Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Novel AIE columnar liquid crystals: the influence of the number of diphenylacrylonitrile groups on the mesomorphic and fluorescence properties†
Liangbin Lina,
Hongyu Guo*a,
Xiaoting Fanga and
Fafu Yang *abc
*abc
aCollege of Chemistry and Chemical Engineering, Fujian Normal University, Fuzhou 350007, P. R. China. E-mail: yangfafu@fjnu.edu.cn
bFujian Key Laboratory of Polymer Materials, Fuzhou 350007, P. R. China
cFujian Provincial Key Laboratory of Advanced Materials Oriented Chemical Engineering, Fuzhou 350007, P. R. China
First published on 6th April 2017
Abstract
The novel triphenylene derivatives with one, two or three diphenylacrylonitrile groups on the side chain were prepared in yields of 75–83%. The influence of the number of diphenylacrylonitrile groups on the mesophase and fluorescence properties were investigated. The triphenylene derivatives with one or two diphenylacrylonitrile groups exhibited not only the ordered hexagonal columnar mesophase but also good AIE fluorescence properties in both solution and solid state.
Columnar discotic liquid crystals, possessing the unique cores with π–π stacking structures, have attracted much commercial and academic research interest over the years.1 They were high ordered columnar mesophases with fast charge carrier mobility, exhibiting application prospects in various fields such as organic field-effect transistors, organic light-emitting diodes, organic photovoltaic cells, gas sensors, and so on.2–5 Recently, columnar luminescent liquid crystals have been paid considerable attention due to their effective combination of an intrinsic luminescence capability, the supramolecular organization and self-healing characteristic within a mesophase.6,7 For example, by using perylene or Bodipy as aromatic conjugated core,8–20 the luminescent liquid crystals could be constructed conveniently. However, although most of reported luminescent liquid crystals showed high fluorescence in solution, they could not emit fluorescence effectively in solid-state due to the aggregation caused quenching (ACQ) effect. Thus, various molecular designs were applied to avoid the ACQ effect,21,22 but often enhanced the expense of ordered molecular packing, making the synthesis of light-emitting liquid crystalline material a difficult task.
Recently, Tang's group discovered the aggregation-induced emission (AIE) effect, which implied the aggregation could enhance the light emission.23–25 Obviously, introduction of AIE-active dye into liquid crystal is a good strategy to solve the above problem. Thus, some liquid crystalline molecules with AIE effect were designed and synthesized by grafting the long alkyl chains on AIE molecules such as diphenylacrylonitrile and tetraphenylethene.26–37 However, most reported AIE liquid crystals were smectic or nematic mesophases, and only one columnar AIE liquid crystal was obtained by mixing tetraphenylethene–triphenylene oligomer with 2,4,7-trinitrofluorenone.37 These AIE liquid crystals were difficult to form columnar mesophase although they usually possessed highly symmetric structures containing a rigid aromatic core with several soft side-chains. The reason might be attributed to that the conjugated aromatic rings of AIE molecules are not the complete coplanar structures, which are against effective π–π packing in column. Based on this speculation, it could be deduced that the columnar AIE liquid crystal could be prepared by using normal discotic coplanar aromatic structure as core which was favourable for columnar mesophase, and controlling the numbers of AIE groups on side chain to avoid the destruction of columnar mesophase. But no paper concerned on this study up to now. In order to confirm this speculation, in this paper, three novel triphenylene liquid crystals with multiple diphenylacrylonitrile groups were designed and synthesized. Moreover, the influences of the numbers of diphenylacrylonitrile groups on mesomorphic and fluorescence properties were investigated. The experimental results confirmed the above speculation that the mesomorphic and fluorescence properties were influenced greatly by the numbers of AIE groups on side chains. The triphenylene liquid crystals with one or two diphenylacrylonitrile groups exhibited not only good AIE-luminescence property but also ordered hexagonal columnar mesophase.
It is well known that the monohydroxytriphenylene was a convenient platform to construct various triphenylene liquid crystals with good columnar mesophase.38–47 On the other hand, the excellent AIE-fluorescence property of diphenylacrylonitrile had been reported extensively.23–25 Thus, by using monohydroxytriphenylene and diphenylacrylonitrile as building blocks, the triphenylene derivatives with one, two or three diphenylacrylonitrile groups were designed to investigate the influences of the numbers of diphenylacrylonitrile groups on mesomorphic and fluorescence properties. The synthetic routes were illustrated in Scheme 1. According to the reported methods,46 By reacting monohydroxytriphenylene 1 with ethyl chloroacetate and then hydrazinolysis with NH2NH2, the triphenylene hydrazide derivative 3 was prepared in total yield of 68% after purification. On the other hand, by condensating 4-hydroxyphenylacetonitrile with benzaldehyde in NaOH/EtOH system, the diphenylacrylonitrile derivative 4 with hydroxyl group was synthesized in yield of 80%. Further refluxing compound 4 with excess 1-bromo-3-chloropropane in K2CO3/MeCN system, the diphenylacrylonitrile derivative 5 containing terminal Cl group was obtained in yield of 72% after recrystallization. Subsequently, by reacting compound 5 with 4-hydroxybenzaldehyde, 3,4-dihydroxybenzaldehyde or 2,3,4-trihydroxybenzaldehyde in K2CO3/MeCN system, the corresponding mono-, di- and tri-diphenylacrylonitrile derivatives 6, 7 and 8 bearing aldehyde group were collected in yields of 85%, 71% and 66%, respectively. Finally, the target triphenylene derivatives 9, 10 and 11 with one, two or three diphenylacrylonitrile groups were prepared in yield of 75–83% by Schiff-based condensation of compounds 6, 7 and 8 with triphenylene hydrazide derivative 3 under the catalysis of several drops of glacial acetic acid.
The structures of target compounds 9, 10 and 11 were confirmed by 1H NMR, 13C NMR, MALDI-TOF-MS and HR-MS spectral analysis. The corresponding molecular ion peaks (M+, MH+ or MNa+, see ESI†) were observed in their mass spectrometry spectra, suggesting the accomplishment of Schiff-based condensation. In their 1H NMR spectra, all protonic signals were in accordance with the corresponding structures (see ESI†). Although compounds 10 and 11 possessed the overlapped protonic signals for the ArH due to their very similar structures of multiple diphenylacrylonitrile units, the clear singlets for NH, HC–Ar and OCH2CO, and the accurate integrals for corresponding protons supported their structures in Scheme 1. The 13C NMR spectra also agreed with their structures. For example, only one peak at about 164 ppm implied one kind of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group in compounds 9, 10 and 11.
O group in compounds 9, 10 and 11.
As triphenylene derivatives with one, two or three diphenylacrylonitrile groups 9, 10 and 11 had been prepared, the influences of the numbers of diphenylacrylonitrile groups on mesomorphic properties were further studied by differential scanning calorimeter (DSC), polarizing optical microscopy (POM) and X-ray diffraction (XRD). Their DSC curves of samples 9, 10 and 11 for second heating and cooling were shown in Fig. 1. The data of phase transition temperatures and corresponding enthalpy changes were summarized in Table 1. It can be seen that the triphenylene derivative 9 with one diphenylacrylonitrile group exhibited two phase transition peaks upon cooling at 123.1 °C and 39.7 °C, and on second heating at 43.7 °C and 129.2 °C, respectively. The triphenylene derivative 10 with two diphenylacrylonitrile groups also showed the similar transition at 113.2 °C and 40.2 °C on cooling, and at 51.7 °C and 117.2 °C upon second heating. However, the triphenylene derivative 11 with three diphenylacrylonitrile groups only possessed one broad peak on cooling and seconding heating at 57.2 °C and 59.8 °C, respectively. These data might suggest that samples 9 and 10 with one or two diphenylacrylonitrile groups had the transition of crystalline phase-mesophase-isotropic phase during heating and cooling, but compound 11 with three diphenylacrylonitrile groups showed only crystalline phase-isotropic phase transition without mesophase. Moreover, these results implied that, as speculated, the rigid and un-coplanar AIE structure of diphenylacrylonitrile group made great influence on the liquid crystalline property. The more numbers of diphenylacrylonitrile groups resulted in the strong destruction to columnar mesophase. The temperature scope of mesophase for sample 9 with one diphenylacrylonitrile group was 85.5 °C, which was higher than that of sample 10 with two diphenylacrylonitrile group (65.5 °C). Three diphenylacrylonitrile groups on triphenylene derivative 11 destroyed the mesomorphic properties completely. These results suggested that, based on the strong columnar-induced packing capability of triphenylene unit, the triphenylene derivatives with one or two diphenylacrylonitrile groups still possessed the columnar mesophase.
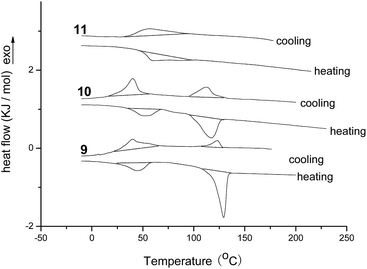 | ||
| Fig. 1 The DSC traces of compounds 9, 10 and 11 upon second heating and cooling (scan rate 10 °C min−1). | ||
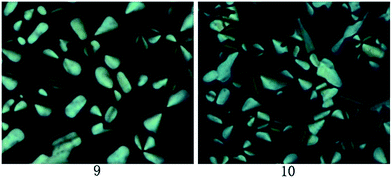
Under the POM observation, sample 11 exhibited the Cr–Iso phase transition upon heating and cooling, and no mesophase was observed. However, compounds 9 and 10 showed the Cr–Col and Col–Iso phase transition at the approximate temperatures of DSC curves. As slowly cooling from isotropic phase, the liquid crystalline textures formed gradually. The corresponding textures for samples 9 and 10 at 70 °C were illustrated in Fig. 2. One can see that these textures were the pseudo-confocal conic ones, indicating the columnar liquid crystals for samples 9 and 10.
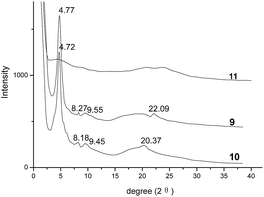
Moreover, the XRD analysis was employed to study the columnar stacking behaviors of mesophase of compounds 9 and 10. Their corresponding XRD traces at 70 °C were exhibited in Fig. 3. In the small-angle region, both samples 9 and 10 showed the three peaks at 4.77°, 8.27°, 9.55° and 4.72°, 8.18°, 9.45°, respectively. Based on these reflections, the d-spacings calculated by formula d = λ/(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin
sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ) were 18.51 Å, 10.68 Å and 9.25 Å for compound 9 and 18.70 Å, 10.80 Å and 9.35 Å for compound 10, respectively. There data were in agreement with the ratio of 1
θ) were 18.51 Å, 10.68 Å and 9.25 Å for compound 9 and 18.70 Å, 10.80 Å and 9.35 Å for compound 10, respectively. There data were in agreement with the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/√3
1/√3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/√4 for (100), (110) and (200) reflections of hexagonal columnar mesophase. Also, the lattice parameter (α) for compounds 9 and 10 could be calculated as 21.36 Å and 20.60 Å based on the strong [100] reflections of column phase. In their wide-angle regions, the broad halos at 15–25° indicated the distances of 3.6–5.9 Å approximately, which could be attributed to the reflections of very short correlation length of the molten alkyl chains. The weak reflections at 22.09° for sample 9 and 20.37° for sample 10, suggesting the spacings of 4.02 Å and 4.36 Å, could be assigned for the typical characteristic of π–π interaction for the intracolumnar distance of columnar liquid crystals. Thus, all these data in small- and wide-angle region implied the hexagonal columnar mesophase for compounds 9 and 10. On the other hand, compound 11 showed no obvious peak, indicating it had been melted at 70 °C without mesophase, which were in accordance with the analysis of DSC and POM. All these XRD analyses supported that, although one or two diphenylacrylonitrile groups were introduced on the side-chains of triphenylene derivatives 9 and 10, they still maintained the ordered hexagonal columnar mesophase based on the strong columnar-induced mesophase of triphenylene unit as expected.
1/√4 for (100), (110) and (200) reflections of hexagonal columnar mesophase. Also, the lattice parameter (α) for compounds 9 and 10 could be calculated as 21.36 Å and 20.60 Å based on the strong [100] reflections of column phase. In their wide-angle regions, the broad halos at 15–25° indicated the distances of 3.6–5.9 Å approximately, which could be attributed to the reflections of very short correlation length of the molten alkyl chains. The weak reflections at 22.09° for sample 9 and 20.37° for sample 10, suggesting the spacings of 4.02 Å and 4.36 Å, could be assigned for the typical characteristic of π–π interaction for the intracolumnar distance of columnar liquid crystals. Thus, all these data in small- and wide-angle region implied the hexagonal columnar mesophase for compounds 9 and 10. On the other hand, compound 11 showed no obvious peak, indicating it had been melted at 70 °C without mesophase, which were in accordance with the analysis of DSC and POM. All these XRD analyses supported that, although one or two diphenylacrylonitrile groups were introduced on the side-chains of triphenylene derivatives 9 and 10, they still maintained the ordered hexagonal columnar mesophase based on the strong columnar-induced mesophase of triphenylene unit as expected.
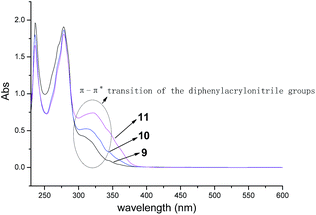
On the other hand, due to one, two or three AIE active groups of diphenylacrylonitrile grafted onto the triphenylene derivatives 9, 10 and 11, respectively, it was interesting to investigate the influences of the numbers of diphenylacrylonitrile groups on absorption spectra and fluorescence properties. The absorption spectra of compounds 9, 10 and 11 were depicted in Fig. 4. All of them showed very similar absorption profiles with three obvious peaks at 235 nm, 277 nm and 320 nm. But the peaks at 320 nm exhibited different absorbance. These results were in accordance with their structures. The similar absorption profiles were attributed to their similar functional structures. The different absorbance at 320 nm were ascribed to the π–π* transition of the different numbers of diphenylacrylonitrile groups. The more diphenylacrylonitrile groups resulted in the stronger absorbance at 320 nm.
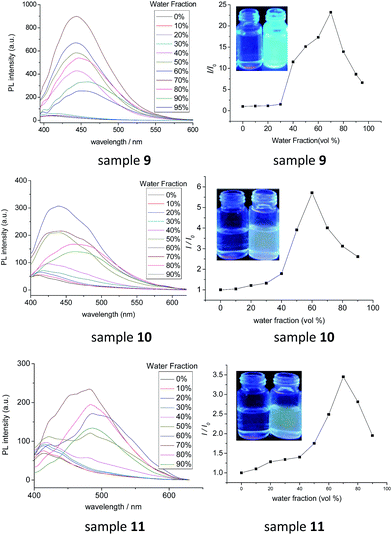
As diphenylacrylonitrile was a well-known AIE luminogen, the fluorescence (FL) spectra of samples 9, 10 and 11 in THF solution and THF/H2O mixture were measured to investigate the influences of the numbers of diphenylacrylonitrile groups on fluorescence properties. The fluorescence spectra of samples 9, 10 and 11 in THF/H2O mixture with different fractions of H2O and their corresponding plot of values (I/I0) versus the fractions of the aqueous mixtures were exhibited in Fig. 5. It can be seen that, in pure THF solution, all of them showed weak fluorescence emission. With the addition of H2O in THF solution, however, the fluorescence emission increased gradually. The increases of intensity were slow at low water fractions (<40%) but became very remarkable afterward. The strongest fluorescence emissions were in the solution of THF/H2O mixtures with 60% of H2O for 10 and 70% of H2O for 9 and 11. These results certainly suggested that compounds 9, 10 and 11 possessed the AIE fluorescence properties as expected. Due to the low solubility resulting in the precipitation, the fluorescence intensities decreased at high water fractions (>60% for 10 and >70% for 9 and 11). This other possibility for these phenomena, such as crystalline-induced emission, could be excluded by the XRD analysis which suggested the amorphous state for the precipitation (Fig. S22†). Moreover, by comparing the fluorescence intensities and I/I0 values of samples 9, 10 and 11, it can be seen that the fluorescence intensities in pure THF solution showed the order of 9 < 10 < 11. But their strongest fluorescence intensities in mixture solutions and their I/I0 values presented the inverse order of 9 > 10 > 11. These phenomena might be explained by their structures. Due to samples 9, 10 and 11 had good solubility and little aggregate in pure THF solution, the more diphenylacrylonitrile groups leaded to the stronger fluorescence intensity, resulted in the order of 9 < 10 < 11 for the fluorescence intensities of samples 9, 10 and 11 with one, two and three diphenylacrylonitrile groups, respectively. On the other hand, the literatures had revealed that the J-aggregate for diphenylacrylonitrile derivatives increased the fluorescence intensity but the H-aggregate might decrease the fluorescence intensity due to the strong π–π* interaction.48–51 The sample 9 with one diphenylacrylonitrile group preferred to J-aggregate in mixture solutions, enhancing the fluorescence intensity greatly by 24 times. However, due to the two or three diphenylacrylonitrile groups grafted onto the closed o-position of one aromatic ring in samples 10 and 11, the diphenylacrylonitrile groups might be more easily to form the H-aggregate in mixture solutions than sample 9, suggesting that samples 10 and 11 might possess not only J-aggregate but also H-aggregate in a certain degree. The existence of some H-aggregate for samples 10 and 11 decreased fluorescence intensity, although the strong J-aggregate for samples 10 and 11 still enhanced their fluorescence intensities. The H-aggregate was also confirmed by some blue shift for UV absorption maximum with the increase of water fractions in the solution of THF/H2O mixtures (Fig. S23†). Thus, although samples 10 and 11 possessed two or three diphenylacrylonitrile groups, their highest fluorescence intensities and maximum I/I0 values in mixture solutions were smaller than that of sample 9. Moreover, the fluorescence peaks of samples 9, 10 and 11 in mixture solutions exhibited obvious red shifts in comparison with that in pure THF solutions. The values for red shift were 45 nm, 51 nm and 70 nm for samples 9, 10 and 11, respectively. The fluorescence red-shift order of 9 < 10 < 11 might also suggest the existence of some H-aggregates for samples 10 and 11 because the stronger π–π* interaction of the H-aggregate leaded to the larger red shift than that of J-aggregate. On the other hand, the fluorescence quantum yield (ΦF) of samples 9, 10 and 11 in solid state were investigated as 15.9%, 7.5% and 5.6%, respectively. These data revealed that compounds 9, 10 and 11 possessed the middle fluorescence emission in solid state, although some H-aggregate for samples 10 and 11 resulting in the order of 9 < 10 < 11. Combining all these photophysical analysis, it could be summarized that the triphenylene derivatives 9, 10 and 11 with one, two or three diphenylacrylonitrile groups had good AIE fluorescence properties. The largest I/I0 values of fluorescence in THF/H2O solution attained 24 times and the highest ΦF in solid state achieved 15.9% for compound 9. The numbers of diphenylacrylonitrile groups had important influence on the fluorescence properties. The multiple diphenylacrylonitrile groups exhibited both the strong AIE fluorescence properties based on the J-aggregate, and weak ACQ effect due to some H-aggregate of multiple diphenylacrylonitrile groups on the closed o-position of one aromatic ring. This kind of novel luminescent LC showed both excellent light-emitting property and ordered columnar mesophase, which exhibited potential application as novel fluorescent liquid crystalline functional materials in liquid crystal display and opto-electronic devices, such as novel organic light-emitting diodes and sensors, etc.
Conclusions
In summary, three novel triphenylene derivatives with one, two or three diphenylacrylonitrile groups on the side chain were designed and synthesized by the Schiff-based condensation in yields of 75–83%. The studies on mesomorphic property revealed that triphenylene derivatives with one or two diphenylacrylonitrile groups exhibited the ordered hexagonal columnar mesophase, but the one with three diphenylacrylonitrile groups showed no mesophase. The photophysical experiments suggested that all of them had good AIE fluorescence properties. The largest fluorescence I/I0 values were 24 times and the highest ΦF in solid state were 15.9% for triphenylene derivative with one diphenylacrylonitrile group. The AIE properties presented the order of 9 > 10 > 11 due to the multiple diphenylacrylonitrile groups exhibited both the strong AIE fluorescence properties and weak ACQ effect. This research confirmed that the columnar AIE liquid crystal could be prepared by using normal discotic coplanar aromatic structure as core and controlling the number of AIE groups on side chain, which is a new strategy to design and synthesis of the novel AIE columnar liquid crystals.Acknowledgements
Financial support from the National Natural Science Foundation of China (No. 21406036), Fujian Natural Science Foundation of China (No. 2017J01571), the encouragement and inspiration from Professor Benzhong Tang were greatly acknowledged.Notes and references
- S. Kumar, Chemistry of discotic liquid crystals: from monomers to polymers, CRC press, London, New York, 2010 Search PubMed
.
- T. Wöhrle, I. Wurzbach, J. Kirres, A. Kostidou, N. Kapernaum, J. Litterscheidt, J. C. Haenle, P. Staffeld, A. Baro, F. Giesselmann and S. Laschat, Chem. Rev., 2016, 116, 1139–1241 CrossRef PubMed
.
- R. J. Bushby and K. Kawata, Liq. Cryst., 2011, 38, 1415–1426 CrossRef CAS
.
- S. Sergeyev, W. Pisula and Y. H. Geerts, Chem. Soc. Rev., 2007, 36, 1902–1929 RSC
.
- T. Kato, N. Mizoshita and K. Kishimoto, Angew. Chem., Int. Ed., 2006, 45, 38–68 CrossRef CAS PubMed
.
- T. Yasuda, H. Ooi, J. Morita, Y. Akama, K. Minoura, M. Funahashi, T. Shimomura and T. Kato, Adv. Funct. Mater., 2009, 19, 411–419 CrossRef CAS
.
- X. Q. Li, X. Zhang, S. Ghosh and F. Würthner, Chem.–Eur. J., 2008, 14, 8074–8078 CrossRef CAS PubMed
.
- M. G. Zhu, H. Y. Guo, F. F. Yang and Z. S. Wang, Liq. Cryst., 2016, 43, 1875–1883 CrossRef CAS
.
- H. Y. Guo, M. G. Zhu, Z. S. Wang and F. F. Yang, Tetrahedron Lett., 2016, 57, 4191–4195 CrossRef CAS
.
- M. G. Zhu, H. Y. Guo, F. F. Yang and Z. S. Wang, RSC Adv., 2017, 7, 4320–4328 RSC
.
- N. Mizoshita, T. Tani and S. Inagaki, Adv. Funct. Mater., 2011, 21, 3291–3298 CrossRef CAS
.
- Z. An, J. Yu, S. C. Jones, S. Barlow, S. Yoo, B. Domercq, P. Prins, L. D. A. Siebbeles, B. Kippelen and S. R. Marder, Adv. Mater., 2005, 17, 2580–2581 CrossRef CAS
.
- Z. Chen, U. Baumeister, C. Tschierske and F. Würthner, Chem.–Eur. J., 2007, 13, 450–465 CrossRef CAS PubMed
.
- X. T. Fang, H. Y. Guo, J. R. Lin and F. F. Yang, Tetrahedron Lett., 2016, 57, 4939–4943 CrossRef CAS
.
- L. Meng, Q. M. Wu, F. F. Yang and H. Y. Guo, New J. Chem., 2015, 39, 72–76 RSC
.
- G. A. Bhavsar and S. K. Asha, Chem.–Eur. J., 2011, 17, 12646–12658 CrossRef CAS PubMed
.
- M. Bagui, T. Dutta, H. Z. Zhong, S. H. Li, S. Chakraborty, A. Keightley and Z. H. Peng, Tetrahedron, 2012, 68, 2806–2818 CrossRef CAS
.
- M. G. Zhu, Z. S. Wang, F. F. Yang and H. Y. Guo, Dyes Pigm., 2016, 133, 387–394 CrossRef CAS
.
- S. K. Gupta, S. Setia, S. Sidiq, M. Gupta, S. Kumar and S. K. Pal, RSC Adv., 2013, 3, 12060–12065 RSC
.
- X. F. Kong, Z. Q. He, Y. N. Zhang, L. P. Mu, C. J. Liang, B. Chen, X. P. Jing and A. N. Cammidge, Org. Lett., 2011, 13, 764–767 CrossRef CAS PubMed
.
- C. L. Chiang, S. M. Tseng, C. T. Chen, C. P. Hsu and C. F. Shu, Adv. Funct. Mater., 2008, 18, 248–257 CrossRef CAS
.
- B. S. Gaylord, S. Wang, A. J. Heeger and G. C. Bazan, J. Am. Chem. Soc., 2001, 123, 6417–6418 CrossRef CAS PubMed
.
- Y. Hong, J. W. Y. Lam and B. Z. Tang, Chem. Soc. Rev., 2011, 40, 5361–5388 RSC
.
- M. Wang, G. Zhang, D. Zhang, D. Zhu and B. Z. Tang, J. Mater. Chem., 2010, 20, 1858–1867 RSC
.
- Y. Hong, J. W. Y. Lam and B. Z. Tang, Chem. Commun., 2009, 28, 4332–4335 RSC
.
- J. H. Wan, L. Y. Mao, Y. B. Li, Z. F. Li, H. Y. Qiu, C. Wang and G. Q. Lai, Soft Matter, 2010, 6, 3195–3210 RSC
.
- W. Z. Yuan, Z. Q. Yu, P. Lu, C. M. Deng, J. W. Y. Lam, Z. M. Wang, E. Q. Chen, Y. G. Ma and B. Z. Tang, J. Mater. Chem., 2012, 22, 3323–3326 RSC
.
- Y. F. Chen, J. S. Lin, W. Z. Yuan, Z. Q. Yu, J. W. Lam and B. Z. Tang, Sci. China: Chem., 2013, 56, 1191–1196 CrossRef CAS
.
- S. L. Yoon, J. H. Kim, K. S. Kim, J. W. Chung, B. Heinrich, F. Mathevet, P. Kim, B. Donnio, A. J. Attias and D. Kim, Adv. Funct. Mater., 2012, 22, 61–69 CrossRef CAS
.
- D. Y. Zhao, F. Fan, J. Cheng, Y. L. Zhang, K. S. Wong, V. G. Chigrinov, H. D. Kwok, L. Guo and B. Z. Tang, Adv. Opt. Mater., 2015, 3, 199–202 CrossRef CAS
.
- M. M. Stephen, M. Q. Malik and J. G. Damian, Opt. Mater., 2013, 35, 837–842 CrossRef
.
- S. J. Yoon, J. H. Kim, K. S. Kim, J. W. Chung, B. Heinrich, F. Mathevet, P. Kim, B. Donnio and A. J. Attias, Adv. Funct. Mater., 2012, 22, 61–69 CrossRef CAS
.
- W. P. Jin, N. Shusaku, Y. Seong-Jun, D. Tomoki, S. Jangwon, S. Takahiro and Y. P. Soo, Adv. Mater., 2014, 26, 1354–1359 CrossRef
.
- M. Martinez-Abadía, B. Robles-Hernandez, B. Villacampa, M. R. de la Fuente, R. Giménez and M. B. Ros, J. Mater. Chem. C, 2015, 3, 3038–3048 RSC
.
- M. Martinez-Abadía, S. Varghese, B. Milian-Medina, J. Gierschner, R. Giménez and M. B. Ros, Phys. Chem. Chem. Phys., 2015, 17, 11715–11724 RSC
.
- M. Martinez-Abadía, S. Varghese, R. Giménez and M. B. Ros, J. Mater. Chem. C, 2016, 4, 2886–2893 RSC
.
- W. H. Yu, C. Chen, P. Hu, B. Q. Wang, C. Redshaw and K. Q. Zhao, RSC Adv., 2013, 3, 14099–14106 RSC
.
- F. F. Yang, H. Y. Guo, J. W. Xie and J. R. Lin, Eur. J. Org. Chem., 2011, 26, 5141–5145 CrossRef
.
- S. Kumar, Liq. Cryst., 2009, 36, 607–638 CrossRef CAS
.
- F. F. Yang, B. T. Xu, H. Y. Guo and J. W. Xie, Tetrahedron Lett., 2012, 53, 1598–1602 CrossRef CAS
.
- A. Zelcer, B. Donnio, C. F. Bourgogne, D. Cukiernik and D. Guillon, Chem. Mater., 2007, 19, 1992–2006 CrossRef CAS
.
- F. F. Yang, J. W. Xie, H. Y. Guo, B. T. Xu and C. C. Li, Liq. Cryst., 2012, 39, 1368–1374 CrossRef CAS
.
- S. Kumar, S. K. Pal, P. S. Kumar and V. Lakshminarayanan, Soft Matter, 2007, 3, 896–900 RSC
.
- F. F. Yang, X. Y. Bai, H. Y. Guo and C. C. Li, Tetrahedron Lett., 2013, 54, 409–413 CrossRef CAS
.
- F. F. Yang, Y. M. Zhang, H. Y. Guo and X. Y. Bai, Tetrahedron Lett., 2013, 54, 4953–4956 CrossRef CAS
.
- F. F. Yang, J. Yuan, C. C. Li, H. Y. Guo and X. Y. Yan, Liq. Cryst., 2014, 41, 137–143 CrossRef CAS
.
- C. Imrie and P. Henderson, Chem. Soc. Rev., 2007, 36, 2096–2124 RSC
.
- P. Chen, R. Lu, P. C. Xue, T. H. Xu, G. J. Chen and Y. Y. Zhao, Langmuir, 2009, 25, 8395–8399 CrossRef CAS PubMed
.
- S. Y. Li, L. M. He, F. Xiong, Y. Li and G. Q. Yang, J. Phys. Chem. B, 2004, 108, 10887–10892 CrossRef CAS
.
- H. Q. Zhang, S. M. Wang, Y. Q. Li, B. Zhang, C. X. Du, X. J. Wan and Y. S. Chen, Tetrahedron, 2009, 65, 4455–4463 CrossRef CAS
.
- M. Kasha, H. R. Rawls and M. A. El-Bayoumi, Pure Appl. Chem., 1965, 11, 371–392 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, supporting figures and table. See DOI: 10.1039/c7ra02338c |
| This journal is © The Royal Society of Chemistry 2017 |