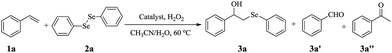
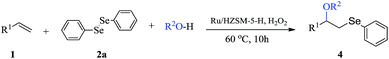Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Acidic hierarchical zeolite ZSM-5 supported Ru catalyst with high activity and selectivity in the seleno-functionalization of alkenes†
Hai Dong,
Lei Zhang,
Zhongxue Fang,
Wenqian Fu*,
Ting Tang,
Yu Feng and
Tiandi Tang *
*
Advanced Catalysis and Green Manufacturing Collaborative Innovation Center, School of Petrochemical Engineering, Changzhou University, Changzhou, Jiangsu 213164, P. R. China. E-mail: fuwenqian@cczu.edu.com; tangtiandi@cczu.edu.cn; tangtiandi@wzu.edu.cn; Tel: +86-519-86330253
First published on 19th April 2017
Abstract
The acidic hierarchical zeolite ZSM-5 (HZSM-5-H) was synthesized for the preparation of a supported Ru catalyst (Ru/HZSM-5-H). The obtained Ru/HZSM-5-H catalyst shows high activity and product selectivity in the seleno-functionalization of alkenes compared to γ-Al2O3, basic ETS-10 and acidic microporous zeolite ZSM-5 supported Ru catalysts (Ru/γ-Al2O3, Ru/ETS-10 and Ru/HZSM-5, respectively), as well as a homogeneous RuCl3 catalyst. The relatively strong acidic sites in Ru/HZSM-5-H could benefit the adsorption of styrenes and the activation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond. Meanwhile, Ru4+ in Ru/HZSM-5-H could facilitate the formation of electrophilic selenium species as compared to Ru0 species. In addition, the Ru/HZSM-5-M catalyst exhibits broad substrate compatibility in the difunctionalization of alkenes.
C bond. Meanwhile, Ru4+ in Ru/HZSM-5-H could facilitate the formation of electrophilic selenium species as compared to Ru0 species. In addition, the Ru/HZSM-5-M catalyst exhibits broad substrate compatibility in the difunctionalization of alkenes.
1. Introduction
The difunctionalization of alkenes is a flexible approach for constructing various functional compounds in modern organic synthesis.1–3 For example, seleno-functionalized compounds are valuable intermediates as they can be used as building blocks in the preparation of various valuable, biologically active and natural products.4–6 The difunctionalization of alkenes can be realized through attacking the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of alkenes with electrophilic selenium species followed by reacting with other nucleophilic reagents.1,7,8 Until now, this type of reaction was almost achieved in homogeneous catalytic systems. Also, to improve the reaction activity, various additives such as expensive metal organic salts,1,9,10 trimethylsilyl trifluoromethanesulfonate11 and toxic halogens12 were required to generate the electrophilic selenium species from diaryl diselenides. In these cases, the reaction systems were not only limited by the range of substrates, but they also had disadvantages, such as the tedious work-up separation and purification of the products and difficulty in reusing the catalysts,13,14 which severely limits their potential application. From a practical point of view, to realize a simple and clean process for the seleno-functionalization of a broad range of alkenes, developing functional heterogeneous catalysts with high efficiencies is of great significance. To achieve this aim, the following basic but critical aspects should be taken into account. (1) The catalyst should contain two active sites, where one can benefit the activation of the C
C bond of alkenes with electrophilic selenium species followed by reacting with other nucleophilic reagents.1,7,8 Until now, this type of reaction was almost achieved in homogeneous catalytic systems. Also, to improve the reaction activity, various additives such as expensive metal organic salts,1,9,10 trimethylsilyl trifluoromethanesulfonate11 and toxic halogens12 were required to generate the electrophilic selenium species from diaryl diselenides. In these cases, the reaction systems were not only limited by the range of substrates, but they also had disadvantages, such as the tedious work-up separation and purification of the products and difficulty in reusing the catalysts,13,14 which severely limits their potential application. From a practical point of view, to realize a simple and clean process for the seleno-functionalization of a broad range of alkenes, developing functional heterogeneous catalysts with high efficiencies is of great significance. To achieve this aim, the following basic but critical aspects should be taken into account. (1) The catalyst should contain two active sites, where one can benefit the activation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond in alkenes and the other can facilitate the formation of the electrophilic selenium species using eco-friendly oxidizing agents (H2O2 or O2). This would lead to the easier occurrence of the electrophilic selenium species attacking the activated C
C bond in alkenes and the other can facilitate the formation of the electrophilic selenium species using eco-friendly oxidizing agents (H2O2 or O2). This would lead to the easier occurrence of the electrophilic selenium species attacking the activated C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond in alkenes on the catalyst. (2) The catalyst should contain a porous structure that benefits the fast diffusion of the bulk reactants, and presents good chemical and mechanical stability under reaction conditions.
C bond in alkenes on the catalyst. (2) The catalyst should contain a porous structure that benefits the fast diffusion of the bulk reactants, and presents good chemical and mechanical stability under reaction conditions.
It is well known that crystalline porous aluminosilicate zeolites have good chemical and mechanical stability, modifiable surface properties (acid-basicity) and unique framework structures, and are widely used as supports for metal catalysts in the petrochemical and fine chemical industries.15–17 Recent studies have shown that organic substrates can interact with the surface acidic or basic sites of zeolites,18,19 and the electronic properties of the metal species could be modified by the zeolite’s framework,20–22 leading to the catalysts showing high activity and selectivity. Furthermore, the hierarchically porous structure of the zeolite crystals could benefit the mass transfer of the reactive substrates and products,23–25 improving the reaction activity and selectivity. Therefore, zeolites with hierarchically porous structures should be good candidates for the preparation of functional metal catalysts with high activity and good selectivity.
In this work, we prepared an acidic hierarchically porous zeolite ZSM-5 (HZSM-5-H) for the preparation of a supported Ru catalyst (Ru/HZSM-5-H), and applied it in alkene di-functionalization to synthesize seleno- and sulfur-containing compounds. As a comparison, γ-Al2O3, H-form microporous zeolite ZSM-5 (HZSM-5) and basic ETS-10 supported Ru catalysts (Ru/γ-Al2O3, Ru/HZSM-5, and Ru/ETS-10, respectively) were also prepared. The catalytic results show that the Ru/HZSM-5-H catalyst has higher reaction activity and product selectivity compared to that of the Ru/γ-Al2O3, Ru/HZSM-5, Ru/ETS-10 and RuCl3 catalysts. These features could be attributed to the fact that the relatively strong acidic sites in Ru/HZSM-5-H benefit the adsorption of styrene and activate its C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond, improving the reaction activity. In addition, the Ru4+ species in the form of an oxide on the Ru/HZSM-5-H catalyst could favor the transformation of diaryl diselenides to electrophilic selenium species that can attack the activated C
C bond, improving the reaction activity. In addition, the Ru4+ species in the form of an oxide on the Ru/HZSM-5-H catalyst could favor the transformation of diaryl diselenides to electrophilic selenium species that can attack the activated C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond in styrene to form the desired product.
C bond in styrene to form the desired product.
2. Experimental
2.1. Material synthesis
The hierarchical zeolite ZSM-5 (ZSM-5-H) was synthesized in a gel with a composition of Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 89 SiO2
89 SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 Na2O
24 Na2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 TPAOH
3 TPAOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.07 COPQA
0.07 COPQA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3200 H2O in a 100 L stainless steel autoclave. TPAOH is tetrapropylammonium hydroxide and was used as a microporous template agent. COPQA is a mesoscale template, and is a cationic copolymer containing quaternary ammonium groups that are synthesized from diallylamine and dimethyl diallylammonium chloride.26 In a typical run, 8.85 L of water glass, 1.4 L of TPAOH (25.0 wt%) and 5.6 L of H2O were sequentially added to the autoclave and stirred for 1 h, then 4.3 L of COPQA was slowly added, and the mixture was further stirred for 2 h. Then, 19 L of acidic aluminum sulfate solution (0.03 mol L−1) was slowly added and the obtained mixture was stirred for 2 h. After that, the obtained aluminosilicate gel underwent dynamic crystallization in the autoclave at 170 °C for 44 h. The solid product was collected through filtration, washing, and drying, followed by calcination in air at 550 °C for 5 h to remove the template agent. The microporous zeolite ZSM-5 was synthesized by the same procedure except for the addition of the mesoscale template COPQA. Titanosilicate zeolite ETS-10, which consists of corner-sharing tetrahedral (SiO4) and octahedral (TiO6)2− links through bridging oxygen atoms, was prepared according to the previously reported literature.27 γ-Al2O3 was purchased from Shanghai HENGYE Chemical Industry Co., Ltd. The zeolite was ion-exchanged with 1 M NH4NO3 solution at 80 °C for 1 h, followed by filtration, drying and calcination at 500 °C for 4 h. This procedure was repeated twice to obtain H-form zeolites (HZSM-5-H and HZSM-5).
3200 H2O in a 100 L stainless steel autoclave. TPAOH is tetrapropylammonium hydroxide and was used as a microporous template agent. COPQA is a mesoscale template, and is a cationic copolymer containing quaternary ammonium groups that are synthesized from diallylamine and dimethyl diallylammonium chloride.26 In a typical run, 8.85 L of water glass, 1.4 L of TPAOH (25.0 wt%) and 5.6 L of H2O were sequentially added to the autoclave and stirred for 1 h, then 4.3 L of COPQA was slowly added, and the mixture was further stirred for 2 h. Then, 19 L of acidic aluminum sulfate solution (0.03 mol L−1) was slowly added and the obtained mixture was stirred for 2 h. After that, the obtained aluminosilicate gel underwent dynamic crystallization in the autoclave at 170 °C for 44 h. The solid product was collected through filtration, washing, and drying, followed by calcination in air at 550 °C for 5 h to remove the template agent. The microporous zeolite ZSM-5 was synthesized by the same procedure except for the addition of the mesoscale template COPQA. Titanosilicate zeolite ETS-10, which consists of corner-sharing tetrahedral (SiO4) and octahedral (TiO6)2− links through bridging oxygen atoms, was prepared according to the previously reported literature.27 γ-Al2O3 was purchased from Shanghai HENGYE Chemical Industry Co., Ltd. The zeolite was ion-exchanged with 1 M NH4NO3 solution at 80 °C for 1 h, followed by filtration, drying and calcination at 500 °C for 4 h. This procedure was repeated twice to obtain H-form zeolites (HZSM-5-H and HZSM-5).
2.2. Catalyst preparation
The catalyst was prepared using the incipient wetness method through the impregnation of the supports with an aqueous solution containing an appropriate amount of ruthenium chloride (RuCl3·3H2O). The Ru loading was 3.0 wt%. The impregnated sample was dried at room temperature for 24 h and subsequently dried in an oven at 100 °C for 12 h. After that, the sample was calcined at 450 °C for 4 h. The resulting samples with different supports were denoted as Ru/HZSM-5-H, Ru/HZSM-5, Ru/ETS-10 and Ru/γ-Al2O3.2.3. Characterization
X-Ray powder diffraction (XRD) patterns were recorded on a D/MAX 2500/PC powder diffractometer (Rigaku) using a Cu Kα radiation source operating at 40 kV and 200 mA. The crystallite size of the ruthenium oxide particles was determined using the peak at 2θ = 34.9° and the Scherrer equation, Dc = Kλ/β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos(θ), where K is a constant taken as 0.9, λ is the wavelength of the X-ray radiation, β is the width of the peak at half-maximum, and 2θ is the Bragg angle. Nitrogen physisorption was conducted at −196 °C using Micromeritics ASAP2020M apparatus. The sample was degassed for 8 h at 300 °C before the measurements were taken. The specific surface area was calculated from the adsorption data using the Brunauer–Emmett–Teller (BET) equation.
cos(θ), where K is a constant taken as 0.9, λ is the wavelength of the X-ray radiation, β is the width of the peak at half-maximum, and 2θ is the Bragg angle. Nitrogen physisorption was conducted at −196 °C using Micromeritics ASAP2020M apparatus. The sample was degassed for 8 h at 300 °C before the measurements were taken. The specific surface area was calculated from the adsorption data using the Brunauer–Emmett–Teller (BET) equation.
The acidities of the supports and catalysts were measured using ammonia temperature-programmed desorption (NH3-TPD) on a Micromeritics ASAP2920 instrument. Typically, 200 mg of the sample was placed in a quartz tube and pretreated in a helium stream at 450 °C for 2 h. After the sample was cooled to 120 °C, an NH3–He gas mixture (10 vol% NH3) was flowed over the sample for 30 min. After removing the physically adsorbed NH3 by flowing helium for 2 h at 120 °C, the sample was heated from 120 to 530 °C at a rate of 10 °C min−1. The desorbed NH3 was collected in dilute hydrochloric acid and titrated with a dilute sodium hydroxide solution to determine the acidic site density of the sample. The obtained NH3-TPD curve of the supports was deconvoluted at different maximum peak temperatures with a Gaussian function for fitting, and the peak areas were calculated.28,29 The acidic nature (Brønsted/Lewis) of the supports and catalysts was investigated through pyridine adsorption infrared spectroscopy (Py-IR) on a Bruker TENSOR 27 spectrophotometer equipped with a reactor cell. The experiment procedure was as follows: the sample was pressed into self-supporting wafers and degassed under vacuum (1 × 10−2 Pa) at 100 °C for 1 h, and subsequently exposed to pyridine vapor after being cooled to 30 °C. The Py-IR spectrum was then recorded at 30 °C after the sample was placed under vacuum at 30 °C for 30 min.
Temperature-programmed reduction (TPR) of the catalyst was performed with a Micromeritics ASAP2920 instrument using a H2–Ar gas mixture (10 vol% H2). The calcined sample (40 mg) was heated from room temperature to 800 °C at a heating rate of 10 °C min−1. The ratio of Si/Al(Ti) of the zeolite as well as the Ru content of the sample were determined using inductively coupled plasma optical emission spectroscopy (ICP-OES) with a Perkin-Elmer 3300DV emission spectrometer.
Scanning electron microscopy (SEM) images of the sample were obtained on a field-emission scanning electron microscope (SUPRA55) operating at an acceleration voltage of 5 kV. Transmission electron microscopy (TEM) images were obtained on a JEM-2100 microscope with a limited line resolution capacity of 1.4 Å at 200 kV. Before it was characterized, the sample was cut into thin slices and dropped onto a Cu grid that was coated with carbon membrane.
The infrared (IR) spectrum of the styrene-adsorbed Ru/HZSM-5-H sample was obtained on a Bruker TENSOR 27 infrared spectrophotometer equipped with a reactor cell. Before the measurements were taken, the sample was evacuated to 10−2 Pa at 50 °C for 20 h. The spectrum was obtained in the absorbance mode and was shown after the subtraction of a background spectrum that was obtained on the corresponding HZSM-5-H sample. For comparison, the spectra of styrene and ethylbenzene were recorded at room temperature. The ultraviolet-visible diffuse reflectance spectrum (UV-Vis) was obtained using a Shimadzu UV-3600 spectrometer. X-ray photoelectron spectroscopy (XPS) experiments were performed using an ESCALAB MK II system.
2.4. Activity tests
All of the materials were of analytical grade and were used as received without further purification. The typical experimental procedure for the seleno-functionalization of styrene was as follows: catalyst (25 mg), styrene 1a (1.0 mmol), diaryl diselenides 2a (0.6 mmol), H2O2 (1.5 mmol, 30% aqueous solution), acetonitrile (1 mL) and H2O (1 mL) were placed in a sealed tube (10 mL). The reaction proceeded at 60 °C for the desired time. After the reaction finished, the reaction mixture was separated using centrifugation and extraction to obtain the products in the liquid phase. The liquid products were analyzed using an Agilent 1260 Infinity Liquid Chromatogram. The pure product was obtained with flash column chromatography on silica gel using petroleum ether (60–90 °C) and ethyl acetate as the eluents. The compounds that are described in the literature were characterized by comparing their 1H NMR (500, 400 and 300 MHz) and 13C NMR (125, 100 and 75 MHz) spectra, which were recorded with spectrometers at 20 °C using CDCl3 as the solvent. The chemical shifts were given in parts per million relative to TMS as the internal standard at room temperature.2.5. Adsorption experiments
The typical adsorption experimental procedure was carried out as follows: 0.1 g of the catalyst and 4 mg of styrene were dissolved in acetonitrile (10 mL) in a 25 mL sealed tube with stirring. The adsorption time was 3 h at 60 °C. After cooling to room temperature, the liquid phase was separated from the reaction mixture using centrifugation, and was analyzed with an Agilent 7890B GC. The solid sample was washed with acetonitrile (15 mL) as many times as possible to eliminate the physically adsorbed styrene, and was dried at 50 °C for 24 h and evacuated to 10−2 Pa at 50 °C for 20 h. The resulting solid sample was used for UV-Vis and IR characterization.3. Results and discussion
3.1. Characterization
In Fig. 1a, the XRD patterns of ZSM-5-H and ZSM-5 show typical diffraction peaks in the range 5–50° associated with an MFI structure,26 which is in line with the reference ZSM-5 (PDF#44-0003). The isotherms of ZSM-5-M show the appearance of a hysteresis loop at P/P0 = 0.6–0.96, suggesting the presence of a mesoporous structure in the zeolite crystals (Fig. 1b), and the corresponding mesoporous size is mainly centered at 22 nm (Fig. 1b, inset). The texture parameters for all of the samples are listed in Table S1.† ZSM-5-H has an external surface area of 208 m2 g−1 and a mesoporous volume of 0.47 cm3 g−1, while the bulk ZSM-5 only has a low external surface area (45 m2 g−1, Table S1 and Fig. S1†). The SEM image shows that the large ZSM-5-H particles are formed by the aggregation of small nanocrystals with sizes of 50–250 nm, and meso- and macropores are formed between these aggregated particles (Fig. 2a). Interestingly, the TEM image shows that many intra-crystalline mesopores (light areas) also existed in the ZSM-5-H crystals (Fig. 2b). The abundant mesopores and macropores in ZSM-5-H could benefit the diffusion of the bulky reactant molecule.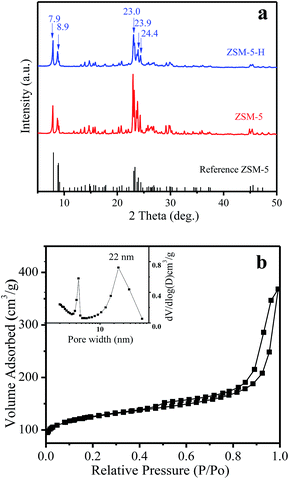 | ||
| Fig. 1 (a) XRD patterns of the reference ZSM-5 (PDF#44-0003), ZSM-5 and ZSM-5-H samples, (b) N2 adsorption isotherms of the ZSM-5-H sample (pore size distribution, inset). | ||
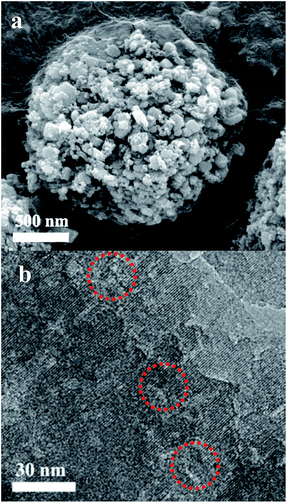 | ||
| Fig. 2 (a) SEM image of the ZSM-5-H sample and (b) a TEM image of a thin slice of the ZSM-5-H zeolite. | ||
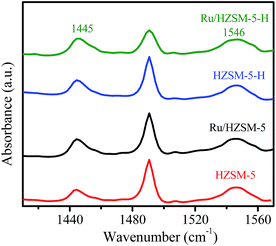
The NH3-TPD curves in Fig. 3a show that HZSM-5-H and HZSM-5 have similar desorption profiles with peaks around 224, 325 and 406 °C, indicating the presence of sites with weak, medium and strong acidity on the HZSM-5-H and HZSM-5 samples, respectively. In contrast, a desorption profile with only a peak at 243 °C and a shoulder peak at 357 °C appears in the NH3-TPD curve of γ-Al2O3, suggesting the presence of sites with weak and medium acidity on γ-Al2O3, respectively. The acid–base titration results in Table S2† show that HZSM-5 (580 μmol g−1) and HZSM-5-H (500 μmol g−1) have higher total acidic site densities than γ-Al2O3 (480 μmol g−1). After the loading of Ru, the total acidic site density on the catalysts is lower than that on the corresponding supports. Nevertheless, the acidic site densities on Ru/HZSM-5 (480 μmol g−1) and Ru/HZSM-5-H (420 μmol g−1) are higher than that on Ru/γ-Al2O3 (340 μmol g−1). It is notable that the NH3-desorption peak appears at 262, 296 and 300 °C for Ru/γ-Al2O3, Ru/HZSM-5-H and Ru/HZSM-5, respectively, which indicates that the acidic strength of Ru/HZSM-5 and Ru/HZSM-5-H is stronger than that of Ru/γ-Al2O3 (Fig. 3b). The Py-IR spectra in Fig. 4 show absorption bands at 1445 and 1546 cm−1, which are attributed to pyridine that is adsorbed on the Lewis acid and Brønsted acid sites of the zeolites and the catalysts,29,30 while there are only Lewis sites on the γ-Al2O3 and Ru/γ-Al2O3 samples (Fig. S2†).
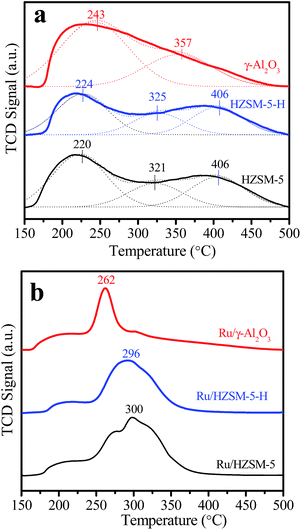 | ||
| Fig. 3 (a) NH3-TPD curves and Gaussian deconvoluted peaks of the different supports and (b) the NH3-TPD curves of the different catalysts. | ||
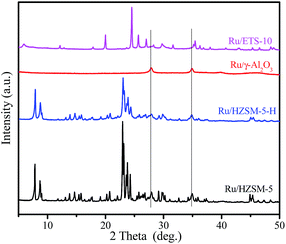
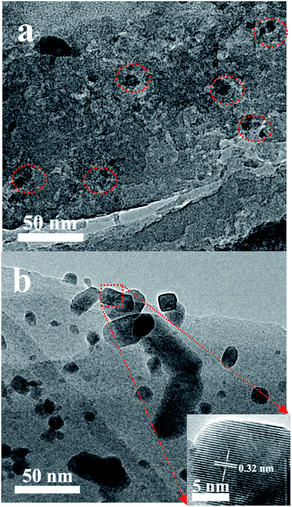
Fig. 5 shows the XRD patterns of the various catalysts. Clearly, two diffraction peaks at 2θ = 27.9 and 34.9° that are associated with ruthenium oxide are present in the XRD patterns of the Ru/HZSM-5, Ru/HZSM-5-H and Ru/γ-Al2O3 catalysts,31 indicating that relatively large ruthenium oxide particles were formed after the catalyst precursor was calcined at 450 °C. The corresponding particle sizes that were calculated using the Scherrer equation are 20.4, 17.2 and 16.5 nm for the Ru/HZSM-5, Ru/HZSM-5-H and Ru/γ-Al2O3 catalysts, respectively (for details please see the ESI, Fig. S3 and S4†). In contrast, the diffraction peaks of ruthenium oxide were not detected in the XRD spectra for the Ru/ETS-10 catalyst (for details please see the ESI, Fig. S5†), indicating that relatively small Ru particles were formed. When the Ru loading was reduced in Ru/HZSM-5-H, the particle size of the ruthenium oxide decreased (Fig. S6†). The TEM images of the supported Ru catalysts show that Ru particles with sizes of 5–25 nm were irregularly located in the mesopores and on the outer surface of Ru/HZSM-5-H (Fig. 6a), and relatively large Ru particles were dispersed on the outer surface of Ru/HZSM-5 (Fig. 6b). The Ru particles having a crystal lattice spacing of 0.32 nm is consistent with the d-spacing of the RuO2 {110} crystallographic plane (Fig. 6b, inset).32,33 In addition, the size distributions of RuO2 on the Ru/HZSM-5-H and Ru/HZSM-5 catalysts were also obtained using statistical analyses from the TEM images (Fig. S7†), and the average RuO2 particle size (Daver.) was 17.9 nm on Ru/HZSM-5-H and 23.3 nm on Ru/HZSM-5.
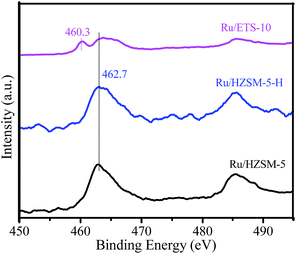
The electronic states of the Ru species on the different catalysts were investigated using XPS, and the results are shown in Fig. 7. The binding energy at 462.7 eV is related to Ru4+ in the form of RuO2 on Ru/HZSM-5-H, Ru/HZSM-5 and Ru/ETS-10.34–36 However, another binding energy at 460.3 eV that is assigned to Ru0 is also present in the XPS spectrum of Ru/ETS-10, indicating some Ru0 species existed on Ru/ETS-10.34–36 This could be due to the fact that the strong Lewis basic sites on ETS-10 (ref. 27) can partially reduce ruthenium oxide to Ru0 species during the catalyst preparation process.
3.2. Catalytic performance
The catalytic performance of the Ru/HZSM-5-H catalyst in alkene difunctionalization was tested firstly by choosing the hydroxyselenation of styrene with diaryl diselenide (Table 1). The initial results show that moderate styrene conversion (51%) and product 3a selectivity (72%) were achieved without the use of a catalyst (entry 1). When using RuCl3 salt as the catalyst, although the styrene conversion did not change, the 3a selectivity increased to 96%, indicating that the Ru3+ species is beneficial for the improvement of reaction selectivity. When acidic HZSM-5-H was employed as the catalyst, the styrene conversion (65%) slightly increased, but the 3a selectivity did not obviously improve (78%, entry 3). It is notable that the styrene conversion (94%) and 3a selectivity (95%) significantly improved when the strongly acidic Ru/HZSM-5-H catalyst was used (entry 4). The reduced Ru/HZSM-5-H catalyst was also tested in this reaction, and the reaction activity (48%) and product selectivity (67%) did not improve (entry 5). These results indicate that, compared with Ru0 species in reduced Ru/HZSM-5-H (Fig. S8†), Ru4+ in the form of RuO2 on the Ru/HZSM-5-H catalyst can significantly improve the reaction activity and product selectivity. In addition, although Ru/HZSM-5 has a similar acidity to Ru/HZSM-5-H, the reaction activity is relatively lower (67%, entry 6), which could be due to the fact that, compared with Ru/HZSM-5 with a low external area (37 m2 g−1), Ru/HZSM-5-H has a large mesoporous surface area (162 m2 g−1) and mesoporous volume (0.4 cm3 g−1, Table S1†), and the abundant mesopores not only benefit the reactants’ easy access to the acidic sites, but also facilitate the mass transfer of the bulk reactant and product, improving its catalytic performance. Compared to strongly acidic Ru/HZSM-5-H, weakly acidic Ru/γ-Al2O3 has a large mesoporous surface area (332 m2 g−1, Table S1†) and presents similar RuO2 particles (Fig. 5), but the styrene conversion on this catalyst is lower (48%, entry 7). Furthermore, the styrene conversion on the strongly basic Ru/ETS-10 catalyst is very low (entry 8), which indicates that a catalyst with basicity could disfavour this transformation. In addition, the effect of Ru loading in the catalyst on the catalytic performance was also investigated (Table S3†). The styrene conversion increased with the Ru loading in the Ru/HZSM-5-H catalyst.| Entry | Catalyst | Conv.b (%) | Selectivityc (%) | Ad. capacityf (mg gcat.−1) | ||
|---|---|---|---|---|---|---|
| 3a | 3a′ | 3a′′ | ||||
| a Reaction conditions: 25 mg of solid catalyst, styrene (1.0 mmol), diaryl diselenides (0.6 mmol), H2O2 (1.5 mmol), H2O (1.0 mL), CH3CN (1.0 mL), and 7 h. The carbon in the reaction mixture is balanced.b The conversion was analyzed using LC.c The product selectivity was analyzed using LC.d The Ru content was equal to the Ru content in the 25 mg of solid catalyst.e Ru/HZSM-5-H was reduced at 150 °C for 2 h in a H2 stream (the temperature programmed reduction profile is shown in Fig. S7).f The adsorption capacity of styrene (1a) on the catalysts. | ||||||
| 1 | — | 51 | 72 | 25 | 3 | — |
| 2 | RuCl3d | 50 | 96 | 2 | 2 | — |
| 3 | HZSM-5-H | 65 | 78 | 20 | 2 | — |
| 4 | Ru/HZSM-5-H | 94 | 95 | 2 | 3 | 20 |
| 5 | Ru/HZSM-5-He | 48 | 67 | 11 | 22 | — |
| 6 | Ru/HZSM-5 | 67 | 80 | 10 | 10 | — |
| 7 | Ru/γ-Al2O3 | 48 | 52 | 22 | 20 | 9.5 |
| 8 | Ru/ETS-10 | 15 | 78 | 18 | 4 | 1.2 |
The above results imply that the strongly acidic sites and the Ru4+ species on the Ru/HZSM-5-H catalyst could play synergistic catalytic roles, which enhance the reaction activity and selectivity. In the difunctionalization of styrene with diaryl diselenide, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond activation in styrene should be a key step. In this case, the activated C
C bond activation in styrene should be a key step. In this case, the activated C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond is easily attacked by electrophilic selenium species. In our case, the abundant strongly acidic sites on HZSM-5-H favor the styrene adsorption and activation of the C
C bond is easily attacked by electrophilic selenium species. In our case, the abundant strongly acidic sites on HZSM-5-H favor the styrene adsorption and activation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond. This suggestion was supported by the investigation of styrene adsorption experiments, as well as the UV-Vis and IR spectra of styrene adsorbed on the Ru/HZSM-5-H sample (Ru/HZSM-5-H-1a).
C bond. This suggestion was supported by the investigation of styrene adsorption experiments, as well as the UV-Vis and IR spectra of styrene adsorbed on the Ru/HZSM-5-H sample (Ru/HZSM-5-H-1a).
From Table 1, the adsorption capacity of styrene (1a) on Ru/HZSM-5-H (20 mg gcat.−1) is much higher than that on Ru/γ-Al2O3 (9.5 mg gcat.−1) and Ru/ETS-10 (1.2 mg gcat.−1). More valuable information is obtained from the UV-Vis and IR spectra of Ru/HZSM-5-H-1a. From Fig. 8a, the absorption band at 258 nm for pure styrene, assigned to the conjugate π bond resulting from the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond and benzene ring in styrene, was shifted to 245 nm for the Ru/HZSM-5-H-1a sample. This could be due to the fact that the conjugate π bond between the C
C bond and benzene ring in styrene, was shifted to 245 nm for the Ru/HZSM-5-H-1a sample. This could be due to the fact that the conjugate π bond between the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond and benzene ring in styrene was destroyed when the styrene was adsorbed on Ru/HZSM-5-H, resulting in the occurrence of a blue shift. Thus, the C
C bond and benzene ring in styrene was destroyed when the styrene was adsorbed on Ru/HZSM-5-H, resulting in the occurrence of a blue shift. Thus, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond in styrene could be activated on the acidic sites of the Ru/HZSM-5-H catalyst. This suggestion was further confirmed using the IR characterization of the Ru/HZSM-5-H-1a sample. The IR spectrum of pure styrene 1a shows an absorption band in the 3004–3104 cm−1 region, which is characteristic of the stretching vibrations of C–H in a benzene ring,17 and the band at 2978 cm−1 is assigned to the stretching vibration of C–H in the C
C bond in styrene could be activated on the acidic sites of the Ru/HZSM-5-H catalyst. This suggestion was further confirmed using the IR characterization of the Ru/HZSM-5-H-1a sample. The IR spectrum of pure styrene 1a shows an absorption band in the 3004–3104 cm−1 region, which is characteristic of the stretching vibrations of C–H in a benzene ring,17 and the band at 2978 cm−1 is assigned to the stretching vibration of C–H in the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond (Fig. 8b).37–39 In contrast, two new bands at 2862 and 2937 cm−1 appear in the spectrum of the Ru/HZSM-5-H-1a sample. Similar absorption bands at 2873 and 2931 cm−1 are observed in the spectrum of ethylbenzene, which are related to the C–H stretching vibrations in methyl and methylene in ethylbenzene (Fig. 4b).37–39 These results confirm that the C
C bond (Fig. 8b).37–39 In contrast, two new bands at 2862 and 2937 cm−1 appear in the spectrum of the Ru/HZSM-5-H-1a sample. Similar absorption bands at 2873 and 2931 cm−1 are observed in the spectrum of ethylbenzene, which are related to the C–H stretching vibrations in methyl and methylene in ethylbenzene (Fig. 4b).37–39 These results confirm that the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond in styrene was activated on the acidic Ru/HZSM-5-H catalyst.
C bond in styrene was activated on the acidic Ru/HZSM-5-H catalyst.
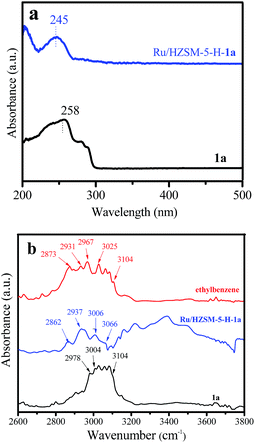 | ||
| Fig. 8 (a) UV-Vis spectra of the styrene (1a) and Ru/HZSM-5-H-1a samples, and (b) the IR spectra of the 1a, ethylbenzene and Ru/HZSM-5-H-1a samples. | ||
The activated C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond in styrene could be easily attacked by the electrophilic selenium species to form an intermediate that was reacted with a nucleophilic reagent to form the target product (the proposed mechanism is shown in Fig. S9†). Meanwhile, compared with the Ru0 species, the electron-poor Ru4+ species can act as a Lewis acid or co-oxidant that could more easily facilitate the formation of electrophilic selenium species in the case of H2O2 oxidizing the diaryl diselenides (Fig. S9†). As a result, the reaction activity and selectivity (94 and 95%) on Ru/HZSM-5-H is much higher than those (48 and 67%) on reduced Ru/HZSM-5-H.
C bond in styrene could be easily attacked by the electrophilic selenium species to form an intermediate that was reacted with a nucleophilic reagent to form the target product (the proposed mechanism is shown in Fig. S9†). Meanwhile, compared with the Ru0 species, the electron-poor Ru4+ species can act as a Lewis acid or co-oxidant that could more easily facilitate the formation of electrophilic selenium species in the case of H2O2 oxidizing the diaryl diselenides (Fig. S9†). As a result, the reaction activity and selectivity (94 and 95%) on Ru/HZSM-5-H is much higher than those (48 and 67%) on reduced Ru/HZSM-5-H.
The scope of this hydroxyselenation reaction over the Ru/HZSM-5-H catalyst was investigated, and the results are summarized in Table 2. The Ru/HZSM-5-H catalyst tolerates various styrenes with electron-donating substituents, such as methoxy, methyl, tertiary butyl and acetoxy groups, leading to high activity and target product selectivity (3b–3f). Meanwhile, styrenes with halogen substituents (3g–3i) at the ortho, meta and para positions also afforded the desired products in good to high yields. Other types of alkenes with polycyclic components such as biphenyl, naphthalene and diyldibenzene were compatible, affording good yields of the target products (3j–3l). Interestingly, this protocol was also applicable to α-methylstyrene, affording the desired product in gratifying yield (3m). Meanwhile, the substrates of cyclohexene and methylenecyclopentane were also suitable for this transformation and gave the products in good yields (3n, 3o). Notably, the reagent 4-pentenoic acid containing an internal nucleophile was also successfully applied to this transformation, resulting in the cyclo-functionalization of alkenes and the formation of seleno-lactone in moderate yield (3p).
| Entry | Alkenes | Products | Conv. (%) |
|---|---|---|---|
| a Reaction conditions: 25 mg of solid catalyst, alkenes (1.0 mmol), diaryl diselenides (0.6 mmol), H2O2 (1.5 mmol), H2O (1.0 mL), CH3CN (1.0 mL), and 60 °C for 10 h. The data outside of the parentheses is the conversion, and the data in parentheses is the selectivity. | |||
| 1 |  |
 |
80 (76) |
| 2 |  |
 |
82 (74) |
| 3 |  |
 |
77 (71) |
| 4 | 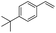 |
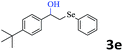 |
77 (76) |
| 5 | 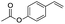 |
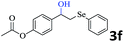 |
79 (78) |
| 6 |  |
 |
89 (93) |
| 7 |  |
 |
90 (91) |
| 8 |  |
 |
94 (89) |
| 9 |  |
 |
83 (85) |
| 10 |  |
 |
89 (83) |
| 11 |  |
 |
89 (87) |
| 12 |  |
 |
94 (92) |
| 13 |  |
 |
89 (89) |
| 14 |  |
 |
94 (93) |
| 15 |  |
 |
74 (60) |
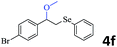
The generality of the Ru/HZSM-5-H catalyst in the difunctionalization of alkenes was further investigated through choosing alkoxy and carbethoxy groups as nucleophiles (Table 3). From Table 3, different alcohols such as methanol, ethanol, iso-propanol and phemethylol were used as reaction solvents, and they were also shown to be very good nucleophiles for this transformation, giving the corresponding seleno-functionalization compounds in satisfactory yields (4a–4d). In addition, styrenes with methoxyl or bromine substituent groups were also successfully reacted with methanol and diaryl diselenides, delivering the desired products in 62–66% yield (4e, 4f). Furthermore, 2-vinylnaphthalene with large molecular dimensions and cyclohexene were also smoothly reacted with methanol and diaryl diselenides (4g, 4h). Very interestingly, the displacement of the alcohol by the nucleophilic acetic acid could also be successfully applied to different alkenes such as styrene, p-bromo-styrene, 2-vinylnaphthalene and cyclohexene in the seleno-functionalization reactions, which furnished good substrate conversions and product selectivities (4i–4l). Notably, the styrenes with 4-CH3- and 4-Cl-substituent groups also smoothly reacted with diaryl disulfides in the presence of the methoxyl nucleophile, giving the sulfur-functionalization compounds in good yields (Table S4†).
| Entry | Alkenes | Solvent | Products | Conv. (%) |
|---|---|---|---|---|
| a Reaction conditions: 25 mg of solid catalyst, alkenes (1.0 mmol), diaryl diselenides (0.6 mmol), H2O2 (1.5 mmol), alcohols or acetic acid (2.0 mL), and 60 °C for 10 h. The data outside of the parentheses is the conversion, and the data in parenthesis is the selectivity. | ||||
| 1 |  |
CH3OH |  |
88 (83) |
| 2 | CH3CH2OH |  |
87 (75) | |
| 3 |  |
 |
84 (75) | |
| 4 |  |
 |
85 (83) | |
| 5 |  |
CH3OH | 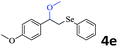 |
79 (84) |
| 6 | 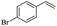 |
 |
74 (84) | |
| 7 | 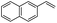 |
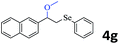 |
75 (81) | |
| 8 | 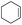 |
 |
79 (79) | |
| 9 | 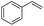 |
CH3COOH | 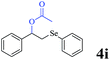 |
87 (89) |
| 10 | 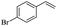 |
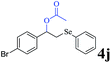 |
82 (81) | |
| 11 | 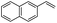 |
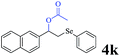 |
79 (84) | |
| 12 |  |
 |
72 (88) | |
The results from Tables 2 and 3 indicate that Ru/HZSM-5-H has a broad scope in the difunctionalization of alkenes. In addition, the reusable ability of the Ru/HZSM-5-H catalyst in the hydroxyselenation of styrenes with diaryl diselenides was also performed (Table S5†). The Ru/HZSM-5-H catalyst exhibits high activity (90%) and product selectivity (91%) even after it was recycled seven times, indicating that the Ru/HZSM-5-H catalyst has a good reusability. Nevertheless, the Ru content in the reused Ru/HZSM-5-H catalyst that was recycled seven times is only 2.5 wt%. The parallel experiments over the Ru/HZSM-5-H catalyst in the hydroxyselenation of styrene with diaryl diselenides were performed, and the results are shown in Table S6.† The reproducibility over the Ru/HZSM-5-H catalyst is good, and the error bars for the styrene conversion and selectivity are 0.41 and 0.48.
4. Conclusions
In summary, an acidic Ru/HZSM-5-H catalyst shows high activity and product selectivity in the hydroxyselenation of styrenes with diaryl diselenides compared to Ru/ETS-10, Ru/γ-Al2O3, Ru/HZSM-5 and homogeneous RuCl3 catalysts. The relatively strong acidic sites on Ru/HZSM-5-H could benefit the adsorption of styrene and activate its C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond, increasing the reaction activity. Meanwhile, the Ru4+ in Ru/HZSM-5-H facilitates the transformation from diaryl diselenides to electrophilic selenium species, improving the catalytic activity and selectivity. Furthermore, Ru/HZSM-5-H not only has a broad scope in the difunctionalization of alkenes with diaryl diselenides in the presence of hydroxyl, alkoxy and carbethoxy groups, but also has relatively good reusability.
C bond, increasing the reaction activity. Meanwhile, the Ru4+ in Ru/HZSM-5-H facilitates the transformation from diaryl diselenides to electrophilic selenium species, improving the catalytic activity and selectivity. Furthermore, Ru/HZSM-5-H not only has a broad scope in the difunctionalization of alkenes with diaryl diselenides in the presence of hydroxyl, alkoxy and carbethoxy groups, but also has relatively good reusability.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (U1463203, 21476030 and U1662139) and the Natural Science Foundation of Jiangsu Province of China (BK20150258).Notes and references
- K. Okamoto, Y. Nishibayashi, S. Uemura and A. Toshimitsu, Angew. Chem., Int. Ed., 2005, 44, 3588–3591 CrossRef CAS PubMed.
- F. Wang, D. Wang, X. Mu, P. Chen and G. Liu, J. Am. Chem. Soc., 2014, 136, 10202–10205 CrossRef CAS PubMed.
- X. Sun, X. Li, S. Song, Y. Zhu, Y.-F. Liang and N. Jiao, J. Am. Chem. Soc., 2015, 137, 6059–6066 CrossRef CAS PubMed.
- C. W. Nogueira, G. Zeni and J. B. T. Rocha, Chem. Rev., 2004, 104, 6255–6285 CrossRef CAS PubMed.
- B. C. Ranu and T. Mandal, J. Org. Chem., 2004, 69, 5793–5795 CrossRef CAS PubMed.
- B. Das, V. S. Reddy and R. Ramu, J. Mol. Catal. A: Chem., 2007, 263, 276–278 CrossRef CAS.
- T. Wirth, G. Fragale and M. Spichty, J. Am. Chem. Soc., 1998, 120, 3376–3381 CrossRef CAS.
- T. G. Back and Z. Moussa, Org. Lett., 2000, 2, 3007–3009 CrossRef CAS PubMed.
- B. C. Ranu, T. Mandal and S. Samanta, Org. Lett., 2003, 5, 1439–1441 CrossRef CAS PubMed.
- M. Tingoli, R. Diana and B. Panunzi, Tetrahedron Lett., 2006, 47, 7529–7531 CrossRef CAS.
- E. Tang, Y. Zhao, W. Li, W. Wang, M. Zhang and X. Dai, Org. Lett., 2016, 18, 912–915 CrossRef CAS PubMed.
- A. A. Vieira, J. B. Azeredo, M. Godoi, C. Santi, E. N. S. Júnior and A. L. Braga, J. Org. Chem., 2015, 80, 2120–2127 CrossRef CAS PubMed.
- M. J. Climent, A. Corma and S. Iborra, Chem. Rev., 2011, 111, 1072–1133 CrossRef CAS PubMed.
- À. Molnár, Chem. Rev., 2011, 111, 2251–2320 CrossRef PubMed.
- Q. Wu, X. Wang, G. Qi, Q. Guo, S. Pan, X. Meng, J. Xu, F. Deng, F. Fan, Z. Feng, C. Li, S. Maurer, U. Müller and F.-S. Xiao, J. Am. Chem. Soc., 2014, 136, 4019–4025 CrossRef CAS PubMed.
- Y. Wei, T. E. Parmentier, K. P. Jong and J. Zečević, Chem. Soc. Rev., 2015, 44, 7234–7261 RSC.
- T. Tang, L. Zhang, H. Dong, Z. Fang, W. Fu, Q. Yu and T. Tang, RSC Adv., 2017, 7, 7711–7717 RSC.
- L. Wang, J. Zhang, X. Yi, A. Zheng, F. Deng, C. Chen, Y. Ji, F. Liu, X. Meng and F.-S. Xiao, ACS Catal., 2015, 5, 2727–2734 CrossRef CAS.
- S. Chen, Z. Shao, Z. Fang, Q. Chen, T. Tang, W. Fu, L. Zhang and T. Tang, J. Catal., 2016, 338, 38–46 CrossRef CAS.
- W. Fu, T. Liu, Z. Fang, Y. Ma, X. Zheng, W. Wang, X. Ni, M. Hu and T. Tang, Chem. Commun., 2015, 51, 5890–5893 RSC.
- X. Zheng, W. Fu, J. Xiong, J. Xi, X. Ni and T. Tang, Catal. Today, 2016, 264, 152–157 CrossRef CAS.
- W. Fu, Y. Feng, Z. Fang, Q. Chen, T. Tang, Q. Yu and T. Tang, Chem. Commun., 2016, 52, 3115–3118 RSC.
- T. Liu, W. Fu, X. Zheng, J. Jiang, M. Hu and T. Tang, RSC Adv., 2014, 4, 18217–18221 RSC.
- H. Li, Y. Wang, F. Meng, H. Chen, C. Sun and S. Wang, RSC Adv., 2016, 6, 99129–99138 RSC.
- A. Li, C. Huang, C.-W. Luo, W.-J. Yia and Z.-S. Chao, RSC Adv., 2017, 7, 9551–9561 RSC.
- W. Fu, L. Zhang, D. Wu, Q. Yu, T. Tang and T. Tang, Ind. Eng. Chem. Res., 2016, 55, 7085–7095 CrossRef CAS.
- M. Xiang, X. Ni, X. Yi, A. Zheng, W. Wang, M. He, J. Xiong, T. Liu, Y. Ma, P. Zhu, X. Zheng and T. Tang, ChemCatChem, 2015, 7, 521–525 CrossRef CAS.
- M. D. Romero, J. A. Calles and A. Rodríguez, Ind. Eng. Chem. Res., 1997, 36, 3533–3540 CrossRef CAS.
- Y. Wang, Z. Tao, B. Wu, J. Xu, C. Huo, K. Li, H. Chen, Y. Yang and Y. Li, J. Catal., 2015, 322, 1–13 CrossRef CAS.
- H. Song, J. Wang, Z. Wang, H. Song, F. Li and Z. Jin, J. Catal., 2014, 311, 257–265 CrossRef CAS.
- C.-P. Lo and V. Ramani, ACS Appl. Mater. Interfaces, 2012, 4, 6109–6116 CAS.
- N. Taniguchi, J. Org. Chem., 2006, 71, 7874–7876 CrossRef CAS PubMed.
- R. Mu, D. C. Cantu, X. Lin, V.-A. Glezakou, Z. Wang, I. Lyubinetsky, R. Rousseau and Z. Dohnálek, J. Phys. Chem. Lett., 2014, 5, 3445–3450 CrossRef CAS PubMed.
- X. Zhang and K.-Y. Chan, Chem. Mater., 2003, 15, 451–459 CrossRef CAS.
- J. Yang, J. Y. Lee, T. C. Deivaraj and H.-P. Too, J. Colloid Interface Sci., 2004, 271, 308–312 CrossRef CAS PubMed.
- K. Qadir, S. H. Joo, B. S. Mun, D. R. Butcher, J. R. Renzas, F. Aksoy, Z. Liu, G. A. Somorjai and J. Y. Park, Nano Lett., 2012, 12, 5761–5768 CrossRef CAS PubMed.
- A. Mallmann and D. Barthomeuf, Zeolites, 1988, 8, 292–301 CrossRef.
- W. P. Addiego, C. A. Estrada, D. W. Goodman and M. P. Rosynek, J. Catal., 1994, 146, 407–414 CrossRef CAS.
- I. Retzko, J. F. Friedrich, A. Lippitz and W. E. S. Unger, J. Electron Spectrosc. Relat. Phenom., 2001, 121, 111–129 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra01732d |
| This journal is © The Royal Society of Chemistry 2017 |