara, J. Ishii and A. Kondo, Bioresour. Technol., 2013, 135, 513–522 CrossRef CAS PubMed.
- A. V. Gusakov, Trends Biotechnol., 2011, 29, 419–425 CrossRef CAS PubMed.
- V. Balan, ISRN Biotechnol., 2014, 2014, 1–31 CrossRef PubMed.
- E. A. Bayer, R. Lamed and M. E. Himmel, Curr. Opin. Biotechnol., 2007, 18, 237–245 CrossRef CAS PubMed.
- E. A. Johnson, M. Sakajoh, G. Halliwell, A. Madia and A. L. Demain, Appl. Environ. Microbiol., 1982, 43, 1125–1132 CAS.
- T. Sheng, L. Zhao, L. F. Gao, W. Z. Liu, M. H. Cui, Z. C. Guo, X. D. Ma, S. H. Ho and A. J. Wang, Biotechnol. Biofuels, 2016, 9, 1–11 CrossRef.
- J. Cheng and M. Zhu, Bioresour. Technol., 2013, 144, 623–631 CrossRef CAS PubMed.
- X.-S. Qu and M.-J. Zhu, J. Biobased Mater. Bioenergy, 2016, 10, 362–369 CrossRef.
- R. Waeonukul, A. Kosugi, C. Tachaapaikoon, P. Pason, K. Ratanakhanokchai, P. Prawitwong, L. Deng, M. Saito and Y. Mori, Bioresour. Technol., 2012, 107, 352–357 CrossRef CAS PubMed.
- P. Prawitwong, R. Waeonukul, C. Tachaapaikoon, P. Pason, K. Ratanakhanokchai, L. Deng, J. Sermsathanaswadi, K. Septiningrum, Y. Mori and A. Kosugi, Biotechnol. Biofuels, 2013, 6, 1–11 CrossRef PubMed.
- H.-N. Lin, B.-B. Hu and M.-J. Zhu, Int. J. Hydrogen Energy, 2016, 41, 2383–2390 CrossRef CAS.
- S. Brethauer and M. H. Studer, Chimia, 2015, 69, 572–581 CrossRef CAS PubMed.
- D. W. Templeton, E. J. Wolfrum, J. H. Yen and K. E. Sharpless, BioEnergy Res., 2016, 9, 303–314 CrossRef PubMed.
- G. Gefen, M. Anbar, E. Morag, R. Lamed and E. A. Bayer, PNAS, 2012, 109, 10298–10303 CrossRef CAS PubMed.
- H. Zheng, J. Yin, Z. Gao, H. Huang, X. Ji and C. Dou, Appl. Biochem. Biotechnol., 2011, 164, 1215–1224 CrossRef CAS PubMed.
- T. K. Ghose, Pure Appl. Chem., 1987, 59, 257–268 CAS.
- Q. Q. Tian, L. Liang and M. J. Zhu, Bioresour. Technol., 2015, 197, 422–428 CrossRef CAS PubMed.
- J. Cheng, Y. Yu and M. Zhu, Green Chem., 2014, 16, 2689–2695 RSC.
- L. Feinberg, J. Foden, T. Barrett, K. W. Davenport, D. Bruce, C. Detter, R. Tapia, C. Han, A. Lapidus, S. Lucas, J. F. Cheng, S. Pitluck, T. Woyke, N. Ivanova, N. Mikhailova, M. Land, L. Hauser, D. A. Argyros, L. Goodwin, D. Hogsett and N. Caiazza, J. Bacteriol., 2011, 193, 2906–2907 CrossRef CAS PubMed.
- Y. Lu, Y. Wang, J. C. Guoqian Xu, Y. Zhuang and S. Zhang, Appl. Biochem. Biotechnol., 2008, 160, 360–369 CrossRef PubMed.
- A. G. Cruz, C. Scullin, C. Mu, G. Cheng, V. Stavila, P. Varanasi, D. Xu, J. Mentel, Y.-D. Chuang, B. A. Simmons and S. Singh, Biotechnol. Biofuels, 2013, 6, 1–9 CrossRef PubMed.
- G. Gefen, M. Anbar, E. Morag, R. Lamed and E. A. Bayer, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 10298–10303 CrossRef CAS PubMed.
- S. Morais, J. Stern, A. Kahn, A. P. Galanopoulou, S. Yoav, M. Shamshoum, M. A. Smith, D. G. Hatzinikolaou, F. H. Arnold and E. A. Bayer, Biotechnol. Biofuels, 2016, 9, 1–12 CrossRef PubMed.
- M. G. Resch, B. S. Donohoe, J. O. Baker, S. R. Decker, E. A. Bayer, G. T. Beckham and M. E. Himmel, Energy Environ. Sci., 2013, 6, 1858–1867 CAS.
| This journal is © The Royal Society of Chemistry 2017 |