Open Access Article
Open Access ArticleNiX2 (X = Cl and Br) sheets as promising spin materials: a first-principles study†
Muhammad Mushtaq,
Yungang Zhou * and
Xia Xiang*
* and
Xia Xiang*
School of Physical Electronics, University of Electronic Science and Technology of China, Chengdu, 610054, P. R. China. E-mail: zhouyungang1@126.com; xiaxiang@uestc.edu.cn
First published on 24th April 2017
Abstract
In order to achieve paper-like spin devices, it is rather critical to develop new two-dimensional (2D) spin materials. In this study, the geometrical structure, stability, and electronic and magnetic properties of 2D nickel dihalides of NiX2 (X = Cl and Br) type were investigated using density functional theory (DFT) calculations. We found that after optimization, geometries of NiCl2 and NiBr2 sheets that are obtained from their bulk counterparts are well kept. Phonon dispersion calculations demonstrated that both NiCl2 and NiBr2 sheets are dynamically stable. Magnetism calculations showed that ferromagnetic (FM) coupling is ground state for both structures in which per NiCl2 and NiBr2 unit cells can possess the moments of 1.91 and 1.88 μB, respectively. Density of states (DOS) and band structure calculations revealed that both structures are magnetic semiconductors with large band gaps. In addition, strain effect also showed that the moments of NiCl2 and NiBr2 sheets can be effectively tuned by applying the biaxial strain. A unique combination of integrated geometry, dynamical stability, intrinsic ferromagnetism, a magnetic semiconductor and tunable magnetism makes NiCl2 and NiBr2 sheets promising candidates for next-generation paper-like spin devices.
Introduction
Driven by the enormous demand of the microprocessor market all over the world, a paper-like spin device has become one of the most exciting and challenging research areas. Paper-like spin devices can not only utilize spin degree of freedom of the electron to sense, store, process and transfer information with high density and high sensitivity but can also overcome the fundamental limits, such as heat dissipation and energy loss, of the device architecture caused by the diminishing size of the devices in current semiconductor technologies. Thus, the development of paper-like spin devices is important to both fundamental scientific research and industrial applications. It is well known that the performance of a device strongly depends on the properties of the material. Consequently, the progress of paper-like spin devices drives us to further develop advanced 2D spin materials.Thus far, different families of 2D materials, such as the group IVA family (graphene, silicene and germanene),1,2 group IIIA (boronene),3 group VA family (phosphorene, arsenene and antimonene),4,5 group IIIA–VA family (BN sheet),6,7 group IVA–VIA family (GeS, GeSe, SnS and SnSe sheets),8 transition metal dichalcogenides (MoS2, MoSe2, WS2, WSe2, TaS2, TaSe2, VS2, VSe2, NbS2 and NbSe2 sheets),9–11 MXenes (Ti2C, V2C, Cr2C, Nb2C, Zr2C, Hf2C and Ta2C sheets),12,13 organo-metal porous materials (Co-PP, Cr-PP, Cu-PP, Fe-PP, Mn-PP, Ni-PP and Zn-PP sheets),14,15 metal-halides16 and metal-oxides,17,18 have been reported theoretically and experimentally. Among these, all IVA, IIIA, VA, IIIA–VA, IVA–VIA groups and most transition metal dichalcogenides in their pristine forms are nonmagnetic, thus hindering their applications in spin-based devices. Great efforts have been made in order to explore the application of 2D structures in this field.19–27 For example, creating carbon vacancies in graphene,19 doping metallic or nonmetallic atoms in graphene,20,21 silicone,22 germanene22 and phosphorene,23 and using strain in defect-interacted MoS2 sheet,25 have been confirmed as effective methods to generate spin. Particularly, FM, the important property for the application of 2D materials in spintronic devices, was predicted in half-brominated silicone26 and half-fluorinated BN and ZnO sheets.27 Nevertheless, experimental realisation of promising spin with the methods mentioned above still is difficult because precise modulations of vacancy, doping, strain and surface-functionalization on 2D structures is still challenging. Recently, there has been an increasing interest for exploring 2D structures with intrinsic magnetism.28–31 Some MXenes,28 organo-metallic porous sheets,29 metal-halide sheets30 and metal-oxide sheets31 have been identified as excellent spin materials. In particular, robust FM was reported in pristine Ti2C,32 Ti2N,32 Cr2C,33 FeC2,34 VCl3,35 VI3 (ref. 35) and NiCl3 (ref. 36) sheets. While great efforts have been made in previous studies, researching new promising 2D spin structures is still important for further development of paper-like spin devices.
NiCl2 and NiBr2 crystals have been prepared for several years with methods such as vapor phase dynamical transport method,37 laser beam38,39 and thermal evaporation.40 It should be noted that NiCl2 and NiBr2 crystals are formed by the stacking of layers with the interlayer separations of 3.08 and 3.24 Å, respectively. The existence of large interlayer separation in such two materials provides a possibility of their exfoliations into single-layer sheets, as reported in graphene1 and MoS2.9 Thus, in this study, we focused on the study of stability, geometrical structure, electronic and magnetic properties of NiCl2 and NiBr2 sheets. As mentioned above, NiCl2 and NiBr2 sheets can be viewed as 2D counterparts of NiCl2 and NiBr2 crystals, respectively.41 Our paper is organized as follows: (1) we first discuss the geometric structures of NiCl2 and NiBr2 sheets; (2) the stabilities of NiCl2 and NiBr2 sheets are then examined by performing phonon dispersion curve calculations; (3) next, magnetic properties of NiCl2 and NiBr2 sheets, including magnetic moment, magnetic coupling, exchange parameter and Curie temperature, are studied in detail; (4) finally, based on the calculations of DOS and band structures, we analyze the electronic properties of NiCl2 and NiBr2 sheets; (5) in addition, we also explore the effect of strain on the magnetic properties of NiCl2 and NiBr2 sheets. Our aim in so doing is to assess the suitability of NiCl2 and NiBr2 sheets as excellent spin materials.
Theory and methods
First principles calculations were performed using projector augmented wave (PAW) type potentials42 executed in the Vienna ab initio Simulation Package (VASP).43,44 The Perdew–Burke–Ernzerhof (PBE)45 pseudo potential inside the general gradient approximation (GGA) was chosen for the exchange correlation. The 3s23p5, 4s24p5 and 3d84s2 atomic orbitals are treated as valence states for Cl, Br and Ni atoms, respectively. A plane wave basis set with threshold value of 600 eV was used. In order to avoid the interactions between neighboring sheets, we set a vacuum space of about 17 Å for NiCl2 and NiBr2 sheets. The k-point sampling in reciprocal space was represented with 10 × 10 × 1 grid meshes. The convergence criterion for the atomic position and lattice vector optimizations was set as 1 × 10−4 eV in energy. To account for strong correlation of the unfilled d orbitals of the Ni atom, the U scheme was used to respect on-site electron correlation. Herein, the effective Coulomb exchange interaction, Ueff = U − J, where U and J are on-site Coulomb energy and exchange energy parameters, respectively, was set as 3 eV. The veracity of Ueff = 3 eV for 3d transition metal compounds has been confirmed in previous studies.46,47 Moreover, in this study, the spin-resolved total DOS, atom projected density of states (PDOS) and band structure calculations were performed with a more accurate and reliable Heyd–Scouseria–Ernzerhof (HSE06) scheme.48,49Results and discussions
We first performed the structural relaxations, including volume and atom position, for NiX2 (X = Cl, Br) sheets. A 2 × 2 supercell that contains four Ni atoms and eight X atoms was applied for NiX2 sheets. The optimized atomic structures of NiX2 sheets are shown in Fig. 1. NiX2 sheets show a close resemblance in structure with 1T-MoS2.50 Calculated lattice constants are about 3.50 and 3.70 Å for NiCl2 and NiBr2 sheets, respectively, which are comparable to the values of 3.48 and 3.72 Å for NiCl2 and NiBr2 in their bulk structures.51,52 The “thickness” of NiCl2 and NiBr2 sheets are found to be about 2.69 and 2.85 Å, respectively, which also are comparable to the values of 2.72 and 2.87 Å for NiCl2 and NiBr2 in their bulk structures.51,52 In addition, we found that the Cl–Ni–Cl bond angle in NiCl2 sheet is about 92.80°, which is only 1.18° larger than the value of 91.62° found in its bulk structure; the calculated Br–Ni–Br bond angle in NiBr2 sheet is about 92.17°, which is only 0.07° larger than the value of 92.10° found in its bulk structure.51,52 Thus, the difference of structural parameters between 2D and their 3D structures is very small, indicating that geometries of NiCl2 and NiBr2 sheets obtained from their bulk counterparts are well kept.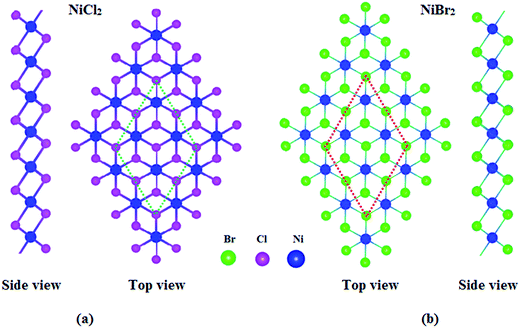 | ||
| Fig. 1 Optimized geometric structures of (a) NiCl2 and (b) NiBr2 sheets. The selected 2 × 2 supercell is marked by the dotted lines. | ||
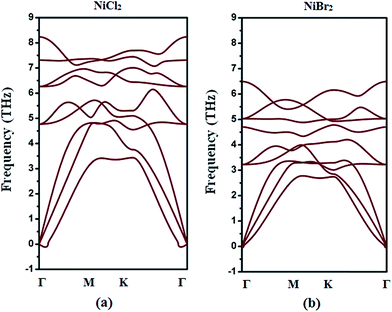
The stabilities of the structures are important for their practical applications. To check whether NiCl2 and NiBr2 sheets are stable enough, we performed phonon dispersion curve calculations. During the calculations a 1 × 1 unit cell was used for both cases. Since per unit cell contains three atoms (1Ni atom and 2X atoms), 9 phonon branches (3 acoustic branches and 6 optical branches) were observed in Fig. 2. Clearly, for NiBr2 sheet, we found that there are no imaginary bands. Thus, NiBr2 sheet is dynamically stable. As for NiCl2 sheet, the lowest transverse branch has quite few imaginary frequencies as Γ → 0. Nevertheless, we also conclude that NiCl2 sheet is dynamically stable for two reasons: (1) the obtained imaginary frequencies might arise from the numerical artifacts because phonon dispersion calculations are highly sensitive to the choices of parameter and supercell and36,53 (2) the obtained imaginary frequencies might be of acoustic nature because they are derived from a collective vibration mode with a long wavelength.36,54
Next, we carried out spin-polarized calculations to study the magnetic properties of NiX2 sheets. In order to decide the most favorable state, a 2 × 2 supercell consisting of four Ni and eight X atoms was used. Three different coupling structures, including FM, AFM and nonmagnetic (NM) structures, were considered as shown in Fig. 3(a). Our calculations showed that for both structures, NM coupling is the least stable coupling, and FM coupling is somewhat more stable than AFM coupling. Calculated energy differences between AFM and FM couplings, ΔE = EAFM − EFM, are about 63 and 71 meV for NiCl2 and NiBr2 sheets, respectively. Calculated net magnetic moment for NiCl2 unit cell is about 1.91 μB, in which each Ni atom contributes 1.57 μB and each Cl atom contributes 0.16 μB; calculated net magnetic moment for NiBr2 unit cell is about 1.88 μB, in which each Ni atom contributes 1.50 μB and each Br atom contributes 0.18 μB. Corresponding spin charge distributions for NiCl2 and NiBr2 sheets with FM coupling are shown in Fig. 3(b). Long-range magnetic ordering observed here makes NiCl2 and NiBr2 sheets promising candidates for spin applications. In order to estimate the robustness of FM coupling, we calculated the values of the exchange parameter J and Curie temperature Tc. The value of J was obtained from the Ising model with the expression H = −J∑mimj, where H is the Hamiltonian in the absence of external magnetic field, J is the exchange parameter for two nearest neighbor Ni atoms located at sites i and j, and mi and mj are the magnetic moments of Ni atoms at sites i and j, respectively.14 For NiCl2 and NiBr2 sheets, J can be represented as J = ΔE/16m2.14,55 Calculated values of J are about 1.59 and 1.94 meV for NiCl2 and NiBr2 sheets, respectively. The positive sign of the constants confirm that Ni atoms in both cases prefer to be formed as FM coupling instead of AFM coupling. Furthermore, comparing with the values of other dihalides, such as 0.63 meV in CrCl3 sheet and 0.81 meV in CrBr3 sheet,30 we believe the J values obtained here are large enough. The value of Tc was further evaluated based on a classical Heisenberg model with the formula Tc = 2/3(ΔE/kβ),56 where kβ is the Boltzmann constant. Calculated values of Tc are about 121 and 136 K for NiCl2 and NiBr2 sheets, respectively. Comparing with the values of 66 K reported in CrCl3 sheet and 86 K reported in CrBr3 sheet,30 the obtained values of Tc here are also somewhat higher.
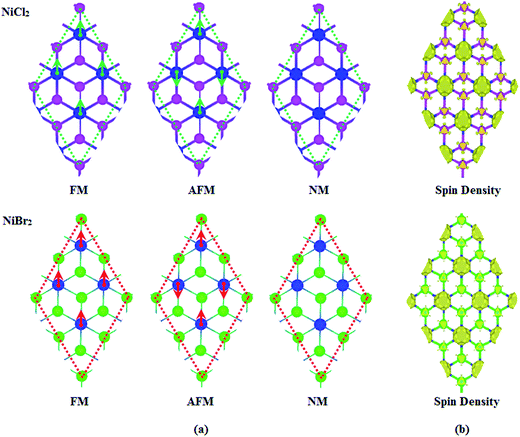 | ||
| Fig. 3 (a) Ferromagnetic, antiferromagnetic, nonmagnetic configurations for NiCl2 and NiBr2 sheets. (b) The spin density distributions for ferromagnetic NiCl2 and NiBr2 sheets. | ||
Finally, we explored the electronic properties of NiCl2 and NiBr2 sheets. Spin-polarized band structures based on HSE06 functional are shown in Fig. 4. Two important features were observed for NiCl2 and NiBr2 sheets. First, both sheets present a semiconducting property. Calculated band gaps are about 5.0 and 4.0 eV for NiCl2 and NiBr2 sheets, respectively. For both structures, the valence band maximum (VBM) and conduction band minimum (CBM) are contributed by spin-up and spin-down channels, respectively. In order to understand the origin of VBM and CBM, PDOS of Ni and Cl atoms in NiCl2 sheet and Ni and Br atoms in NiBr2 sheet were calculated, as shown in Fig. 5. It can be seen that for NiCl2 sheet, the VBM mainly comes from the Cl atom and the CBM mainly comes from the Ni atom, and for NiBr2 sheet, VBM mainly comes from the Br atom and the CBM mainly comes from the Ni atom. Further studies showed that VBM and CBM in NiCl2 sheet are caused by the Cl-3p and Ni-3d orbitals, respectively, and VBM and CBM in NiBr2 sheet are caused by the Br-4p and Ni-3d orbitals, respectively. In addition, we also calculated the band structures of NiCl2 and NiBr2 sheets with different Ueff, shown in Fig. 1S in the ESI.† Clearly, the change of band structure for both structures is very small with the increase of Ueff, indicating that the semiconducting property with large band gap found in NiCl2 and NiBr2 sheets is rather strong. Second, as shown in Fig. 4, a distinct asymmetry between the spin-up and spin-down channels was observed for NiCl2 and NiBr2 sheets. The asymmetry of spin channels mainly comes from the bands located at the energy region from −8.0 to 0 eV for both sheets. As shown in Fig. 5, we found that Ni atoms have a dominant contribution in the introduction of asymmetry of spin channels, and the contributions of Cl/Br atoms are small. Thus, for both sheets, the moment was caused mainly by Ni atoms, in accord with the moment calculations mentioned above. Further examination reveals that all d orbitals including dxy, dyz, dz2, dxz and dx2−y2 orbitals contribute to the magnetism for both sheets. As a result, the solid semiconductor and remarkable magnetism show great potential for the application of NiCl2 and NiBr2 sheets in the spin field.
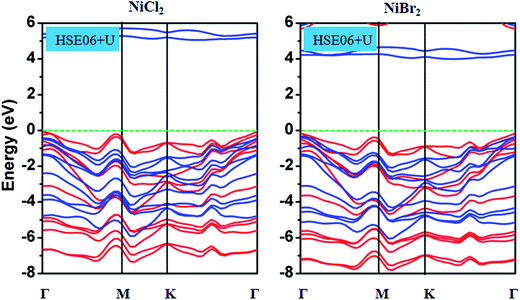 | ||
| Fig. 4 Spin-polarized HSE06 + U band structures of NiCl2 and NiBr2 sheets. The red and blue solid lines correspond to the spin-up and spin-down states. | ||
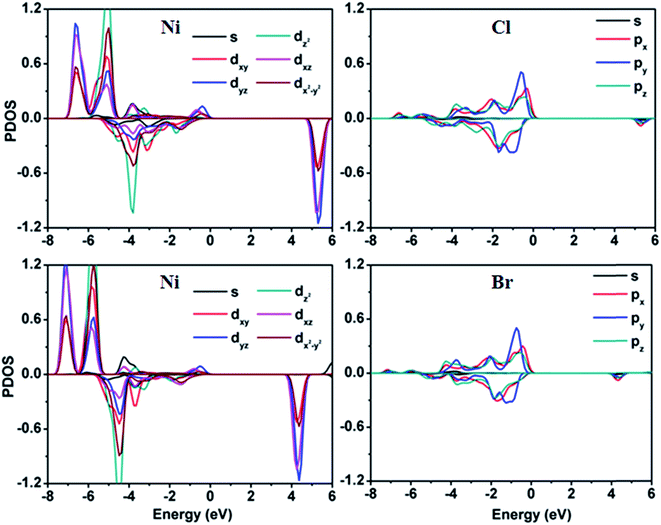 | ||
| Fig. 5 Spin-polarized partial density of states of Ni and Cl atoms in NiCl2 sheet and Ni and Br atoms in NiBr2 sheet. | ||
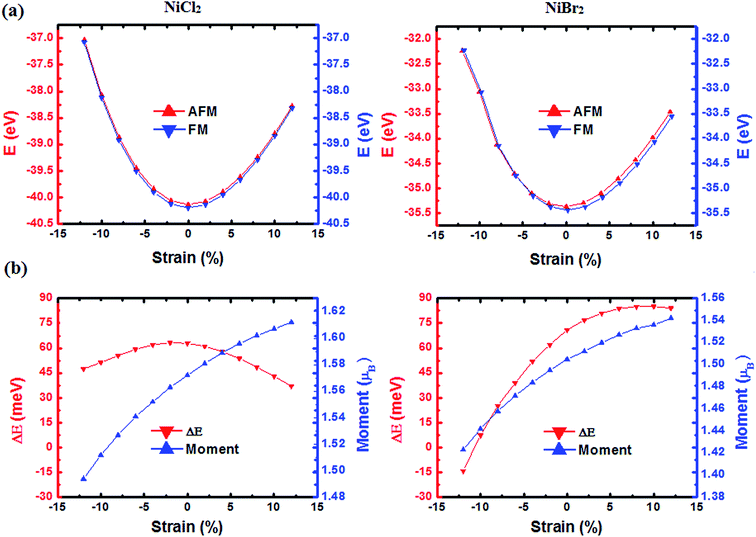
It has been proven that the magnetic property of materials can be tuned by strain.57 Thus, we also investigated the strain effect on the magnetic properties of NiCl2 and NiBr2 sheets by varying the biaxial strain from −12–12%. As shown in Fig. 6(a), total energies of NiCl2 and NiBr2 sheets increase continuously with the increase of tensile strain or compression strain, indicating that fracture process will not happen when NiCl2 and NiBr2 sheets are deformed. Variations of the spin moment and the energy difference between AFM and FM states, ΔE, as a function of strain are shown in Fig. 6(b). One can see that the responses of ΔE against the strain for NiCl2 and NiBr2 sheets are somewhat different. For NiCl2 sheet, the ΔE first increases and then decreases with the increase of strain, and for NiBr2 sheet, the ΔE presents a tendency towards monotonic increase. Nevertheless, except for 12% compression strain applied in NiBr2 sheet, ΔE is always positive for both cases, indicating that FM coupling is more stable than AFM coupling for different deformed structures. Moreover, for both cases, the moment increases almost linearly as we increase the strain. For NiCl2 sheet, the moment increases from 1.49 to 1.61 μB when the strain increased from −12–12%, and, for NiBr2 sheet, the moment increases from 1.42 to 1.54 μB when the strain increased from −12–12%. In addition, we also calculated their band structures with different strains, as shown in Fig. 2S in the ESI.† The change of band structure is very small with the increase of strain for both structures. Tunable magnetism and stable FM coupling and the semiconducting property indicate great potential applications of NiCl2 and NiBr2 in flexible spin nanoelectronics.
Conclusions
In conclusion, using DFT, we constructed NiX2 (X = Cl, Br) sheets and studied their geometrical structures, stabilities, electronic and magnetic properties. Both sheets show a tri-layered structure in which the central layer is formed by Ni atoms and the top and bottom layers are formed by X atoms. The phonon dispersion calculations show that ultrathin NiX2 structures are dynamically stable. Spin polarized calculations revealed that FM coupling is the ground state for NiX2 sheets. Calculated energy differences between AFM and FM couplings are about 63 and 71 meV for NiCl2 and NiBr2 sheets, respectively. The spin-polarized DOS and band structure calculations based on HSE06 functional revealed that both sheets are solid semiconductors with remarkable magnetisms. In addition, strain effect calculations show that FM coupling is always more stable than AFM coupling for NiCl2 and NiBr2 sheets, and the moments of NiCl2 and NiBr2 sheets can be effectively tuned by applying biaxial strain. These results open the possibility for two new promising 2D structures in the application of spin devices.Acknowledgements
This project was supported by the National Natural Science Foundation of China (No. 11504044), the Fundamental Research Funds for the Central Universities (No. ZYGX2015KYQD012) and the New Academic Researcher Award (No. Y03111023901014002).References
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS PubMed.
- H. Şahin, S. Cahangirov, M. Topsakal, E. Bekaroglu, E. Akturk, R. T. Senger and S. Ciraci, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 80, 155453 CrossRef.
- H. Tang and S. Ismail-Beigi, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 82, 115412 CrossRef.
- H. Liu, A. T. Neal, Z. Zhu, Z. Luo, X. Xu, D. Tománek and P. D. Ye, ACS Nano, 2014, 8, 4033–4041 CrossRef CAS PubMed.
- S. Zhang, Z. Yan, Y. Li, Z. Chen and H. Zeng, Angew. Chem., Int. Ed., 2015, 54, 3112–3115 CrossRef CAS PubMed.
- C. Jin, F. Lin, K. Suenaga and S. Iijima, Phys. Rev. Lett., 2009, 102, 195505 CrossRef PubMed.
- R. Ponce-Perez, G. H. Cocoletzi and N. Takeuchi, J. Mol. Model., 2016, 22, 226 CrossRef CAS PubMed.
- L. C. Gomes, A. Carvalho and A. H. Castro Neto, Phys. Rev. B: Condens. Matter Mater. Phys., 2015, 92, 214103 CrossRef.
- K. S. Novoselov, D. Jiang, F. Schedin, T. J. Booth, V. V. Khotkevich, S. V. Morozov and A. K. Geim, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 10451–10453 CrossRef CAS PubMed.
- M. Chhowalla, H. S. Shin, G. Eda, L.-J. Li, K. P. Loh and H. Zhang, Nat. Chem., 2013, 5, 263–275 CrossRef PubMed.
- J. N. Coleman, M. Lotya, A. O'Neill, S. D. Bergin, P. J. King, U. Khan, K. Young, A. Gaucher, S. De, R. J. Smith, I. V. Shvets, S. K. Arora, G. Stanton, H.-Y. Kim, K. Lee, G. T. Kim, G. S. Duesberg, T. Hallam, J. J. Boland, J. J. Wang, J. F. Donegan, J. C. Grunlan, G. Moriarty, A. Shmeliov, R. J. Nicholls, J. M. Perkins, E. M. Grieveson, K. Theuwissen, D. W. McComb, P. D. Nellist and V. Nicolosi, Science, 2011, 331, 568–571 CrossRef CAS PubMed.
- M. Khazaei, M. Arai, T. Sasaki, C. Y. Chung, N. S. Venkataramanan, M. Estili, Y. Sakka and Y. Kawazoe, Adv. Funct. Mater., 2013, 23, 2185–2192 CrossRef CAS.
- G. Gao, G. Ding, J. Li, K. Yao, M. Wu and M. Qian, Nanoscale, 2016, 8, 8986–8994 RSC.
- J. Tan, W. Li, X. He and M. Zhao, RSC Adv., 2013, 3, 7016 RSC.
- M. Abel, S. Clair, O. Ourdjini, M. Mossoyan and L. Porte, J. Am. Chem. Soc., 2011, 133, 1203–1205 CrossRef CAS PubMed.
- J. He, S. Ma, P. Lyu and P. Nachtigall, J. Mater. Chem. C, 2016, 4, 2518–2526 RSC.
- M. Topsakal, S. Cahangirov, E. Bekaroglu and S. Ciraci, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 80, 235119 CrossRef.
- M. Kan, J. Zhou, Q. Sun, Y. Kawazoe and P. Jena, J. Phys. Chem. Lett., 2013, 4, 3382–3386 CrossRef CAS PubMed.
- M. M. Ugeda, I. Brihuega, F. Guinea and J. M. Gomez-Rodriguez, Phys. Rev. Lett., 2010, 104, 096804 CrossRef CAS PubMed.
- E. J. G. Santos, A. Ayuela and D. Sánchez-Portal, New J. Phys., 2010, 12, 053012 CrossRef.
- L. Xie, X. Wang, J. Lu, Z. Ni, Z. Luo, H. Mao, R. Wang, Y. Wang, H. Huang, D. Qi, R. Liu, T. Yu, Z. Shen, T. Wu, H. Peng, B. Ozyilmaz, K. Loh, A. T. S. Wee, Ariando and W. Chen, Appl. Phys. Lett., 2011, 98, 193113 CrossRef.
- X. Q. Wang, H. D. Li and J. T. Wang, Phys. Chem. Chem. Phys., 2012, 14, 3031–3036 RSC.
- H. Zheng, J. Zhang, B. Yang, X. Du and Y. Yan, Phys. Chem. Chem. Phys., 2015, 17, 16341–16350 RSC.
- A. Ramasubramaniam and D. Naveh, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 87, 195201–195207 CrossRef.
- P. Tao, H. Guo, T. Yang and Z. Zhang, J. Appl. Phys., 2014, 115, 054305 CrossRef.
- F.-B. Zheng and C.-W. Zhang, Nanoscale Res. Lett., 2012, 7, 422 CrossRef PubMed.
- E. J. Kan, H. J. Xiang, F. Wu, C. Tian, C. Lee, J. L. Yang and M. H. Whangbo, Appl. Phys. Lett., 2010, 97, 122503 CrossRef.
- S. Zhao, W. Kang and J. Xue, Appl. Phys. Lett., 2014, 104, 133106 CrossRef.
- E. Kan, X. Wu, C. Lee, J. H. Shim, R. Lu, C. Xiao and K. Deng, Nanoscale, 2012, 4, 5304–5307 RSC.
- J. Liu, Q. Sun, Y. Kawazoe and P. Jena, Phys. Chem. Chem. Phys., 2016, 18, 8777–8784 RSC.
- W. Cheng, J. He, T. Yao, Z. Sun, Y. Jiang, Q. Liu, S. Jiang, F. Hu, Z. Xie, B. He, W. Yan and S. Wei, J. Am. Chem. Soc., 2014, 136, 10393–10398 CrossRef CAS PubMed.
- G. Gao, G. Ding, J. Li, K. Yao, M. Wu and M. Qian, Nanoscale, 2016, 8, 8986–8994 RSC.
- C. Si, J. Zhou and Z. Sun, ACS Appl. Mater. Interfaces, 2015, 7, 17510–17515 CAS.
- T. Zhao, J. Zhou, Q. Wang, Y. Kawazoe and P. Jena, ACS Appl. Mater. Interfaces, 2016, 8, 26207–26212 CAS.
- J. He, S. Ma, P. Lyua and P. Nachtigall, J. Mater. Chem. C, 2016, 4, 2518–2526 RSC.
- J. He, X. Li, P. Lyu and P. Nachtigall, Nanoscale, 2017, 9, 2246–2252 RSC.
- M. Kozielski, I. Pollini and G. Spinolo, J. Phys. C: Solid State Phys., 1972, 5, 1253 CrossRef CAS.
- H. Y. Rosenfeld, R. Popovitz-Biro, Y. Prior, S. Gemming, G. Seifert and R. Tenne, Phys. Chem. Chem. Phys., 2003, 5, 1644–1651 RSC.
- H. Y. Rosenfeld, R. Popovitz-Brio, E. Grunbaum, Y. Prior and R. Tenne, Adv. Mater., 2002, 14, 1075–1078 CrossRef.
- M. Bar-Sadan, R. Popovitz-Biro, Y. Prior and R. Tenne, Mater. Res. Bull., 2006, 41, 2137–2146 CrossRef CAS.
- M. R. Tubbs, Phys. Status Solidi B, 1972, 49, 11–50 CrossRef CAS.
- P. E. Blöchl, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 17953–17979 CrossRef.
- G. Kresse and J. Hafner, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 47, 558–561 CrossRef CAS.
- G. Kresse and F. Jürgen, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- H. S. Kim and H. Y. Kee, Phys. Rev. B: Condens. Matter Mater. Phys., 2015, 92, 235121 CrossRef.
- M. Arita, K. Shimada, Y. Utsumi, O. Morimoto, H. Sato, H. Namatame, M. Taniguchi, Y. Hadano and T. Takabatake, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 83, 246116 CrossRef.
- J. Heyd, G. E. Scuseria and M. Ernzerhof, J. Chem. Phys., 2003, 118, 8207–8215 CrossRef CAS.
- F. Fuchs, J. Furthmüller, F. Bechstedt, M. Shishkin and G. Kresse, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 76, 115109 CrossRef.
- G. Eda, T. Fujita, H. Yamaguchi, D. Voiry, M. Chen and M. Chhowalla, ACS Nano, 2012, 6, 7311–7317 CrossRef CAS PubMed.
- J. A. A. Ketelaar, Z. Kristallogr., 1934, 88, 26–34 CAS.
- A. Ferrari, A. Braibanti and G. Bigliardi, Acta Crystallogr., 1963, 16, 846–847 CrossRef CAS.
- S. H. Lin and J. L. Kuo, Phys. Chem. Chem. Phys., 2014, 16, 20763–20771 RSC.
- X. Li, Y. Dai, M. Li, W. Wei and B. Huang, J. Mater. Chem. A, 2015, 3, 24055–24063 CAS.
- J. Zhou and Q. Sun, J. Am. Chem. Soc., 2011, 133, 15113–15119 CrossRef CAS PubMed.
- Y. W. Ma, Y. H. Lu, J. B. Yi, Y. P. Feng, T. S. Herng, X. Liu, D. Q. Gao, D. S. Xue, J. M. Xue, J. Y. Ouyang and J. Ding, Nat. Commun., 2012, 3, 727 CrossRef CAS PubMed.
- J. Zhou, Q. Wang, Q. Sun, Y. Kawazoe and P. Jena, J. Phys. Chem. Lett., 2012, 3, 3109–3114 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra02218b |
| This journal is © The Royal Society of Chemistry 2017 |