Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Fabrication of durable and flexible single-walled carbon nanotube transparent conductive films†
Subramani Devaraju a,
Taeheon Leea,
Aruna Kumar Mohantya,
Young Kun Honga,
Kwan Han Yoonb,
Young Sil Leec,
Jong Hun Han*d and
Hyun-jong Paik
a,
Taeheon Leea,
Aruna Kumar Mohantya,
Young Kun Honga,
Kwan Han Yoonb,
Young Sil Leec,
Jong Hun Han*d and
Hyun-jong Paik *a
*a
aDepartment of Polymer Science and Engineering, Pusan National University, 2, Busandaehak-ro 63beon-gil, Geumjeong-gu, Busan 609-735, Korea. E-mail: hpaik@pusan.ac.kr
bDepartment of Polymer Science and Engineering, Kumoh National Institute of Technology, 1 Yangho-dong, Gumi, Gyeongbuk 730-701, Korea
cIndustry-Academic Cooperation Foundation, Kumoh National Institute of Technology, 1 Yangho-dong, Gumi, Gyeongbuk 730-701, Korea
dSchool of Chemical Engineering, Chonnam National University, 77 Yongbong-ro, Buk-gu, Gwangju 500-757, Korea. E-mail: jhhan@jnu.ac.kr
First published on 31st March 2017
Abstract
In the present work, flexible, durable, transparent, conductive films (TCFs) were fabricated with the use of aqueous dispersed single-walled carbon nanotubes (SWCNTs). A small amount of the synthesized sulfonated poly(ether sulfone) (SPES) was used to effectively disperse SWCNTs with high aspect ratios. The lengths and heights of the dispersed SWCNTs were 2.5 ± 1.0 μm and 2 ± 1 nm, as determined by TEM and AFM, respectively. TCFs were fabricated using spray coating on a PET substrate; the best performance among the TCFs was achieved with a sheet resistance (Rs) of 125 Ω sq−1 and optical transmittance of 87.1%. Moreover, no appreciable change in the Rs was observed after repeated bending cycles and adhesive peel-off tests, which indicate that SPES acts as a good dispersant and effective binder for the improvement in durability and adhesion behavior of the resulting TCFs in the absence of additional binders. Therefore, this material holds great potential for scalable and facile production of flexible SWCNT-TCFs for various electronic applications.
Introduction
Transparent conductive films with flexible character have been the focus of much attention for next-generation thin, lightweight, foldable and bendable electronic applications. Flexible displays are expected to be used in a wide range of applications such as televisions, monitors, mobile devices, e-publications, fabrics, and medical equipment. As uses of technology advance, the demands for user-friendly and universal displays as human interfaces are also fast increasing in the upcoming years. Currently, indium tin oxide (ITO) is used commercially to produce TCFs for the electrodes of displays and touch screen panels. However, there is a need for an alternative material which can overcome the disadvantages associated with the use of ITO such as high processing costs, inflexibility, and insufficiency of rare metals.For past few decades, carbon nanotubes (CNTs) are considered as a promising material in various fields including energy, environment, medicine, and textiles because of their unique and outstanding properties such as good electrical properties, high mechanical and thermal stability.1–11 In particular, single-walled carbon nanotubes (SWCNTs), which have remarkable electrical mobility, conductivity6,12 and work functions,13 are deliberated favorable in electrical applications such as transistors,6,14,15 sensors,16,17 memory devices18 and conducting films.4–7,19–21 Thus, SWCNTs are expected as the best candidate material to replace current ITO due to outstanding flexibility, good stability, high conductivity and good optical transparency, ability of low-temperature printing and coating process; and thanks to its abundant availability which has great potential for the cost reduction.
However, SWCNTs exist as thick bundles due to their high surface energy and strong van der Waals force, and exhibit poor dispersibility in water and common organic solvents. To overcome this issue, several strategies have been adopted over the years to de-bundle SWCNTs, which include the covalent22,23 and the non-covalent methods.24–26 Among the techniques, noncovalent functionalization method using polymeric dispersants is seen as an effective strategy to de-bundle SWCNTs with high aspect ratio while minimizing the structural defects.27–29 High aspect ratio SWCNTs are expected to decrease the network resistivity because of a smaller number of contacts in series. However, the non-covalent functionalization methods have been reported with the use of a higher amount of polymers or surfactants for exfoliation of SWCNTs. The residual dispersants increase the contact resistance between CNTs, which needs further processing steps to remove the residual dispersant.30,31
In our previous reports,32–34 polymeric dispersants (CNTs to polymer ratio, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) were employed to disperse SWCNTs individually with high aspect ratio in various organic solvents. Various post-treatments of the TCFs such as chemical doping (HNO3 and SOCl2) and thermal treatment were also used to improve the conductivity. However, high-temperature treatment was not generally deliberated upon the low-temperature plastic substrate. Though, chemical doping treatment of CNT TCFs are used in the improvement of charge carrier and the decrease of contact resistance between CNT junctions, the doped CNT films become sensitive to air, temperature, and humidity. Thus, most of the reported CNT-based TCFs properties are not stable as a serious drawback in many applications.27,35,36
10) were employed to disperse SWCNTs individually with high aspect ratio in various organic solvents. Various post-treatments of the TCFs such as chemical doping (HNO3 and SOCl2) and thermal treatment were also used to improve the conductivity. However, high-temperature treatment was not generally deliberated upon the low-temperature plastic substrate. Though, chemical doping treatment of CNT TCFs are used in the improvement of charge carrier and the decrease of contact resistance between CNT junctions, the doped CNT films become sensitive to air, temperature, and humidity. Thus, most of the reported CNT-based TCFs properties are not stable as a serious drawback in many applications.27,35,36
In recent years, CNT TCFs have been reported with high transmittance and high conductivity. However, they lack in good durability due to weak adhesion between the CNT films and the polymer substrate which limits their practical and scalable use in electronic fields.37,38 Binder/or additives are commonly used to increase the adhesion between CNT and polymer substrate which, however, results with disadvantages in terms of higher resistance due to their insulating behavior in the CNT junctions.39–42 Moreover, the main drawback lies with the fact that the excess binder/additive can't be easily removed by water washing or acid-treatments.40–42
There is a special requirement to disperse CNTs in the aqueous medium due to economic and environmental reasons. Besides, as a key requirement for high-performance applications in electronics, it is important to fabricate the TCFs with good transmittance, high conductivity as well as excellent durability without the use of any binder/additive or post treatments. No post treatment also implies that the manufacturing process of TCFs can be more simplified by eliminating the rinsing equipment (for removing the binder or additive), which enables low-cost fabrication of TCFs. So, the simplified fabrication of low-cost CNT TCFs needs essential a new polymeric dispersant which imparts the combined properties including good dispersion ability, doping agent to enhance the conductivity and also as a binder to enhance the durability.
In this regard, we synthesized sulfonated poly(ether sulfone) (SPES) to disperse SWCNTs with high aspect ratio using a significantly lower amount of the polymer (CNTs to polymer ratio, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in aqueous medium and fabricated TCFs thereof for the best performance in terms of transmittance, conductivity, and durability without any post-treatments. Sulfonated poly(ether sulfone) have been extensively investigated as proton exchange membrane material for fuel cell and other membrane applications because of their good thermal, mechanical, solvent resistant and ionic conductivity properties. Thus, the synthesized SPES which consists of both hydrophobic and hydrophilic moieties is highly soluble in water and able to disperse SWCNTs individually for long term stability (more than 6 months). Hydrophobic fluorinated aromatic moieties in SPES backbone structure are expected to act as the anchor to the hydrophobic CNT surfaces by π–π interactions. Meanwhile, hydrophilic sulfonic acid groups (–SO3H) of SPES are also expected to be an effective stabilizer and dopant for individual SWCNTs in the solution.19 The stability of the SWCNT dispersion is determined by TEM and AFM. The structural quality of the SWCNT dispersion is checked with Raman spectroscopy. Finally, the mechanical durability of TCFs in terms of bending and adhesive peel-off test are investigated in the absence of any kind of binder/additive and discussed.
1) in aqueous medium and fabricated TCFs thereof for the best performance in terms of transmittance, conductivity, and durability without any post-treatments. Sulfonated poly(ether sulfone) have been extensively investigated as proton exchange membrane material for fuel cell and other membrane applications because of their good thermal, mechanical, solvent resistant and ionic conductivity properties. Thus, the synthesized SPES which consists of both hydrophobic and hydrophilic moieties is highly soluble in water and able to disperse SWCNTs individually for long term stability (more than 6 months). Hydrophobic fluorinated aromatic moieties in SPES backbone structure are expected to act as the anchor to the hydrophobic CNT surfaces by π–π interactions. Meanwhile, hydrophilic sulfonic acid groups (–SO3H) of SPES are also expected to be an effective stabilizer and dopant for individual SWCNTs in the solution.19 The stability of the SWCNT dispersion is determined by TEM and AFM. The structural quality of the SWCNT dispersion is checked with Raman spectroscopy. Finally, the mechanical durability of TCFs in terms of bending and adhesive peel-off test are investigated in the absence of any kind of binder/additive and discussed.
Results and discussions
Water soluble SPES (Fig. S1†) was synthesized (detailed synthesis procedure has been given in the ESI†) from the base-mediated condensation polymerization of 6FBPA with SDCDPS in NMP. The chemical structure of the SPES was confirmed from the 1H NMR (Fig. S2†) and ATR FT-IR spectra (Fig. S3†). The SPES with both hydrophobic (fluorinated aromatic backbone) and hydrophilic groups (sulfonic acid) was found to be an effective polymeric dispersant as SWCNTs were individually dispersed in aqueous medium with long term stability (more than 6 months). Aqueous dispersion of SWCNTs (1 g L−1) by non-covalent functionalization with different weight concentration of SPES (0.0, 0.1, 0.5 and 1.0 weight%) are shown in Fig. S4.† Non-covalent functionalization was achieved through π–π interactions between the SWCNTs surface and the hydrophobic fluorinated aromatic backbone moieties in the SPES.29,30,43 Further, the sulfonic acid groups (–SO3H) in SPES could be effective stabilizer and dopant for the dubundled SWCNTs.19 The hydrophilic nature of charged sulfonate groups being accessible by bulk water helps the exfoliation and the stabilization of individual SWCNTs come through their electrostatic repulsion.9TEM and AFM were utilized to characterize the length and diameter of the SWCNTs dispersion. Fig. 1a shows the TEM images of dispersed SWCNTs using SPES as a dispersant. From the TEM images, it was evident that SWCNTs were well dispersed with reasonably higher length. Further, in the SWCNTs length distribution (Fig. 1b), the average length of dispersed SWCNTs was ca. 2.5 ± 1.0 μm. The estimated length of dispersed SWCNTs was 2 to 2.5 times higher than the previous reports.44–48 AFM was used to determine the diameter of dispersed SWCNTs. AFM image and height profiles of SWCNT dispersion are shown in Fig. 2. From the height profile, the average diameter of dispersed SWCNTs was observed to be 1–2 nm (0.1% SPES), 2–4 nm (0.5% SPES) and 2–3 nm (1% SPES). The AFM image clearly indicates that the SWCNTs were dispersed individually or small bundle diameter. It is important to maintain a high aspect ratio after dispersion because the length of the SWCNTs influences the electrical properties of the resulting SWCNT network. Dispersion of SWCNTs with high aspect ratio results in a smaller number of junctions between SWCNTs in the network, lowering the contact resistance.32–34,49 The AFM and TEM results revealed that the SWCNTs were individually dispersed and maintained their high aspect ratios while using SPES as a dispersant.
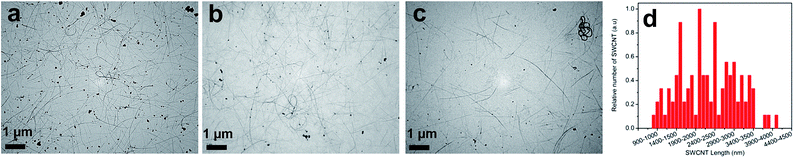 | ||
| Fig. 1 TEM images of SWCNTs dispersed in water with SPES as the polymeric dispersant (a) 0.1%, (b) 0.5% (c) 1.0% SPES and (d) SWCNT length distribution (0.1 wt% SPES). | ||
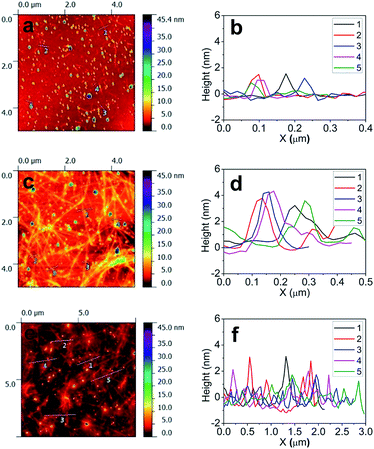 | ||
| Fig. 2 AFM phase and height images for the SWCNT dispersions (a and b) 0.1%, (c and d) 0.5% and (e and f) 1.0% SPES as the polymeric dispersant. | ||
UV-vis-NIR spectroscopy is a powerful tool for the characterization of SWCNT for dispersion and efficiency.50,51 In the UV-vis region, SWCNTs dispersed as individuals show characteristic absorption bands corresponding to additional absorption due to van Hove singularities. On the other hand, bundled CNTs in which tunneling of carriers occurs between the nanotubes hardly show the absorption bands due to quenching of the photoluminescence properties.32–34 Fig. 3a shows the UV-vis absorption spectrum in the range of 400–1200 nm for SWCNT dispersions. The peaks at 900–1200 nm and 550–800 nm were assigned to the first and second van Hove transitions of semiconducting SWCNTs (S11 and S22) respectively, and the peak at 400–550 was attributed to the first van Hove transitions of metallic SWCNTs (M11).32 Well-defined absorption peaks observed in the 900–1100 nm range reveal that SWCNTs were well dispersed individually for each solution. In-particular, the SWCNTs solution with 0.1% SPES with the highest intensity indicates the effective individual dispersion of SWCNTs with the lower amount of dispersant. Raman spectroscopy was also successfully used to characterize the SWCNT structural properties and determines the level of defects in SWCNTs. Fig. 3b shows the Raman spectra for SWCNT dispersions using SPES as the polymeric dispersant. The peak at 171 cm−1 was attributed to the radial breathing mode (RBM) caused by the expansion and the contraction of the nanotube. The RBM frequency is specifically important for the determination of the diameter distribution of SWCNTs.33,34 The RBM frequency can be empirically related to the diameter of the SWCNT by
| RBM = C1/d | (1) |
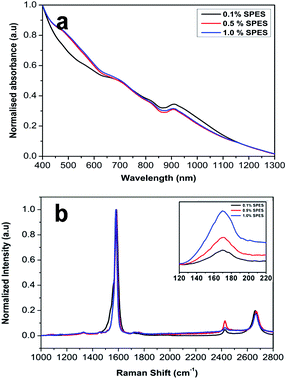 | ||
| Fig. 3 UV-vis-NIR (a) and Raman (b) spectra for SWCNT dispersions using SPES as the polymeric dispersant (the inserted image indicates the RBM region). | ||
| Sample | ID/IG | Defect [%] |
|---|---|---|
| 0.1% SPES | 0.020 | 2% |
| 0.5% SPES | 0.022 | 2.2% |
| 1.0% SPES | 0.025 | 2.5% |
The TCFs were fabricated by spray-coating the SWCNT dispersed solutions onto the flexible PET substrates. The thickness CNT film in final TCF films was measured as ∼100 nm by surface profile meter. The sheet resistance (Rs) of the TCFs fabricated using 0.1, 0.5 and 1.0% polymer dispersant were 125 Ω sq−1 (transmittance, 87.1%), 21.4 kΩ sq−1 (transmittance, 88.6%) and 9.6 kΩ sq−1 (transmittance, 84.9%), respectively. The SWCNT dispersions using 0.5 and 1.0% SPES showed a higher value of Rs than that with 0.1% SPES. As weight fractions of SWCNTs were the same for the three solutions and the SWCNTs in the solutions were dispersed with almost similar high aspect ratio, the difference in the Rs values might be due to the following reason: as the higher amount (0.5 and 1.0%) of SPES dispersant was used, residual polymer and excess polymer wrapping of SWCNTs could function as insulators resulting in increased contact resistance in the CNT network for the fabricated TCFs.53 Since, the SWCNT dispersion with 0.1% SPES showed better results in terms of the lowest resistance with good transmittance, was chosen for further detailed study. Fig. 4 correlates the average Rs values with the transmittance of TCFs made with a number of spray coating of SWCNT solution (0.1% SPES) and compares with values for various other previously reported TCFs. The TCF showing the lower Rs (125 Ω sq−1) and high transmittance (87.1%) for the first time without any post treatment is a considerable achievement when compared with the results of previous research.9,54–58 Here, the sulfonic acid groups in the SPES could act as a dopant for the CNT film to increase the charge carrier and reduce the contact resistance of CNT film resulting in a reduction of resistance without the use of any chemical treatment.19 The observed value was quite comparable with the values recently reported by Harris et al.,59 where the SWCNT film was prepared using chlorosulfonic acid. In this work, it was thus, confirmed that SWCNT dispersion prepared using 0.1 wt% SPES was more suitable for lower cost fabrication of TCFs with a simplified processing.
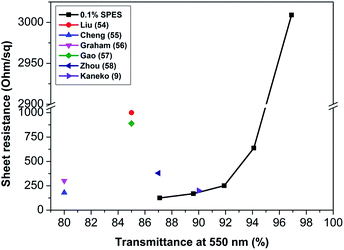 | ||
| Fig. 4 Correlation between sheet resistance and transmittance at 550 nm for SWCNT dispersions based TCFs. | ||
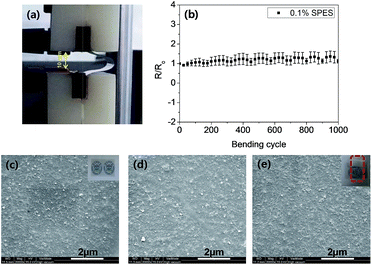
The mechanical stability of the SWCNT TCF (from 0.1% SPES) was investigated by bending test (Fig. 5a). The TCF film was bent for 1000 times and the Rs were measured from the average of every 25 bending cycles. As shown in Fig. 5b, there was no significant increase in Rs after 1000 bending cycles. An increase of less than 15% in the Rs value of the SWCNT TCF was observed after 1000 bending cycles. Fig. 5c–e shows the SEM images (and inset optical images) of the TCF as prepared and compares with the TCF after repeated bending and peel-off tape test. The mechanical durability of the TCF was checked for adhesion property by peel-off tape test. In this test, the Scotch™ tape (3M) was pasted to the film with constant pressure, and then the tape was peeled out to remove CNT film from the polymer substrate. The adhesion was evaluated by measuring the resistance of the films for as-prepared (R0) and after a peel-off tape test (R). After adhesion test with the Scotch™ tape for 5 times, almost no change in Rs was observed; this may be due to the presence of SPES, which imparts a strong interfacial bonding between SWCNTs and the polymer substrate to enhance the adhesion. The hydrophobic rigid aromatic structures of SPES dispersant could provide a sufficient π–π interactions with both CNT surface and the PET substrate in the absence of any binders/additives. On the other hand, there are reports in which SWCNTs were removed from the TCF substrate while using small molecule surfactants or polymeric dispersants in the absence of binders or additives in peel-off test.8,40,41 This indicates the effectiveness of SPES as a polymeric dispersant to induce good dispersion of individual SWCNTs with high aspect ratio as well as outstanding mechanical durability in flexible TCFs. We believe that this approach may be used to develop the large-scale production of transparent electrodes with good flexibility, stability, and durability, suitable for touch panel applications. Fabricated CNT TCFs may be suitable for touch panel applications (touch screen needs a sheet resistance in the range of 50–300 Ω sq−1) to replace the ITO market in a low cost process. However, the fabricated CNT TCFs are not well comparable to the ITO for low resistance (<50 Ω sq−1) applications. In this context, further developments would be required to enhance the performances of CNT TCFs. Fabrication of CNT hybrid TCFs with graphene or metal nanowires may be a suitable approach to overcome the conductivity limits in the current CNT-based TCFs to replace the ITO in the display applications.60–62
Conclusion
Flexible and bendable CNT TCFs were successfully fabricated with high conductivity, good transmittance, and mechanical durability from the de-bundled SWCNTs in the aqueous solvent using a small amount of SPES. The synthesized SPES was able to disperse SWCNTs individually with high aspect ratio for long-term stability (>6 months). Flexible TCFs fabricated from the spray coatings of SWCNT dispersion (with 0.1% SPES) on PET film substrate showed the first ever best performance with sheet resistance as low as 125 Ω sq−1 with a good optical transmittance of 87.1% without any chemical doping treatment as post-processing. Further, the TCF was very stable and durable as there was only a mild increase in the resistance value after 1000 bending cycles and tape peel-off test. This indicated that the SPES dispersant was inducing the good adhesion property between the SWCNTs and the PET substrate in the absence of binder/additives or any other post-treatments. We believe that the SWCNT dispersions using SPES dispersant in environment-friendly aqueous system holds much potential as a material to fabricate durable and flexible TCFs for touch displays in a facile and economic way.Acknowledgements
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (2013R1A2A2A01068818), BK21 PLUS Centre for Advanced Chemical Technology (21A20131800002), and Industrial Strategic Technology Development Program (10045051, 10052838) funded by the Ministry of Trade, Industry and Energy (MOTIE) of Korea. Also, this work was supported by Nano Material Technology Development Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (2017M3A7B4014045).Notes and references
- S. Iijima, Nature, 1991, 354, 56 CrossRef CAS.
- T. W. Odom, J. L. Huang, P. Kim and C. M. Lieber, Nature, 1998, 391, 62 CrossRef CAS.
- R. H. Baughman, A. A. Zakhidov and W. A. De Heer, Science, 2002, 297, 787 CrossRef CAS PubMed.
- T. Fujigaya and N. Nakashima, Sci. Technol. Adv. Mater., 2015, 16, 024802 CrossRef PubMed.
- C. Biswas and Y. H. Lee, Adv. Funct. Mater., 2011, 21, 3806 CrossRef CAS.
- V. M. F. L. De, S. H. Tawfick, R. H. Baughman and A. J. Hart, Science, 2013, 339, 535 CrossRef PubMed.
- Z. Song, J. Dai, S. Zhao, Y. Zhou, F. Su, J. Cui and Y. Yan, RSC Adv., 2014, 4, 2327 RSC.
- S. K. Kim, T. Liu and X. Wang, ACS Appl. Mater. Interfaces, 2015, 7, 20865 CAS.
- T. Matsuda, D. Minami, F. Khoerunnisa, M. Sunaga, M. Nakamura, S. Utsumi, T. Itoh, T. Fujimori, T. Hayashi, Y. Hattori, M. Endo, H. Isobe, H. Onodera and K. Kaneko, Langmuir, 2015, 31, 3194 CrossRef CAS PubMed.
- M. Adeli, R. Soleyman, Z. Beiranvand and F. Madani, Chem. Soc. Rev., 2013, 42, 5231 RSC.
- H. C. Wu, X. Chang, L. Liu, F. Zhao and Y. Zhao, J. Mater. Chem., 2010, 20, 1036 RSC.
- T. Dürkop, S. A. Getty, E. Cobas and M. S. Fuhrer, Nano Lett., 2003, 4, 35–39 CrossRef.
- R. Gao, Z. Pan and Z. L. Wang, Appl. Phys. Lett., 2001, 78, 1757–1759 CrossRef CAS.
- D. R. Kauffman and A. Star, Chem. Soc. Rev., 2008, 37, 1197–1206 RSC.
- B. R. Lee, J. S. Kim, Y. S. Nam, H. J. Jeong, S. Y. Jeong, G.-W. Lee, J. T. Han and M. H. Song, J. Mater. Chem., 2012, 22, 21481–21486 RSC.
- C. Hu and S. Hu, J. Sens., 2009, 1–40 CrossRef CAS.
- B. L. Allen, P. D. Kichambare and A. Star, Adv. Mater., 2007, 19, 1439–1451 CrossRef CAS.
- J. Y. Son, S. Ryu, Y.-C. Park, Y.-T. Lim, Y.-S. Shin, Y.-H. Shin and H. M. Jang, ACS Nano, 2010, 4, 7315–7320 CrossRef CAS PubMed.
- H.-Z. Geng, K. K. Kim, C. Song, N. T. Xuyen, S. M. Kim, K. A. Park, D. S. Lee, K. H. An, Y. S. Lee, Y. Chang, Y. J. Lee, J. Y. Choi, A. Benayad and Y. H. Lee, J. Mater. Chem., 2008, 18, 1261–1266 RSC.
- K. K. Kim, S.-M. Yoon, H. K. Park, H.-J. Shin, S. M. Kim, J. J. Bae, Y. Cui, J. M. Kim, J.-Y. Choi and Y. H. Lee, New J. Chem., 2010, 34, 2183–2188 RSC.
- J. T. Han, J. S. Kim, H. D. Jeong, H. J. Jeong, S. Y. Jeong and G.-W. Lee, J. Mater. Chem., 2010, 20, 8557–8562 RSC.
- S. Banerjee, T. H. Benny and S. S. Wong, Adv. Mater., 2005, 17, 17–29 CrossRef CAS.
- C. A. Dyke and J. M. Tour, Chem.–Eur. J., 2004, 10, 812 CrossRef CAS PubMed.
- M. F. Islam, E. Rojas, D. M. Bergey, A. T. Johnson and A. G. Yodh, Nano Lett., 2003, 3, 269 CrossRef CAS.
- K. K. Kim, S. M. Yoon, J. Y. Choi, J. Lee, B. K. Kim, J. M. Kim, J. H. Lee, U. Paik, M. H. Park, C. W. Yang, K. H. An, Y. Chung and Y. H. Lee, Adv. Funct. Mater., 2007, 17, 1775 CrossRef CAS.
- K. Y. Cho, Y. S. Yeom, H. Y. Seo, Y. H. Park, H. N. Jang, K. Y. Baek and H. G. Yoon, ACS Appl. Mater. Interfaces, 2015, 7, 9841 CAS.
- R. Wang, J. Sun, L. Gao and J. Zhang, ACS Nano, 2010, 4, 4890 CrossRef CAS PubMed.
- J. U. Lee, J. Huh, K. H. Kim, C. Park and W. H. Jo, Carbon, 2007, 45, 1051 CrossRef CAS.
- M. Tunckol, S. Fantini, F. Malbosc, J. Durand and P. Serp, Carbon, 2013, 57, 209 CrossRef CAS.
- H. Z. Geng, K. K. Kim, K. P. So, Y. S. Lee, Y. Chang and Y. H. Lee, J. Am. Chem. Soc., 2007, 129, 7758 CrossRef CAS PubMed.
- U. D. Weglikowska, V. Skakalova, R. Graupner, S. H. Jhang, B. H. Kim, H. J. Lee, L. Ley, Y. W. Park, S. Berber, D. Tomanek and S. Roth, J. Am. Chem. Soc., 2005, 127, 5125 CrossRef PubMed.
- T. Lee, B. Kim, S. Kim, J. H. Han, H. B. Jeon, Y. S. Lee and H. J. Paik, Nanoscale, 2015, 7, 6745 RSC.
- T. Lee, J. Park, K. Kim, A. K. Mohanty, B. Kim, J. H. Han, H. B. Jeon, Y. S. Lee and H. J. Paik, RSC Adv., 2015, 5, 69410 RSC.
- B. S. Kim, D. Kim, K. W. Kim, T. Lee, S. Kim, K. Shin, S. Chun, J. H. Han, Y. S. Lee and H. J. Paik, Carbon, 2014, 72, 57 CrossRef CAS.
- R. Graupner, J. Abraham, A. Vencelova, T. Seyller, F. Hennrich, M. M. Kappes, A. Hirsch and L. Ley, Phys. Chem. Chem. Phys., 2003, 5, 5472 RSC.
- H. Z. Geng, K. K. Kim, C. Song, N. T. Xuyen, S. M. Kim, K. A. Park, D. S. Lee, K. H. An, Y. S. Lee, Y. K. Chang, Y. J. Lee, J. Y. Choi, A. Benayad and Y. H. Lee, J. Mater. Chem., 2008, 18, 1261 RSC.
- A. Rahy, P. Bajaj, I. H. Musselman, S. H. Hong, Y. P. Sun and D. J. Yang, Appl. Surf. Sci., 2009, 255, 7084 CrossRef CAS.
- H. Jung, J. S. Yu, H. P. Lee, J. M. Kim, J. Y. Park and D. A. Kim, Carbon, 2013, 52, 259 CrossRef CAS.
- H. Jung, S. Y. An, J. S. Lim and D. Kim, J. Nanosci. Nanotechnol., 2011, 11, 6345 CrossRef CAS PubMed.
- B. T. Liu and C. H. Hsu, J. Colloid Interface Sci., 2011, 359, 423 CrossRef CAS PubMed.
- Y. Wang, H. J. Yang, H. Z. Geng, Z. C. Zhang, E. X. Ding, Y. Meng, Z. Luo, J. Wang, X. M. Su and S. X. Da, J. Mater. Chem. C, 2015, 3, 3796 RSC.
- S. Azoubel and S. Magdassi, ACS Appl. Mater. Interfaces, 2014, 6, 9265–9271 CAS.
- F. Wang, T. Chen and T. Xu, Macromol. Chem. Phys., 1998, 199, 1421 CrossRef CAS.
- J. Zhang, L. Gao, J. Sun, Y. Liu, Y. Wang, J. Wang, H. Kajiura, Y. Li and K. Noda, J. Phys. Chem. C, 2008, 112, 16370 CAS.
- M. J. O'Connell, S. M. Bachilo, C. B. Huffman, V. C. Moore, M. S. Strano, E. H. Haroz, K. L. Rialon, P. J. Boul, W. H. Noon, C. Kittrell, J. Ma, R. H. Hauge, R. B. Weisman and R. E. Smalley, Science, 2002, 297, 593 CrossRef PubMed.
- M. F. Islam, E. Rojas, D. M. Bergey, A. T. Johnson and A. G. Yodh, Nano Lett., 2003, 3, 269 CrossRef CAS.
- N. I. Kovtyukhova, T. E. Mallouk, L. Pan and E. C. Dickey, J. Am. Chem. Soc., 2003, 125, 9761 CrossRef CAS PubMed.
- S. Qin, D. Qin, W. T. Ford, J. E. Herrera, D. E. Resasco, S. M. Bachilo and R. B. Weisman, Macromolecules, 2004, 37, 3965 CrossRef CAS.
- B. Zhao, H. Hu, A. Yu, D. Perea and R. C. Haddon, J. Am. Chem. Soc., 2005, 127, 8197 CrossRef CAS PubMed.
- S. Pfeifer, S. H. Park and P. R. Bandaru, J. Appl. Phys., 2010, 108, 024305 CrossRef.
- C. Backes, C. D. Schmidt, F. Hauke, C. Bottcher and A. Hirsch, J. Am. Chem. Soc., 2009, 131, 2172 CrossRef CAS PubMed.
- J. H. Lee, U. Paik, J. Y. Choi, K. K. Kim, S. M. Yoon, J. H. Lee, B. K. Kim, J. M. Kim, M. H. Park, C. W. Yang, K. H. An and Y. H. Lee, J. Phys. Chem. C, 2007, 111, 2477 CAS.
- B. Baykal, V. Ibrahimova, G. Er, E. Bengu and D. Tuncel, Chem. Commun., 2010, 46, 6762 RSC.
- T. Lee, S. Kim, H. Kim, B. S. Kim, Y. S. Lee, J. H. Han and H. J. Paik, Compos. Sci. Technol., 2015, 121, 95 CrossRef CAS.
- K. R. Chen, H. F. Yeh, H. C. Chen, T. J. Liu, S. J. Huang, P. Y. Wu and C. Tiu, Adv. Chem. Eng. Sci., 2013, 3, 105 CrossRef.
- W. B. Liu, S. Pei, J. Du, B. Liu, L. Gao, Y. Su, C. Liu and H. M. Cheng, Adv. Funct. Mater., 2011, 21, 2330 CrossRef CAS.
- R. Jackson, B. Domercq, R. Jain, B. Kippelen and S. Graham, Adv. Funct. Mater., 2008, 18, 2548 CrossRef CAS.
- R. Wang, J. Sun, L. Gao and J. Zhang, J. Mater. Chem., 2010, 20, 6903 RSC.
- J. M. Harris, R. J. Headrick, M. R. Semler, J. A. Fagan, M. Pasqualib and E. K. Hobbie, Nanoscale, 2016, 8, 7969 RSC.
- J. Lee, J. Y. Woo, J. T. Kim, B. Y. Lee and C.-S. Han, ACS Appl. Mater. Interfaces, 2014, 6, 10974 CAS.
- A. L. Gorkina, A. P. Tsapenko, E. P. Gilshteyn, T. S. Koltsova, T. V. Larionova, A. Talyzin, A. S. Anisimov, I. V. Anoshkin, E. I. Kauppinen, O. V. Tolochko and A. G. Nasibulin, Carbon, 2016, 100, 501 CrossRef CAS.
- L. He and S. C. Tjong, J. Mater. Chem. C, 2016, 4, 7043 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: SPES synthesis scheme, 1H NMR and FT-IR spectra for SPES. See DOI: 10.1039/c7ra01180f |
| This journal is © The Royal Society of Chemistry 2017 |