Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Ultra high stable supercapacitance performance of conducting polymer coated MnO2 nanorods/rGO nanocomposites†
K. Hareesh *ab,
B. Shateeshc,
R. P. Joshib,
J. F. Williamsa,
D. M. Phased,
S. K. Haram*c and
S. D. Dhole*b
*ab,
B. Shateeshc,
R. P. Joshib,
J. F. Williamsa,
D. M. Phased,
S. K. Haram*c and
S. D. Dhole*b
aSchool of Physics, University of Western Australia, Crawley, WA 6009, Australia. E-mail: appi.2907@gmail.com
bDepartment of Physics, Savitribai Phule Pune University, Pune – 411007, India. E-mail: sanjay@physics.unipune.ac.in
cDepartment of Chemistry, Savitribai Phule Pune University, Pune – 411007, India. E-mail: haram@chem.unipune.ac.in
dUGC-DAE Consortium for Scientific Research, Indore – 452001, India
First published on 5th April 2017
Abstract
A ternary nanocomposite that consists of MnO2 nanorods and reduced graphene oxide sheets supported on poly(3,4-ethylenedioxythiophene)-poly(styrenesulfonate) (PEDOT:PSS) polymer has been developed for supercapacitor applications. X-ray diffraction, field emission scanning electron microscopy, Raman spectroscopy, X-ray photoelectron spectroscopy, the Brunauer–Emmett–Teller method and X-ray photoelectron spectroscopic analysis confirmed the formation of a ternary nanocomposite of PEDOT:PSS/MnO2 nanorods/rGO. Electrochemical investigation of these materials in acetonitrile containing lithium per chlorate demonstrated an enhanced specific capacitance of 633 F g−1 at a current density of 0.5 A g−1 and 100% stability up to 5000 charging–discharging cycles at 1 A g−1. The enhanced capacitance and working stability of the PEDOT:PSS/MnO2 nanorods/rGO nanocomposite along with the simplicity in making the active materials make this system a promising candidate for the commercial development of supercapacitors.
Introduction
Transition metal oxides have attracted immense attention because of their applications in various fields such as active materials for water splitting catalysts, sensors, pollutant degradation, Li-ion batteries, supercapacitors, etc.1,2 Supercapacitors are alternative energy storage-conversion devices that show rapid charging–discharging rates, a high power density and a long cycle life. Supercapacitors have a variety of applications, such as in memory backup systems, industrial power, consumer electronics, public transportation and military devices.3–5 Among various transition metal oxides, MnO2 has received tremendous attention due to its low cost, environmental friendliness and natural abundance.6 α-MnO2 is a polymorph of MnO2 with different spatial arrangements depending upon linkage of its basic MnO6 octahedral units.7 However, its low inherent electrical conductivity (10−5 to 10−6 S cm−1) is a main constraint for its widespread application.8 Overcoming this limitation has been attempted by combining it with carbon based materials, such as graphene/reduced graphene oxide, to improve its conductivity.9,10Graphene is a layer polymorph of carbon with a 2D hexagonal packing layered structure and can offer a useful synergy by serving both as a conducting agent and as the cathode material.11,12 Reduced graphene oxide (rGO) is considered a physical analogue of graphene that can be obtained by reducing the oxygen functional groups present on the edge and basal plane of graphene oxide (GO) and is considered to be a cost effective substitute for graphene prepared by physical methods.13,14
Recently, researchers have demonstrated many binary systems with the combination of different kinds of MnO2 and rGO/carbon nanotube (CNT). Zhao et al.15 have reported the uniform anchoring of MnO2 nanosheets on macroporous graphene for supercapacitor electrodes. Xia et al.16 have studied a MnO2–CNT nanocomposite and observed an enhancement in supercapacitance compared to the individual MnO2 and CNT components. The carbon nanofibres/MnO2 nanosheets have been synthesized for an asymmetric supercapacitance application by Ning et al.17 Bristle-like α-MnO2 has been grown on multi-walled CNTs by Vinny et al.18 for asymmetric supercapacitance applications. Ma et al.19 have synthesized hierarchical MnO2 nanowire/graphene hybrid fibres with excellent electrochemical performance. Liu et al.20 have reported the in situ chemical synthesis of sandwich structured MnO2/graphene nanoflowers for supercapacitive applications. A three dimensional pompon-like MnO2/graphene hydrogel composite has been synthesized by Zhang et al.21 for supercapacitor applications.
The specific capacitance of these binary nanocomposites can be further enhanced by combining them with a conducting support like viz. PEDOT:PSS, polyaniline, polypyrrole, etc.22 Among many conducting polymers, the PEDOT:PSS polymer has received attention due to its easy processability and its conjugated backbone, which allows an easy conveyance of de-localized electrons through the π orbital system.23–26 The unfilled valence shells of oxygen atoms in PEDOT:PSS act as doping levels that make it important in charge storage materials.27 A plausible reason for this enhancement might be due to the dispersion of binary active materials in the conducting matrix, which may allow all the edges and the corners to be available for charge storage as well as protect the faradic materials from dissolution into the electrolyte. Supercapacitance applications for a graphene/MnO2 nanostructure wrapped by the PEDOT:PSS polymer have been studied by Yu et al.28 Hou et al.29 have improved the specific capacitance of MnO2 nanospheres/CNT nanocomposites by wrapping them with the PEDOT:PSS conducting polymer. The sandwich-structure MnO2/polypyrrole/rGO hybrid composites have been synthesized by Han et al.30 for high performance supercapacitors. The same research group synthesized a combination of MnO2 nanorods/GO nanocomposites and polyaniline to enhance the supercapacitance performance.31 Pan et al.32 have reported the two step synthesis of polyaniline/MnO2/graphene ternary composites for electrochemical supercapacitor applications. The glycol assisted synthesis of a graphene–MnO2–polyaniline ternary nanocomposite for supercapacitor applications has been studied by Mu et al.33 Li et al.34 synthesized the MnO2 nanoflakes/polyaniline nanorods hybrid nanostructures on graphene paper and observed tan enhancement in supercapacitance performance. The electrochemical properties of the MnO2 nanosheet array/rGO/PEDOT:PSS ternary composite electrode material for supercapacitor applications has been studied by Yan et al.35 These composites showed a capacitance retention of 66.2% over 2000 cycles. A decrease in the capacitance retention has been attributed to the dissolution of the MnO2 counterpart into the aqueous electrolyte during cycling. They used a simple sonication method to coat PEDOT:PSS on MnO2/rGO, which perhaps is not enough to coat PEDOT:PSS firmly on MnO2/rGO. Therefore, an alternative approach is needed to coat the PEDOT:PSS polymer on the MnO2/rGO nanocomposite in which the PEDOT:PSS will be coated firmly on MnO2/rGO and prevent dissolution. With this motivation, we developed an alternative chemical approach to coat PEDOT:PSS on MnO2. Besides, to forbid the dissolution we replaced the aqueous electrolyte by organic solvents, which have been found to play a positive role in imparting the outstanding cycling stability.
Herein, we prepared MnO2/rGO composites with hydrothermal methods and the formation of nanorods of MnO2 have been noted in the product. These have been coated successfully with PEDOT:PSS by chemical routes that result in a ternary composite ready for supercapacitor applications. These materials have been characterized thoroughly and their supercapacitance performance has been studied by cyclic voltammetry, galvanostatic charge–discharge cycling and electrochemical impedance spectroscopy in acetonitrile solution containing 1 M lithium perchlorate (LiClO4).
Experimental details
Potassium permanganate (KMnO4), sulfuric acid (H2SO4), PEDOT:PSS, polyvinylidene fluoride (PVDF), acetonitrile and LiClO4 were procured from Sigma-Aldrich. All the other chemicals used were analytical grade unless specified. Milli-Q® (MQ) water was used throughout the experiments.Synthesis of nanocomposites
GO was synthesized by a modified Hummers' method and the procedure is explained elsewhere.36 The MnO2/rGO nanocomposite was prepared by a hydrothermal method and the procedure used is as follows. The synthesized GO was dispersed in double distilled water with a concentration of 1 mg ml−1 and sonicated for an hour. 0.3 g of KMnO4 was added to it and it was stirred for another hour. The mixture was transferred into a Teflon lined autoclave. The autoclave was sealed and heated in an oven at 150 °C for 6 h. After cooling, the product was collected by centrifugation, washed with MQ water, dried overnight at 100 °C, and named as MG nanocomposite. For the preparation of the PEDOT:PSS/MnO2/rGO nanocomposite, two steps were employed. The MnO2/rGO nanocomposite was synthesized as explained above. It was then dispersed in 50 ml of MQ water with concentration of 2 mg ml−1 and subsequently heated with vigorous stirring. Once the temperature reached 80 °C, 5 ml of PEDOT:PSS was added and stirred for an hour at the same temperature. After cooling to room temperature, the product was centrifuged, washed with MQ water, dried overnight, and named as MGP nanocomposite. MnO2 nanorods were synthesized by adding 0.3 g of KMnO4 and 0.2 ml H2SO4 in 25 ml of MQ water and keeping the remaining conditions same as above.Characterization of the nanocomposites
The surface morphologies of the prepared nanocomposites along with the MnO2 nanorods and GO have been determined using field emission scanning electron microscopy (FESEM, Model Nova Nanosem 450) and EDAX analysis (using Bruker XFlash 6130). The structural analysis was done using an X-ray diffractometer having a CuKα source (model Bruker AXS D8 Advance). XPS was performed using an Omicron EA 125 analyser at room temperature in an ultrahigh vacuum chamber. Raman measurements were done with a Renishaw Invia laser Raman microscope with a laser excitation wavelength of 532 nm. A Brunauer–Emmett–Teller (BET) analyser (model Quantachrome, Model Autosorb iQ2) was used to study the surface area, pore size and pore diameter by nitrogen gas absorption–desorption.Electrochemical analysis of the nanocomposites
The electrochemical analysis of the nanocomposites was performed using a BioLogic potentiostat (model: SP 300) workstation with a three-electrode system in acetonitrile solution containing 1 M LiClO4 with platinum wire as a counter electrode and silver wire as a quasi-reference electrode. The working electrodes were prepared by mixing the nanocomposites, carbon block and PVDF (1% wt) in NMP with a mass ratio of 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. This mixture was loaded on a carbon flag having a geometrical area of 1 cm2 and the active mass of the electrode was ∼7% wt (0.3 mg). Cyclic voltammetry (CV) in the range 0–1.1 V was performed at varied scan rates. The galvanostatic charge/discharge (CD) tests for all the nanocomposites were carried out in the potential range of 0–1.1 V at varied current densities. The electrochemical impedance spectra (EIS) were recorded in a frequency range from 6 MHz to 200 mHz.
10. This mixture was loaded on a carbon flag having a geometrical area of 1 cm2 and the active mass of the electrode was ∼7% wt (0.3 mg). Cyclic voltammetry (CV) in the range 0–1.1 V was performed at varied scan rates. The galvanostatic charge/discharge (CD) tests for all the nanocomposites were carried out in the potential range of 0–1.1 V at varied current densities. The electrochemical impedance spectra (EIS) were recorded in a frequency range from 6 MHz to 200 mHz.
Results and discussion
The synthesis of the MGP nanocomposite includes two steps; the first one is the preparation of the MG nanocomposite by a hydrothermal method and the next is coating PEDOT:PSS polymer over that to obtain the MGP nanocomposite.The KMnO4 used in the reaction is dissociated and forms nanocrystalline MnO2 as follows,16
| 4MnO4− + 2H2O → 4MnO2 + 4OH− + 3O2 | (1) |
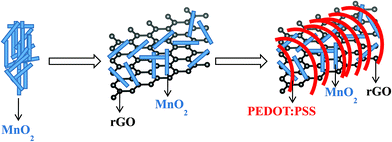
During the hydrothermal reaction, these formed MnO2 nanocrystallites may serve as nucleation sites. The newly formed MnO2 nanocrystallites could deposit on pre-formed MnO2 nanocrystallites and form MnO2 nanorods. Simultaneously, GO will also be reduced into rGO by the removal of oxygen functional groups.37 The flower like morphology of the MG nanocomposite may be due to the preferred growth of the MnO2 nanorods on rGO. The formed MG nanocomposite was mixed with the PEDOT:PSS conducting polymer and heated at 80 °C. During heating, the conducting polymer coated the MG nanocomposite and acts as a supporting layer.22 The schematic representation of the synthesis of the MGP nanocomposite is shown in Scheme 1.
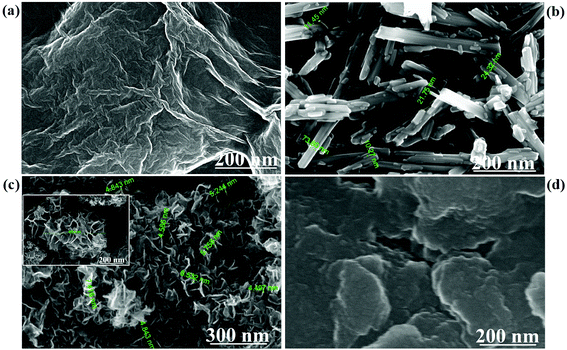
Fig. 1 depicts FESEM images of GO, MnO2 nanorods, and MG and MGP nanocomposites. Fig. 1(a) depicts the homogeneous composition of GO and Fig. 1(b) depicts MnO2 nanorods having a diameter in the range 10–75 nm. Fig. 1(c) shows the decoration of MnO2 nanorods on rGO sheets and they are seen in the form of flower like structures having a diameter of ∼825 nm (shown in the inset of Fig. 1(c)). However, these structures are not legible in the MGP nanocomposite (Fig. 1(d)) as they are coated by the PEDOT:PSS polymer. Fig. 2 shows an elemental mapping of the MnO2 nanorods, and the MG and MGP nanocomposites. It can be seen from Fig. 2 that the elements Mn, O, C and S are uniformly distributed, confirming the presence of MnO2 and PEDOT:PSS polymer in the nanocomposites. The presence of Mn, C, O and S in the nanocomposites is also confirmed by EDS of the MnO2 nanorods, and the MG and MGP nanocomposites, shown in Fig. S1 (ESI†).
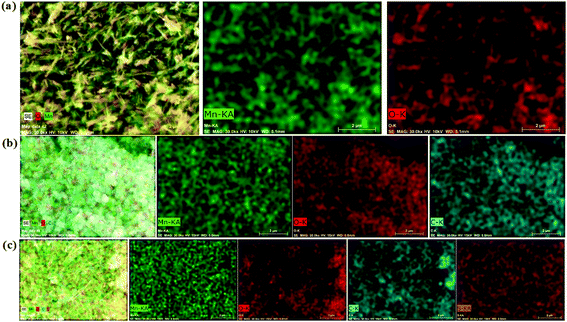 | ||
| Fig. 2 Elemental mapping of (a) MnO2, (b) MG nanocomposite and (c) MGP nanocomposite for Mn, O, C and S. | ||
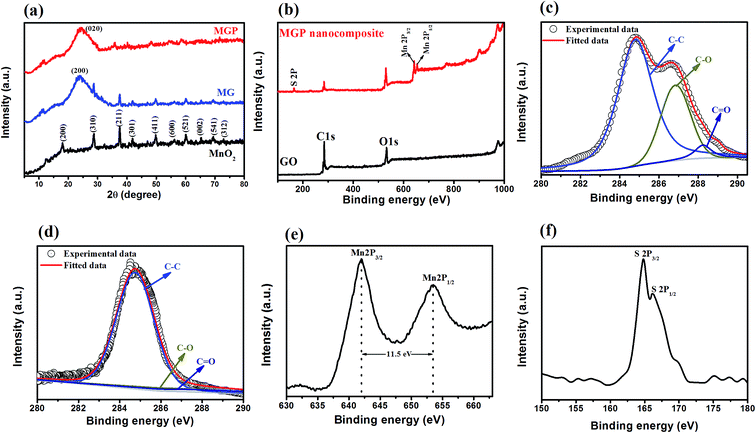
Fig. 3(a) shows the XRD of MnO2 nanorods, and the MG and MGP nanocomposites. The XRD for GO is shown in Fig. S2 (ESI†). MnO2 nanorods showed peaks at 18.12°, 28.75°, 37.51°, 41.89°, 49.9°, 56.18°, 59.99°, 65.33°, and 69.33° corresponding to the Miller indices (200), (310), (211), (301), (411), (600), (521), (002), (541), respectively, indicating the formation of a tetragonal phase of α-MnO2 nanorods10 and is in agreement with JCPDF file no 44-0141. The respective peaks are also observed in the MG and MGP nanocomposites. In addition to this, a broad peak around 24.51° is observed in the MG nanocomposites, representing rGO and corresponding to (200).28 In the case of MGP nanocomposites, a broad peak was observed around 26.1°, corresponding to the (020) plane of the backbone of the PEDOT:PSS polymer.34 It can also be observed that the peak intensity of the MnO2 nanorods decreased in MGP nanocomposites implying the wrapping of MnO2/rGO nanocomposite by PEDOT:PSS polymer.38
XPS analysis was carried out to study the chemical analysis of the nanocomposites. The survey scan of the GO and MGP nanocomposites is shown in Fig. 3(b), which confirmed the presence of C 1s, O 1s, Mn 2p and S 2p. The high resolution XPS of C 1s of GO is shown in Fig. 3(c). It is deconvoluted into three peaks at 284.8 eV, 286.8 eV and 288.4 eV corresponding to C–C, C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, respectively.38 The intensity of these oxygen functional groups is decreased in the MGP nanocomposites as can be seen from Fig. 3(d), confirming the reduction of GO.37 Fig. 3(e) shows the high resolution XPS of Mn 2p. It shows two peaks at 642.1 eV and 653.4 eV, corresponding to Mn 2p3/2 and Mn 2p1/2, which is in good agreement with energy splitting spectrum of standard MnO2.29 The peak to peak separation between Mn 2p3/2 and Mn 2p1/2 is 11.5 eV and is in good agreement with already reported values.29 The S 2p high resolution XPS spectrum is shown in Fig. 3(f). It shows two peaks at 164.94 eV and 166.14 eV, corresponding to S 2p3/2 and S 2p1/2 with peak to peak separation between them as 1.2 eV and is in good agreement with the values reported in the literature.39
O, respectively.38 The intensity of these oxygen functional groups is decreased in the MGP nanocomposites as can be seen from Fig. 3(d), confirming the reduction of GO.37 Fig. 3(e) shows the high resolution XPS of Mn 2p. It shows two peaks at 642.1 eV and 653.4 eV, corresponding to Mn 2p3/2 and Mn 2p1/2, which is in good agreement with energy splitting spectrum of standard MnO2.29 The peak to peak separation between Mn 2p3/2 and Mn 2p1/2 is 11.5 eV and is in good agreement with already reported values.29 The S 2p high resolution XPS spectrum is shown in Fig. 3(f). It shows two peaks at 164.94 eV and 166.14 eV, corresponding to S 2p3/2 and S 2p1/2 with peak to peak separation between them as 1.2 eV and is in good agreement with the values reported in the literature.39
Fig. 4(a) shows Raman spectra of GO, and the MG and MGP nanocomposites. It can be seen that all the spectra show characteristics D and G bands at 1350 and 1590 cm−1, corresponding to vacancies, edge defects, grain boundaries, and disordered carbon species in graphite layers40 and an in-plane bond stretching motion of C sp2 atoms, respectively.41 The intensity ratio of the D to G band, i.e. ID/IG, is a measure of the sp2 domain size of the carbon structure and partially ordered crystal structure of graphene.40 The ID/IG is found to be 0.84 for GO, while it is found to be 1.02 and 1.16 for MG and the MGP nanocomposites, respectively. The increase in ID/IG ratio of the nanocomposites compared to GO indicates an increase in disorderness/defects in rGO.40 As explained by Rusi and Majid, the increment in the D band intensity is due to the combined bands of D1, D2, D3 and D4 in the region 1300–1700 cm−1. The deconvoluted Raman spectra for GO and the MGP nanocomposite are shown in Fig. 4(b) and (c). The deconvoluted bands D1, D2, D3 and D4 correspond to disordered graphitic lattice (graphene layer edges, A1g-symmetry), disordered graphitic lattice (surface graphene layers, E2g-symmetry), amorphous carbon (Gaussian or Lorentzian line shape) and disordered graphitic lattice (A1g-symmetry, polyenes, ionic impurities etc.), respectively. The positions of the D1, D2, D3, D4 bands and IDx/IG ratios of GO and the MGP nanocomposite are listed in Table 1. The increment in D band intensity is mainly due to the overlapping of D1 and D4 bands in the region 1200–1400 cm−1, indicating an increase in disordered carbon in the graphitic lattice.40 For the MG and MGP nanocomposites, a broad peak around 2900 cm−1 appears, corresponding to 2D graphene, confirming the reduction of GO.41 The inset of Fig. 4(a) shows the Raman spectrum of MnO2 of the MGP nanocomposite, showing peaks around 190–380 cm−1 and 575–650 cm−1, corresponding to α-MnO2 (ref. 42) and stretching vibrations of the octahedral MnO6.40 Fig. S3† shows the TGA of GO, MnO2, and the MG and MGP nanocomposites. GO showed an initial weight loss before 200 °C, which is due to the removal of adsorbed water and oxygen functional groups. After 200 °C, GO showed less weight loss to 400 °C and then a sudden weight loss. MnO2 showed good thermal stability, in good agreement with the literature.29 The percentages of MnO2 and rGO in the MG nanocomposite are found to be 13% and 70%, respectively. In the case of the MGP nanocomposite, the percentages of MnO2, rGO and PEDOT:PSS are found to be 13%, 54% and 17%, respectively.
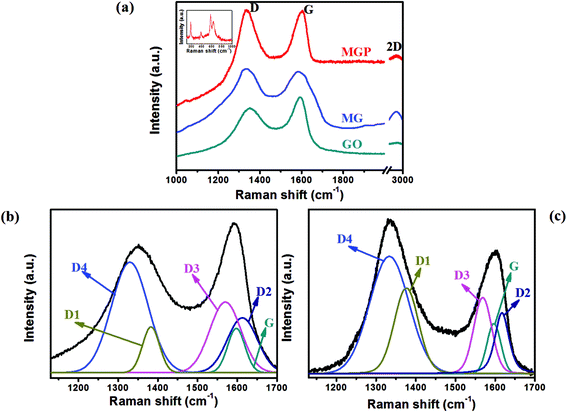 | ||
| Fig. 4 (a) Raman spectrum of GO, MG nanocomposite and MGP nanocomposite; deconvoluted Raman spectrum of (b) GO and (c) MGP. | ||
| Bands | Raman shift (cm−1) | Ratio (IDx/IG) | ||
|---|---|---|---|---|
| MGP | GO | MGP | GO | |
| D1 | 1380 | 1379 | 1.74 | 1.04 |
| D2 | 1618 | 1612 | 1.23 | 1.23 |
| D3 | 1568 | 1569 | 1.56 | 1.51 |
| D4 | 1329 | 1333 | 2.41 | 2.39 |
| G | 1596 | 1598 | — | — |
Fig. 5 shows Nitrogen adsorption–desorption isotherm curves for MnO2, and the MG and MGP nanocomposites. It can be seen from the figure that the hysteresis loop observed in the relative pressure ranges of 0.76–0.94, 0.8–0.9 and above 0.9 for the MGP and MG nanocomposites, and MnO2, respectively. The width of hysteresis is more for the MGP nanocomposite, indicating that it is more mesoporous than the other samples.43 The pore size distribution for the MGP nanocomposite is shown in Fig. 5(d), which shows that the mesopore size mainly falls in the 5–30 nm range. The surface area and pore volume for MnO2, and the MG and MGP nanocomposites are listed in Table 2. From that, the MGP nanocomposite exhibited more surface area, 208 m2 g−1, and those of the MG nanocomposite and MnO2 are 101 m2 g−1 and 58 m2 g−1, respectively. Also, the MGP nanocomposite showed more pore volume than the MG nanocomposite and MnO2 nanorods. The high surface area and high mesoporous nature of the MGP nanocomposite may result in more ionic transportation through the material, leading to enhancement in the supercapacitance performance.
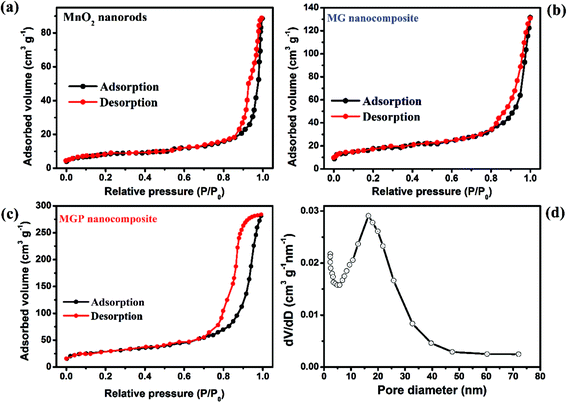 | ||
| Fig. 5 Nitrogen adsorption–desorption curves for (a) MnO2, (b) MG nanocomposite, (c) MGP nanocomposite and (d) pores size distribution of the MGP nanocomposite. | ||
| Electrode materials | Surface area (m2 g−1) | Mesoporous volume (cm3 g−1) | Pore size distribution (nm) |
|---|---|---|---|
| MnO2 nanorods | 58 | 0.308 | 3.17 |
| MG | 101 | 0.391 | 3.42 |
| MGP | 208 | 0.861 | 7.54 |
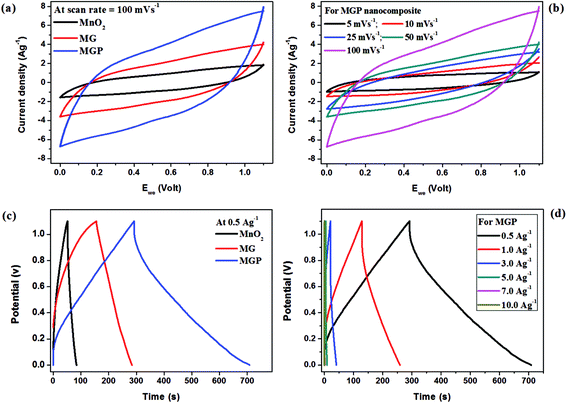
Fig. 6(a) shows typical cyclic voltammograms of MnO2, and the MG and MGP nanocomposites in the potential window of 0 to 1.1 V at a scan rate of 100 mV s−1 carried out in acetonitrile solution containing 1 M LiClO4 electrolyte. All the samples showed asymmetrical CV curves that can be attributed to the combined double-layer and pseudocapacitive contributions to the total capacitance. It is observed that the MGP nanocomposites exhibited a large area under the CV curve, indicating superior supercapacitance behaviour among all. The plausible electrochemical reaction steps governed by the MnO2 are as follows.
| (MnO2)surface + Li+ + e− → (MnO−2Li+)surface | (2) |
| MnO−2 + Li+ + e− → MnOOLi | (3) |
The above equations indicate that the diffusion of Li+ into the interior of MnO2 and charge transfer has a significant effect on the rate capability. The relation between the peak current and the CV scan rate is given by the following equation.44
| ip = 2.69 × 105n3/2AD1/2Cν1/2 | (4) |
Fig. 6(c) shows the galvanostatic charge–discharge for MnO2, and the MG and MGP nanocomposites at a current density of 0.5 A g−1. It is observed from the figure that the all the samples showed nearly triangular shape charge–discharge curves, indicating the electric double layer capacitance behaviour. Also, the ternary nanocomposite showed an ideal capacitive behaviour with very sharp responses and a small internal resistance (IR) drop. The specific capacitance (Cs) of all the samples was calculated using the following equation,46,47
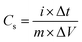 | (5) |
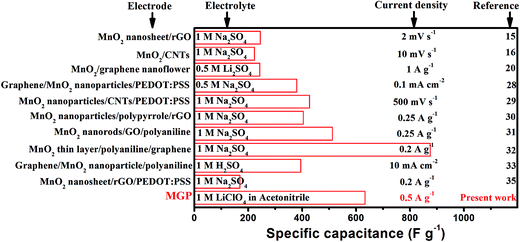 | ||
| Chart 1 Comparison of the specific capacitance of the present work (MGP nanocomposite) with reported electrode materials. | ||
Furthermore, capacitance retention is an important parameter for the practical application of electrode materials. Therefore, the cycling stability of the MGP nanocomposite has been carried out at 1 A g−1 for 5000 cycles. The capacitance retention (%) for the MGP nanocomposite over 5000 cycles is shown in Fig. 7(a). Interestingly, it can be seen that the MGP nanocomposite retained the same specific capacitance even after 5000 cycles, i.e. it showed 100% capacitance retention (%), which is excellent in its class. This excellent capacitance retention may be due to the addition of the PEDOT:PSS polymer, which provides support to the MnO2 nanorods and also restricts the dissolution and aggregation of MnO2 with the help of rGO, which possibly keeps the MnO2/rGO part well separated and provides more interfaces for charge storage.
 | ||
| Fig. 7 (a) Capacitance retention (%) and coulombic efficiency of the MGP nanocomposite over 5000 cycles at a current density of 1 A g−1; (b) Ragone plot for the MGP nanocomposite. | ||
Coulombic efficiency (η) was calculated using eqn (6) and is plotted in Fig. 7(a). It can be observed from the figure that the coulombic efficiency showed an almost constant value over 300 cycles without any fluctuations, suggesting little energy dissipation during the charge–discharge process. The energy density (dE) and power density (dP) of MnO2, and the MG and MGP nanocomposites were calculated using eqn (7) and (8)48,49
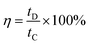 | (6) |
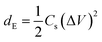 | (7) |
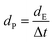 | (8) |
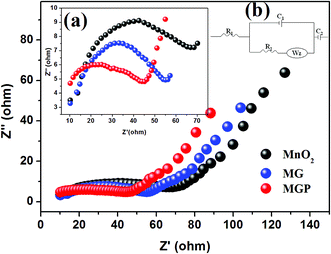
Electrochemical impedance spectroscopy (EIS) was used to study the charge transfer and transport mechanism for MnO2, and the MG and MGP nanocomposites. Fig. 8 shows Nyquist plots for MnO2, and the MG and MGP nanocomposites carried out in acetonitrile solution containing 1 M LiClO4 electrolyte in the frequency range 6 MHz to 200 mHz. It can be seen from the figure that all the curves showed a semicircle at higher frequencies and nearly a straight line at lower frequencies. Among all, the MGP nanocomposite showed the lowest semicircle, suggesting the lowest resistance, which in turn indicates the highest capacitance, in agreement with the galvanostatic charge–discharge analysis. In addition to this, the EIS spectra were analysed using a semi quantitative fitting program supplied along with the BioLogic potentiostat (model: SP 300) on the basis of the Randles equivalent circuit modelling, shown in the inset (Fig. 8(b)) of Fig. 8. The Randles equivalent circuit consists of five elements, internal resistance (R1), electrical double layer capacitance at the interface of electrode and electrolyte (C1), charge transfer resistance (R2), Warburg impedance (WZ) and pseudocapacitance (C2), which is used to account for the faradic reaction. These values were calculated qualitatively from the fittings (mean error of modulus ∼ 0.4%) of experimental EIS spectra and are tabulated in Table 3. It can be observed that the internal resistance (R1), i.e. intrinsic resistance of the MGP nanocomposite, is less compared to MnO2 and the MG nanocomposite. This is due to the development of an internal mesoporous nanorod structure during the MGP nanocomposite synthesis. The lower value of the charge transfer resistance (R2) for the MGP nanocomposite compared to MnO2 and the MG nanocomposite indicates a significant pore size distribution in the MGP nanocomposite. This helps to reduce the intrinsic resistance (R1) via the development of a large number of mesoporous structures. This results in an increase in the kinetics of electron transfer through the redox process, leading to an increase in pseudocapacitance (C2) in the nanocomposite. Moreover, the influence of pseudocapacitance is quite larger than the electrical double layer capacitance (C1), as the conducting polymer PEDOT:PSS itself behaves as a redox system along with MnO2. The lower value of Warburg impedance is attributed to the diffusion of electrolyte in the mesoporous structure of the electrode material at a lower frequency.
| Electrode material | R1 (Ω) | R2 (Ω) | C1 (mF) | C2 (F) | WZ (Ω s−0.5) |
|---|---|---|---|---|---|
| MnO2 nanorods | 1.75 | 0.56 | 2.31 | 0.076 | 0.65 |
| MG | 1.29 | 0.25 | 5.72 | 0.105 | 0.82 |
| MGP | 0.97 | 0.16 | 8.19 | 0.389 | 0.98 |
Conclusions
The synthesized MGP nanocomposite demonstrated an enhanced supercapacitance performance compared to MnO2 and the MG nanocomposite. The specific capacitance of the MGP nanocomposite was found to be 633 F g−1 at a current density of 0.5 A g−1 with 100% capacitance retention even after 5000 cycles. The enhanced specific capacitance of the MGP nanocomposite is due to the synergetic effect of the individual components and also the addition of the PEDOT:PSS conducting polymer is an important factor which may provide numerous active sites for faradic redox reactions as well as support the MnO2 nanorods. Overall, we can conclude that the MGP nanocomposite is an excellent material for supercapacitance application and shows an ultra-high stability even after long term cyclic stability.Acknowledgements
One of the authors, KH, acknowledges a Research Fellowship from the Endeavour Australia-India Education Council.References
- T. Guo, M. S. Yao, Y. H. Lin and C. W. Nan, CrystEngComm, 2015, 17, 3551–3585 RSC.
- C. Yuan, H. B. Wu, Y. Xie and X. W. Lou, Angew. Chem., Int. Ed., 2014, 53, 1488–1504 CrossRef CAS PubMed.
- A. Gonzalez, E. Goikolea, J. A. Barrena and R. Mysyk, Renewable Sustainable Energy Rev., 2016, 58, 1189–1206 CrossRef CAS.
- M. A. Sakka, H. Gualous, J. V. Mierlo and H. Culcu, J. Power Sources, 2009, 194, 581–587 CrossRef.
- W. K. Chee, H. N. Lim, Z. Zainal, N. M. Huang, I. Harrison and Y. Andou, J. Phys. Chem. C, 2016, 120, 4153–4172 CAS.
- X. Liu, C. Chen, Y. Zhao and B. Jia, J. Nanomater., 2013, 2013, 736375 Search PubMed.
- H. Wang, Z. Lu, D. Qian, Y. Li and W. Zhang, Nanotechnology, 2007, 18, 115616 CrossRef.
- D. Belanger, T. Brousse and J. W. Long, Electrochem. Soc. Interface, 2008, 17, 49–52 CAS.
- G. Yu, L. Hu, M. Vosgueritchian, H. Wang, X. Xie, J. R. McDonough, X. Cui, Y. Cui and Z. Bao, Nano Lett., 2011, 11, 2905–2911 CrossRef CAS PubMed.
- Y. Yu, B. Zhang, Y. B. He, Z. D. Huang, S. W. Oh and J. K. Kim, J. Mater. Chem. A, 2013, 1, 1163–1170 CAS.
- D. Chen, H. Feng and J. Li, Chem. Rev., 2012, 112, 6027–6053 CrossRef CAS PubMed.
- Y. B. Tan and J. M. Lee, J. Mater. Chem. A, 2013, 1, 14814–14843 CAS.
- S. Thakur and N. Karak, Carbon, 2015, 94, 224–242 CrossRef CAS.
- D. Chen, H. Feng and J. Li, Chem. Rev., 2012, 112, 6027–6053 CrossRef CAS PubMed.
- Y. Zhao, Y. Meng, H. Wu, Y. Wang, Z. Wei, X. Li and P. Jiang, RSC Adv., 2015, 5, 90307–90312 RSC.
- H. Xia, Y. Wang, J. Lin and L. Lu, Nanoscale Res. Lett., 2012, 7, 33 CrossRef PubMed.
- P. Ning, X. Duan, X. Ju, X. Lin, X. Tong, X. Pan, T. Wang and Q. Li, Electrochim. Acta, 2016, 210, 754–761 CrossRef CAS.
- R. T. Vinny, K. Chaitra, K. Venkatesh, N. Nagaraju and N. Kathyayini, J. Power Sources, 2016, 309, 212–220 CrossRef CAS.
- W. Ma, S. Chen, S. Yang, W. Chen, Y. Cheng, Y. Guo, S. Peng, S. Ramakrishna and M. Zhu, J. Power Sources, 2016, 306, 481–488 CrossRef CAS.
- J. Liu, Y. Zhang, Y. Li, J. Li, Z. Chen, H. Feng, J. Li, J. Jiang and D. Qian, Electrochim. Acta, 2015, 173, 148–155 CrossRef CAS.
- N. Zhang, C. Fu, D. Liu, Y. Li, H. Zhou and Y. Kuang, Electrochim. Acta, 2016, 210, 804–811 CrossRef CAS.
- G. A. Snook, P. Kao and A. S. Best, J. Power Sources, 2011, 196, 1–12 CrossRef CAS.
- V. Singh, S. Arora, M. Arora, V. Sharma and R. P. Tandon, Semicond. Sci. Technol., 2014, 29, 045020 CrossRef.
- C. Yin, C. Yang, M. Jiang, C. Deng, L. Yang, J. Li and D. Qian, Appl. Mater. Interfaces, 2016, 8, 2741–2752 CrossRef CAS PubMed.
- T. Cheng, Y. Z. Zhang, J. D. Zhang, W. Y. Lai and W. Huang, J. Mater. Chem. A, 2016, 4, 10493–10499 CAS.
- C. Yeon, G. Kim, J. W. Lim and S. J. Yun, RSC Adv., 2017, 7, 5888–5897 RSC.
- D. Antiohos, G. Folkes, P. Sherrell, S. Ashraf, G. G. Wallace, P. Aitchison, A. T. Harris, J. Chen and A. I. Minett, J. Mater. Chem., 2011, 21, 15987–15994 RSC.
- G. Yu, L. Hu, N. Liu, H. Wang, M. Vosgueritchian, Y. Yang, Y. Cui and Z. Bao, Nano Lett., 2011, 11, 4438–4442 CrossRef CAS PubMed.
- Y. Hou, Y. Cheng, T. Hobson and J. Liu, Nano Lett., 2010, 10, 2727–2733 CrossRef CAS PubMed.
- G. Han, Y. Liu, E. Kan, J. Tang, L. Zhang, H. Wang and W. Tang, RSC Adv., 2014, 4, 9898–9904 RSC.
- G. Han, Y. Liu, L. Zhang, E. Kan, S. Zhang, J. Tang and W. Tang, Sci. Rep., 2014, 4, 4824 Search PubMed.
- C. Pan, H. Gu and L. Dong, J. Power Sources, 2016, 303, 175–181 CrossRef CAS.
- B. Mu, W. Zhang, S. Shao and A. Wang, Phys. Chem. Chem. Phys., 2014, 16, 7872–7880 RSC.
- H. Li, Y. He, V. Pavlinek, Q. Cheng, P. Sahab and C. Li, J. Mater. Chem. A, 2015, 3, 17165–17171 CAS.
- D. Yan, Y. Liu, Y. Li, R. Zhuo, Z. Wu, P. Ren, S. Li, J. Wang, P. Yan and Z. Geng, Mater. Lett., 2014, 127, 53–55 CrossRef CAS.
- K. Hareesh, B. Shateesh, R. P. Joshi, S. S. Dahiwale, V. N. Bhoraskar, S. K. Haram and S. D. Dhole, Electrochim. Acta, 2016, 201, 106–116 CrossRef CAS.
- Y. Zhou, Q. Bao, L. A. L. Tang, Y. Zhong and K. P. Loh, Chem. Mater., 2009, 21, 2950–2956 CrossRef CAS.
- W. Wang, Q. Hao, W. Lei, X. Xia and X. Wang, J. Power Sources, 2014, 269, 250–259 CrossRef CAS.
- L. Zhang, L. Ji, P. A. Glans, Y. Zhang, J. Zhu and J. Guo, Phys. Chem. Chem. Phys., 2012, 14, 13670–13675 RSC.
- Rusi and S. R. Majid, Sci. Rep., 2015, 5, 16195 CrossRef CAS PubMed.
- L. M. Malard, M. A. Pimenta, G. Dresselhaus and M. S. Dresslhaus, Phys. Rep., 2009, 473, 51–87 CrossRef CAS.
- Y. Wang, H. Guan, S. Du and Y. Wang, RSC Adv., 2015, 5, 8979–88988 Search PubMed.
- E. Raymundo-Pinero, V. Khomenk, E. Frackowiak and F. Beguin, J. Electrochem. Soc., 2005, 152, A229–A235 CrossRef CAS.
- N. Ding, J. Xu, Y. X. Yao, G. Wegner, X. Fang, C. H. Chen and I. Lieberwirth, Solid State Ionics, 2009, 180, 222–225 CrossRef CAS.
- L. L. Zhang, S. Duan, X. L. Yang, G. Peng, G. Liang, Y. H. Huang, Y. Jiang, S. B. Ni and M. Li, Appl. Mater. Interfaces, 2015, 3, 12304–12309 Search PubMed.
- H. Fan, L. Quan, M. Yuan, S. Zhu, K. Wang, Y. Zhong, L. Chang, H. Shao, J. Wang, J. Zhang and C.-N. Cao, Electrochim. Acta, 2016, 188, 222–229 CrossRef CAS.
- X. Li, C. Zhang, S. Xin, Z. C. Yang, Y. Li, D. Zhang and P. Yao, Appl. Mater. Interfaces, 2016, 8, 21373–21380 CrossRef CAS PubMed.
- M. M. Islam, A. T. Chidembo, S. H. Aboutalebi, D. Cardillo, H. K. Liu, K. Konstantinov and S. X. Dou, Frontiers in Energy Research, 2014, 2, 31 CrossRef.
- P. Sen and A. De, Electrochim. Acta, 2010, 55, 4677–4684 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: The EDS analysis for the MnO2 nanorods, and the MG and MGP nanocomposites. XRD of graphene oxide. TGA of graphene oxide, MnO2, and the MG and MGP nanocomposites. See DOI: 10.1039/c7ra01743j |
| This journal is © The Royal Society of Chemistry 2017 |