 Open Access Article
Open Access ArticlePolymeric vesicle formation via temperature-assisted nanoprecipitation†
Junli Zhou‡
 a,
Rong Ni‡bc and
Ying Chau*ab
a,
Rong Ni‡bc and
Ying Chau*ab
aDepartment of Chemical and Biomolecular Engineering, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong, China. E-mail: keychau@ust.hk
bDivision of Biomedical Engineering, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong, China
cInstitute for Advanced Study, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong, China
First published on 24th March 2017
Abstract
We here report an easy and efficient strategy to prepare submicron-sized polymeric vesicles with tetrahydrofuran (THF) as a good solvent through temperature-assisted nanoprecipitation (TAN). While conventional nanoprecipitation did not yield vesicles from block co-polymers (PEG-b-PCL), TAN produced vesicles with morphology and membrane thickness similar to those obtained by film rehydration method. Elevated temperature to allow fast evaporation of THF was identified to be the key process parameter of TAN.
Polymeric vesicles are closed membrane structures self-assembled from amphiphilic block copolymers.1,2 The large hydrophilic reservoir and thick polymer membrane provides a unique environment to encapsulate both hydrophilic and hydrophobic cargoes. This property also enables their broad application in the food industry, cosmetic applications, medical diagnosis and drug delivery.3 In addition, polymeric vesicles are amenable for surface modification and offer certain advantages for controllable drug release.4
The formation of polymeric vesicles usually requires non-spontaneous methods involving high-energy input to break and disperse the bilayer into membrane since the flat bilayer is usually the lowest free energy state, although spontaneous vesicle formation is possible under limited circumstances.5,6 In both cases, it requires molecules to possess certain molecule geometry, described by a dimensionless “packing parameter”, that could lead to desired morphology.7 To obtain polymeric vesicles, extensive efforts have been made to search for polymers with suitable packing parameters. However, the effect of physicochemical properties of the polymer on the resultant morphology was not yet clear. Therefore, some polymers with the same packing parameter can result in different morphologies by different preparation methods and poly(ethylene glycol)-b-poly(ε-caprolactone) (PEG-b-PCL) is one of the typical examples.8
Currently, film rehydration is one of the frequently used methods for polymeric vesicle formation.9 However, the large vesicles require an extra extrusion step to generate submicron-sized ones. This complicated process (including film formation, hydration and extrusion) limits the practical application and scalability of this method. As an alternative strategy, solvent exchange/nanoprecipitation method has been developed to prepare polymeric vesicles.10,11 Specifically, the block copolymer is completely dissolved in a good solvent (usually a water miscible organic solvent which can dissolve all the polymer segments), followed by slow addition of a non-solvent (usually water) into it. The slow exchange of solvent drives the assembly of hydrophobic block, leading to polymeric vesicle formation. However, this process is slow and time-consuming. Therefore, we are motivated to derive a fast and convenient vesicle preparation method.
Nanoprecipitation, by the addition of polymer/organic solution into water/aqueous buffer, is a fast and reproducible method to prepare solid nanospheres at room temperatures.12 In this method, the diffusion of the water-miscible organic solvent into water leads to phase separation and solidification of polymers, which results in the formation of nanoparticles of certain morphology and size.13,14 Temperature has been identified as an important factor to determine nanoparticle size through fastening the solvent diffusion process. However, the temperature studied in nanoprecipitation method so far is up to 50 °C, which is lower than the boiling temperature of most water-miscible organic solvents.15 Therefore, we hypothesize that if the processing temperature is higher than the boiling temperature of the good organic solvent, the addition of polymer/organic solution into hot water may lead to the polymeric vesicle formation through the boiling and fast evaporation of the organic solvent.
To test this hypothesis, we used diblock copolymer PEG-b-PCL as model molecules to study the temperature controlled morphology with modified nanoprecipitation method (TAN). The chemical structure of PEG-b-PCL was shown in Fig. 1. This block copolymer is made from two FDA-approved polymers, so its biodegradability and biocompatibility are advantageous for biomedical applications. However, inconsistency in morphology between methods despite the adoption of appropriate hydrophilic ratio (f) became a barrier to widely produce PEG-b-PCL vesicles.8 It was found that the optimal hydrophilic fraction for PEG-b-PCL polymeric vesicle formation can be as low as 0.12.16,17 A systematical study over a wide range of mPEG-b-PCL polymers using film rehydration suggested that polymers with smaller PEG corona (Mn ≤ 3.8k) were more likely to form polymeric vesicles.18,19 It is also noted that only PEG-b-PCL with molecular weights 2–12k (fEO ≈ 0.13) was recognized as vesicular polymer repeatedly.20 To start with, three poly(ethylene glycol)-b-poly(ε-caprolactone) (PEG-b-PCL) block copolymers with three different hydrophilic fractions (0.25, 0.12 and 0.10) were synthesized for this study, which were reported to form vesicles only via film rehydration in previous references.
Before further investigation, PEG-b-PCL block copolymers with different PEG molecular weights and different hydrophilic fractions were designed based on previous references and synthesized using ring-opening polymerization. Polymer characterization results were summarized in Table 1. The polymer compositions and the molecular weights were analyzed by proton NMR, which showed that all the synthesized polymers closely matched the designed molecular weights. Also, gel permeation chromatography (GPC) showed that all polymers displayed similar polydispersity index (PDI) around 1.2–1.55. Their melting temperatures were measured using differential scanning calorimetry (DSC) and were within 52–56 °C (Table 1), consistent with the previous reference.21
| Polymers | Label | Mna (×104) | fEO | PDIb | Tmc/°C |
|---|---|---|---|---|---|
| a Number-average molecular weight calculated from 1H NMR spectrum.b PDI of polymers measured by GPC (calibrated with polystyrene standards).c Melting temperature of PEG and block copolymers were characterized using DSC. | |||||
| PEG (2k)-b-PCL (6k) | CL26 | 0.80 | 0.25 | 1.52 | 52.3 |
| PEG (2k)-b-PCL (12k) | CL212 | 1.69 | 0.13 | 1.32 | 54.6 |
| PEG (2k)-b-PCL (18k) | CL218 | 2.10 | 0.09 | 1.34 | 54.9 |
The vesicle formation capability of these model polymers was firstly evaluated by film rehydration method (Fig. 2 and S2 and S4†). Therefore, they are good model compounds to test our hypothesis. Tetrahydrofuran (THF) is a routine water-miscible organic solvent used in conventional nanoprecipitation.22 Based on its boiling temperature (66 °C), we setup the processing temperature Tp at 80 °C. To evaluate the efficacy of TAN to fabricate vesicles, the morphology of particle samples was imaged under confocal microscope and cryo-TEM (Fig. 2A and 4, S2 and S4†). It was shown that three block copolymers (CL26, CL212 and CL218) formed vesicles and spherical micelles using the TAN method. According to the captured cryo-TEM images and the observation during the imaging process, the general yield of vesicles is estimated to be 14–50% (Table S3†), which is comparable to those prepared by film rehydration for all three tested polymers (Table S3†). This may be due to the kinetic trap during the fast process in TAN. As a comparison, these polymers were processed with nanoprecipitation at room temperature. Consistent with previous reports, no vesicles but small nanoparticles were obtained (Fig. 2A).8 The results indicate that TAN method (Tp > Tb) can indeed produce submicron-sized vesicles when conventional nanoprecipitation (20 °C) fails to do so.
Polymer concentration and stirring speed are two important factors for the final morphology by TAN. When the polymer concentration was low (0.4 mg ml−1), no vesicles but long tubular-like structures were observed. When the polymer concentration is up to 0.7 mg ml−1, vesicles were generated (Fig. S4†). On the other hand, the stirring speed also affected the vesicle formation. Lower speed is not enough to separate vesicles and the network of vesicles formed, while maximal stirring speed generated dispersed vesicles (Fig. S4†). Therefore, in the later experiment, 1 mg ml−1 polymer concentration and maximal stirring speed were used without further statement.
Membrane thickness is a unique property of polymeric vesicles.4 Therefore, we measured the membrane thickness of polymeric vesicles prepared through TAN and film rehydration methods based on their cryo-TEM images (Fig. 2A and S2†). Similar membrane thickness was observed for the vesicles made by both methods (Fig. 2B). The results indicate that the TAN method is comparable as film rehydration to produce polymeric vesicles.
Next, the vesicle formation temperature required by the TAN method was determined with CL212 (one of the model polymers) under four experimental temperatures: 60 °C, 70 °C, 80 °C and 90 °C. The resultant morphologies were again imaged by cryo-TEM. Fig. 3 demonstrated that no vesicle was observed below 50 °C (Fig. S3†), and the lowest temperature for vesicle formation is around 60 °C. Besides, both polymeric vesicles and fiber-like structures were observed at 60 °C, indicating morphology transition happened around this temperature. This transition temperature is close to the melting temperature of this model polymer CL212 (Tm = 54.6 °C) (Table 1) as well as the boiling temperature of the organic solvent THF (Tb = 66 °C). Therefore, what contributes to the vesicle formation may be the crystallinity change of the polymer chain or the evaporation of THF under this high processing temperature of TAN.
 | ||
| Fig. 3 Cryo-EM images of CL212 particles using TAN method with THF as the good solvent under different temperature: 60 °C (A), 70 °C (B), 80 °C (C) and 90 °C (D) (scale bar = 100 nm). | ||
To understand the temperature-polymer crystallinity correlation on polymeric vesicle formation, several other polymers, either amorphous or higher crystalline, were studied. To this purpose, four amorphous polymers [PEG-PDLLA (2–10k, 5–28k) and PEG-PLGA (2–18k and 5–30k)] and PEG-PLLA (5–20k) of higher crystallinity and melting temperature (Tm = 165.4 °C) were dissolved in THF and processed with TAN. By confocal microscopy, all these polymers showed the formation of vesicles, though the size varied (Fig. 4 and S4†). This suggests that the crystallinity alteration of the polymers is not necessary for vesicle formation.
Then we evaluated the relationship between the boiling temperature of the organic solvent and the processing temperature on polymeric vesicle fabrication by TAN. To this end, water-miscible solvents DMSO (Tb = 189 °C), acetone (Tb = 56 °C) and methanol (Tb = 64.7 °C) of different boiling points were chosen as the good solvents to dissolve polymer, which were processed with TAN at 80 °C. No vesicles but rather amorphous short flakes, long filaments and short filaments were observed for polymer samples in DMSO, acetone and methanol, respectively, under confocal microscope (Fig. S4†). This result supports that not only the processing temperature, but also the properties of organic solvents play important roles on the morphology of polymeric products via TAN. Different organic solvents may have different solubility for the polymers, different diffusion rate in water, and different evaporation rate at high temperature. All these factors can contribute to the final morphology. For the nanoprecipitation method with THF, the interactions among water–polymer, polymer–THF as well as THF–water govern the diffusion process and phase separation of polymer chains, and therefore the final particle size and morphology. When the temperature is close to or higher than the boiling temperature of THF (Tb = 66 °C), the boiling and fast evaporation of THF may result in the final vesicles through: (1) the formation of tiny air bubbles or (2) fastening the solidification process and generating in situ film. By combination with strong agitation, submicron-sized vesicles can be produced.
To evaluate whether the particle size transition is related to the morphology transition, particles were prepared under five operational temperatures: 4 °C, 20 °C, 50 °C, 60 °C and 80 °C and then characterized by DLS. Average diameters of polymeric particles using nanoprecipitation under different temperatures were summarized in Fig. 5. From 4 °C to 20 °C, the size of particles only slightly increased and mainly located in the range of 60–90 nm. From 50 °C to 80 °C, the size of particles increased significantly. The influence of temperature on particle size is non-linear, and higher temperatures (50–80 °C) have more significant effect on particle size than lower temperatures (4–50 °C). Since the temperature threshold for vesicle production is 50 °C, the significant size increase from 50 °C to 80 °C serves to provide addition support on temperature dependent morphology transition.
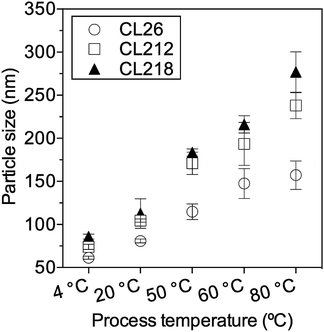 | ||
| Fig. 5 The average diameters of polymeric particles using nanoprecipitation method under different temperatures. Particle sizes were measured by DLS (intensity mean ± SD, n = 5). | ||
Conclusions
A simple and robust polymeric vesicle fabrication method named temperature-assisted nanoprecipitation (TAN) has been developed by using THF as the good organic solvent. This method integrates nanoprecipitation with rational control of the processing temperature, which is set above the boiling temperature of THF. The morphology and membrane thickness of the vesicles prepared through TAN are comparable to those fabricated by film hydration method. Therefore, this method offers us an alternative and fast process to prepare polymeric vesicles.Acknowledgements
This work was supported by the Hong Kong Research Grants Council (GRF 16100014). Junli was supported by Hong Kong PhD Fellowship Scheme.References
- B. M. Discher, Y. Y. Won, D. S. Ege, J. C. M. Lee, F. S. Bates, D. E. Discher and D. A. Hammer, Science, 1999, 284, 1143–1146 CrossRef CAS PubMed.
- P. L. Soo and A. Eisenberg, J. Polym. Sci., Part B: Polym. Phys., 2004, 42, 923–938 CrossRef CAS.
- J. S. Lee and J. Feijen, J. Controlled Release, 2012, 161, 473–483 CrossRef CAS PubMed.
- J. F. Le Meins, O. Sandre and S. Lecommandoux, Eur. Phys. J. E, 2011, 34, 1–17 CrossRef PubMed.
- V. Guida, Adv. Colloid Interface Sci., 2010, 161, 77–88 CrossRef CAS PubMed.
- D. D. Lasic, R. Joannic, B. C. Keller, P. M. Frederik and L. Auvray, Adv. Colloid Interface Sci., 2001, 89, 337–349 CrossRef PubMed.
- A. Ramachandran, T. H. Anderson, L. G. Leal and J. N. Israelachvili, Langmuir, 2011, 27, 59–73 CrossRef CAS PubMed.
- X. F. Sui, P. Kujala, G. J. Janssen, E. de Jong, I. S. Zuhorn and J. C. M. van Hest, Polym. Chem., 2015, 6, 691–696 RSC.
- D. E. Discher and A. Eisenberg, Science, 2002, 297, 967–973 CrossRef CAS PubMed.
- C. E. Mora-Huertas, H. Fessi and A. Elaissari, Int. J. Pharm., 2010, 385, 113–142 CrossRef CAS PubMed.
- C. Sanson, C. Schatz, J. F. Le Meins, A. Brulet, A. Soum and S. Lecommandoux, Langmuir, 2010, 26, 2751–2760 CrossRef CAS PubMed.
- S. Hornig, T. Heinze, C. R. Becer and U. S. Schubert, J. Mater. Chem., 2009, 19, 3838–3840 RSC.
- J. G. J. L. Lebouille, R. Stepanyan, J. J. M. Slot, M. A. C. Stuart and R. Tuinier, Colloids Surf., A, 2014, 460, 225–235 CrossRef CAS.
- A. Mahapatro and D. K. Singh, J. Nanobiotechnol., 2011, 9, 1–11 CrossRef PubMed.
- S. Hornig, T. Heinze, C. R. Becer and U. S. Schubert, J. Mater. Chem., 2009, 19, 3838–3840 RSC.
- J. S. Lee and J. Feijen, J. Controlled Release, 2012, 158, 312–318 CrossRef CAS PubMed.
- Z. Q. Pang, W. Lu, H. L. Gao, K. L. Hu, J. Chen, C. L. Zhang, X. L. Gao, X. G. Jiang and C. Q. Zhu, J. Controlled Release, 2008, 128, 120–127 CrossRef CAS PubMed.
- J. S. Katz, K. A. Eisenbrown, E. D. Johnston, N. P. Kamat, J. Rawson, M. J. Therien, J. A. Burdick and D. A. Hammer, Soft Matter, 2012, 8, 10853–10862 RSC.
- W. Qi, P. P. Ghoroghchian, G. Li, D. A. Hammer and M. J. Therien, Nanoscale, 2013, 5, 10908–10915 RSC.
- P. P. Ghoroghchian, G. Z. Li, D. H. Levine, K. P. Davis, F. S. Bates, D. A. Hammer and M. J. Therien, Macromolecules, 2006, 39, 1673–1675 CrossRef CAS PubMed.
- S. B. Zhou, X. M. Deng and H. Yang, Biomaterials, 2003, 24, 3563–3570 CrossRef CAS PubMed.
- M. M. Niu, Y. W. Naguib, A. M. Aldayel, Y. C. Shi, S. D. Hursting, M. A. Hersh and Z. R. Cui, Mol. Pharmaceutics, 2014, 11, 4425–4436 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: NMR and other information. See DOI: 10.1039/c7ra01959a |
| ‡ Equal contribution. |
| This journal is © The Royal Society of Chemistry 2017 |



