Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Ultrathin TiO2 nanosheets synthesized using a high pressure solvothermal method and the enhanced photoresponse performance of CH3NH3PbI3–TiO2 composite films†
Shilong Jiao‡
a,
Xianwei Fu‡a,
Gang Lian *a,
Laiying Jinga,
Zhenghao Xub,
Qilong Wangb and
Deliang Cui*a
*a,
Laiying Jinga,
Zhenghao Xub,
Qilong Wangb and
Deliang Cui*a
aState Key Lab of Crystal Materials, Shandong University, Jinan 250100, P. R. China. E-mail: liangang@sdu.edu.cn; cuidl@sdu.edu.cn
bKey Laboratory for Special Functional Aggregated Materials of Education Ministry, School of Chemistry & Chemical Engineering, Shandong University, Jinan 250100, P. R. China
First published on 11th April 2017
Abstract
In perovskite–TiO2 composite optoelectronic devices, a large grain size, a high percentage of {001} facets, TiO2 nanocrystals with high crystallinity, and good interconnectivity between both TiO2 and perovskite particles are crucial for the excellent performance of a hybrid photodetector. In this work, a high pressure hydrothermal method was developed to synthesize highly crystalline ultrathin (2–3 nm) TiO2 nanosheets bound with a high percentage of {001} facets. When these TiO2 nanosheets were utilized in the fabrication of perovskite–TiO2 composite photodetectors, a strikingly increased responsivity and detectivity were achieved. Furthermore, when the films were subjected to high pressure thermal treatment, both the particle size and the uniformity of the composite films greatly increased, resulting in a better performance of the hybrid photodetectors.
The power conversion efficiencies (PCEs) of organic–inorganic perovskite solar cells (PSCs) have rapidly reached a remarkable 22.1%,1 while it was only 3.8% (ref. 2) less than a decade ago. The small effective mass and high mobility of the carriers, a low exciton binding energy and an extremely long exciton diffusion length were found to be responsible for the unprecedented performance of the PSCs.3–9 Therefore, organic–inorganic perovskites can be widely utilized in the fields of gas sensors,10 photodetectors11–15 and lithium ion batteries16 etc. The perovskite CH3NH3PbI3 is an ideal active material for photovoltaic devices, as its band gap (∼1.5 eV) matches well with the energy of visible and near-IR light. So, the weakly bound excitons with lifetimes as long as ∼300 ns are another advantage for their application in photodetectors.17,18
TiO2, one of the most commonly used charge separation layer materials in solar cells, has been investigated intensively using both experimental and theoretical methods, including the tailoring of the facets,19 impurity doping,20 surface state simulations,21,22 photovoltaic and photocatalytic applications,22–24 and so on. In fact, the {001} facets of anatase TiO2 are believed to behave better than the {101} facets in both photovoltaic and photocatalytic applications22,25 because of their higher chemical reactivity. When combined with the perovskite, the bound {001} facets of anatase TiO2 nanocrystals exhibited much better performance in PSCs26 and photodetectors.27 Therefore, the successful synthesis of anatase TiO2 containing a high percentage of {001} facets (ref. 28) has attracted much attention. Generally, the introduction of F− is believed to be the most effective strategy to control the growth of the {001} facets of anatase TiO2 nanocrystals.29–34 However, excessive F− can severely etch the {001} facets of TiO2 nanoflakes and create some defects. Even now, it is still a great challenge to grow ultrathin TiO2 nanosheets with high crystallinity. It is known that high crystallinity and a low defect density are of great importance in both facilitating the transportation and reducing the recombination centers of photo-generated carriers at the interfaces among different kinds of materials. Therefore, it is essential to develop a modified strategy to grow highly crystalline ultrathin TiO2 nanosheets with a high percentage of {001} facets combining the effect of F−.
Our former work has shown that a high pressure can greatly increase the crystallinity and effectively control the growth of the nanocrystals in a solvothermal system.35 Herein, the ultrathin TiO2 nanosheets were firstly synthesized using a high pressure solvothermal method. They present high crystallinity and a high percentage of {001} facets. When they were composited with perovskites, the perovskite–TiO2 nanocomposite films exhibited a strikingly improved responsivity as photodetectors. Furthermore, after the film was treated using a thermal-press method, both the particle size and the uniformity of the film were greatly increased, resulting in the further improvement of their photoresponse performance.
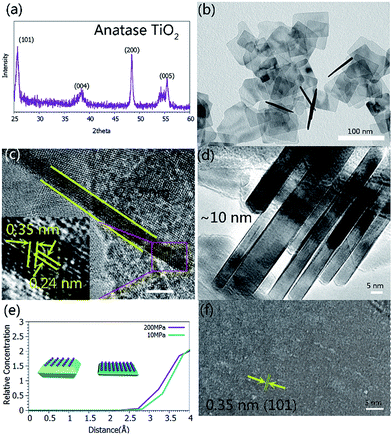
In a typical synthetic process, a certain amount of HF was added to the TBOT (tetrabutyl titanate) solution with stirring for over 30 min. After that, the solution was sealed in a hot-press autoclave (Fig. S1†) and heated to 200 °C at a rate of 5 °C min−1. After the reaction was maintained for 24 h at a pressure of 200 MPa, ultrathin TiO2 nanosheets formed. The details of the synthetic procedures are described in the ESI† (Experimental section). The product was first analyzed using powder X-ray diffraction (XRD). All of the diffraction peaks (Fig. 1a) match with anatase TiO2 (JCPDS no. 12-2172) without any crystalline impurities. As depicted in Fig. 1b and S2,† the as-obtained TiO2 crystals show a uniform rectangular shape and are 50–100 nm in lateral dimension. The high transparency of the TiO2 nanosheets with respect to the electron beam indicates the ultrathin characteristic of the sample. A typical cross-sectional view shows a thickness of 2–3 nm for the TiO2 nanosheets (Fig. 1c), which is much thinner than those synthesized using traditional solvothermal methods (Fig. 1d). The suppressed growth of the {001} facets can be deduced from the enhanced adsorption of F− on the (001) plane at high pressure. Molecular dynamics simulation results indicated that more F− could be strongly absorbed on the {001} facets at high pressure than at a lower pressure (Fig. 1e), which could obviously suppress the further growth of the nanosheets along the [001] direction at high pressure. Then, ultrathin TiO2 highly crystalline nanosheets were obtained. The lattice fringes in the magnified HRTEM images are ascribed to the (001) and (101) planes (the inset in Fig. 1c). The top-view HRTEM image of the TiO2 nanosheets shows the single-crystal character of the nanosheets without any obvious defects. The lattice with a spacing of ∼0.35 nm can be indexed to the {101} set of planes (Fig. 1f). To illustrate the effect of F− on the control of the thickness and defect density, we conducted a series of F− concentration dependent experiments. It was found that thinner nanosheets were obtained when increasing the F− concentration from 8 wt% (HF-2) to 15 wt% (HF-4) (Fig. S2–S4†). However, when it was continually increased to 27 wt% (HF-8), the obtained TiO2 nanosheets were obviously etched and a large amount of dot defects appeared on the {001} facets (Fig. S5†). So, the synergetic effect of F− and high pressure induces the formation of highly crystalline ultrathin TiO2 nanosheets.
After the synthesis of highly crystalline and ultrathin anatase TiO2 nanosheets, they were composited with CH3NH3PbI3 to make a photodetector film device. As mentioned above, firstly, high crystallinity and a low defect density of anatase TiO2 nanosheets can effectively suppress the recombination of the electrons and holes. Secondly, ultrathin TiO2 nanosheets with a high percentage of {001} facets can more effectively achieve photo-generated carrier transfer on the interfaces.36–41 Therefore, the synthesized TiO2 nanosheets are expected to be a promising candidate for enhancing the photoresponse property of CH3NH3PbI3 films. In addition, the interface contact of TiO2 and CH3NH3PbI3 also plays a crucial role in improving the behavior of the device.42–46 It is known that the carriers in the device can easily recombine at the defects and the crystal boundaries that can act as the trapping medium in semiconductors, which is harmful for the device’s performance and should be avoided.47–49 Mohite et al. have successfully fabricated perovskite solar cells with millimeter-scale grains that showed a hysteresis-free photovoltaic response.50 This was attributed to reduced bulk defects and improved charge carrier mobility in the large-grain devices. In addition, Yang et al. believed that the carrier recombination in the absorber could be suppressed by carefully controlling the formation of the perovskite layer with large-area grains and low defect density,51 facilitating carrier injection into the carrier transport layers. So, preparing a detector with a low defect density, few crystal boundaries and large grains will be desirable.
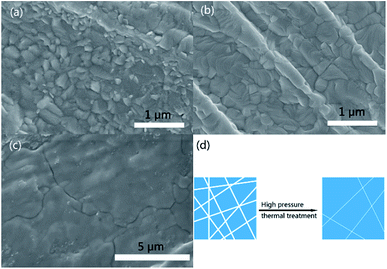
Herein, a novel high pressure thermal treatment method was developed to intensify the interface contact between the TiO2 nanosheets and the CH3NH3PbI3 particles. The details of the procedures are described in the ESI.† As shown in Fig. 2a, the pristine perovskite film mainly consisted of small-sized, non-uniform and irregular particles. The high density grain boundaries, combined with the rough surface of the film, easily resulted in weak photo-generated carrier transfer in the film. When the CH3NH3PbI3–TiO2 films were prepared using a typical spin-coating process, the composite films still presented a similar morphology to the pristine perovskite film (Fig. 2b). In comparison, when the typical film-forming process was followed by a thermal-press treatment, the composite film became closely packed and was constructed of much larger grains that were tens of microns in size (Fig. 2c and d), which facilitates the transport of the photo-generated carriers in the composite film. The thickness of the composite film after the high pressure thermal treatment was 1–2 μm (Fig. S6†). Additionally, the high pressure could smooth the film and undoubtedly promote the interfacial contact between CH3NH3PbI3 and the TiO2 nanosheets. Therefore, an enhanced photoresponse performance can be anticipated for the film. To further illustrate the superiority of the highly crystalline ultrathin nanosheets, TiO2 nanoflakes with different thicknesses and defect densities were also composited with CH3NH3PbI3 for comparison.
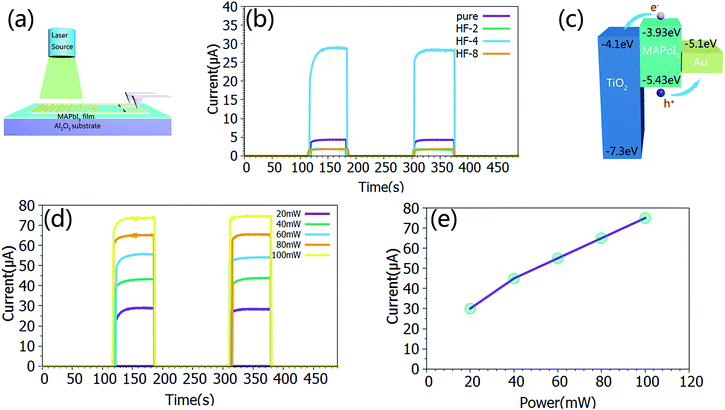
Because of the excellent optoelectronic characteristics of TiO2 nanosheets and CH3NH3PbI3,52–56 the composite film was expected to exhibit a strong response towards light illumination. The photoresponse properties of the composite films were investigated in air at room temperature. Fig. 3a presents a schematic diagram of the photodetector fabricated on an Al2O3 substrate. The dynamic response curves of the film devices under 532 nm laser irradiation with a power intensity of 20 mW cm−2 are presented in Fig. 3b. The responsivity (R), the ratio of the photocurrent to the incident light intensity, indicates how efficiently the detector responds to an optical signal. R is defined as follows:
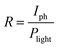 | (1) |
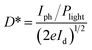 | (2) |
In our case, the calculated responsivity is 0.25 mA W−1 for the pure perovskite and 1.5 mA W−1 for the best performing sample (HF-4) which is about 6 times higher than that of the pure one. The reason for the increase in the responsivity is derived from the well-aligned band structure of the device (Fig. 3c) which allows for rapid charge-carrier separation at the interface, preventing photo-excited electrons in TiO2 from recombining with holes. Interestingly, the other two samples, namely sample (HF-2) and sample (HF-8), showed a lower responsivity than even the pure perovskite. The reason is still not very clear. The calculated specific detectivity for the sample (HF-4) is 2.5 × 1010 Jones (Jones = cm Hz1/2 W−1) with an incident laser wavelength of 532 nm. In addition to the above mentioned two important parameters, the linear relationship of the photocurrent with the incident power of the laser is also very crucial to the device’s performance. Taking sample (HF-4) as the representative sample, its response to a series of laser power intensities is presented in Fig. 3d and S7,† which clearly indicates a nearly linear relationship between the photocurrent and laser power with the R-square value of 0.98907 (Fig. 3e).
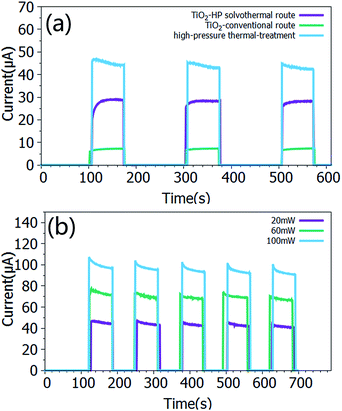
On the other hand, the TiO2 nanosheets, that were synthesized using a conventional solvothermal method and are denoted as “conventional TiO2 nanocrystals” (Fig. S4†), were also applied in the fabrication of perovskite–TiO2 composite film photodetectors (Fig. S8†), in order to compare the photoresponse performances of them with that of the sample (HF-4). The calculated responsivity for the device that was prepared with the conventional TiO2 nanocrystals is only 0.375 mA W−1 (Fig. 4a), which is a little higher than that of the pure perovskite TiO2 nanocrystals. The photocurrent of the former is almost three times higher than that of the latter. Furthermore, when the sample (HF-4) was treated using a high pressure annealing method, it exhibited a more advanced photo-detecting performance with a calculated responsivity of 2.4 mA W−1 (Fig. 4a). The high pressure thermally treated device also showed great linearity between the incident laser power and the photocurrent (Fig. 4b).
As one of the most popular carrier transport materials in perovskite solar cells, anatase TiO2 nanostructures have been intensively investigated in order to achieve an optimized performance. In order to fully understand the interaction between CH3NH3PbI3 and the TiO2 nanosheets in the composite film, and the response mechanism, we conducted molecular dynamics simulations with an NVT ensemble. A detailed description of the computational process can be found in the ESI.† The radial distribution functions of the two-coordinated oxygen atoms (O2c) in TiO2 and the hydrogen atoms on the alkyl- (CH–O2c) and amino- (NH–O2c) groups are presented in Fig. 5a. A strong peak at 1.87 Å can be observed for the NH⋯O2c RDF, while the first peak for the CH⋯O2c RDF appears at 2.08 Å, indicating that the interaction of NH⋯O2c is obviously stronger than that of CH⋯O2c. In comparison, the RDF of I⋯O2c exhibits the first strong peak at 3.01 Å (Fig. 5b), which is much larger than that of CH⋯O2c and NH⋯O2c, revealing a strikingly weaker interaction between the iodine atom and O2c. Similarly, the interactions between the iodine atom and O3c (a three-coordinated oxygen atom in TiO2) and Ti5c (a five-coordinated Ti atom in TiO2) are even weaker. These results reveal that the dominant interfacial interactions in the TiO2–perovskite composite exist between the hydrogen atom on the amino-group and the unsaturated oxygen atom in anatase TiO2. In addition, because there are more unsaturated O2c atoms on the {001} facets of anatase TiO2, stronger interactions exist between the perovskite and the bound {001} facets of the TiO2 nanocrystals, which are inevitably more favorable for the transportation of the photo-generated charge carriers between them.
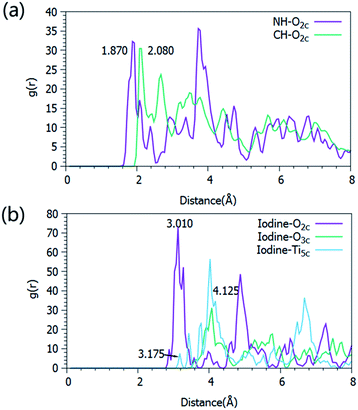 | ||
| Fig. 5 Radial distribution functions (RDFs) of NH⋯O2c and CH⋯O2c (a), and I⋯O2c, I⋯O3c and I⋯Ti5c (b). | ||
In conclusion, we first developed a high pressure solvothermal method for the synthesis of highly crystalline ultrathin (2–3 nm) TiO2 nanosheets bound with a high percentage of {001} facets. The high pressure plays a key role in confining the growth of the nanosheets along the [001] direction, combined with the effect of F−. The CH3NH3PbI3–TiO2 composite films were prepared as hybrid photodetectors. After high pressure thermal treatment of the films, there was obvious growth of the grains, improving their interfacial contact and interconnectivity. As a result, the films presented obviously enhanced responsivity and detectivity, derived from the strikingly improved efficiencies of the photo-generated exciton dissociation and the carrier transportation. Therefore, it is reasonable to believe that the high pressure thermal treatment strategy can be expected to improve the performance of perovskite photovoltaic devices in the future.
Acknowledgements
This work was supported by the Natural Science Foundation of China (NSFC51372143, 51102151, 50990061), Natural Science Foundation of Shandong Province (ZR2011EMQ002, 2013GGX10208) and Independent Innovation Foundation of Shandong University (2012GN051).Notes and references
- M. Saliba, T. Matsui, K. Domanski, J.-Y. Seo, A. Ummadisingu, S. M. Zakeeruddin, J.-P. Correa-Baena, W. R. Tress, A. Abate, A. Hagfeldt and M. Grätzel, Science, 2016, 354, 206–209 CrossRef CAS PubMed.
- A. Kojima, K. Teshima, Y. Shirai and T. Miyasaka, J. Am. Chem. Soc., 2009, 131, 6050–6051 CrossRef CAS PubMed.
- M. B. Johnston and L. M. Herz, Acc. Chem. Res., 2016, 49, 146–154 CrossRef CAS PubMed.
- G. E. Eperon, S. D. Stranks, C. Menelaou, M. B. Johnston, L. M. Herz and H. J. Snaith, Energy Environ. Sci., 2014, 7, 982–988 CAS.
- S. Luo and W. A. Daoud, J. Mater. Chem. A, 2015, 3, 8992–9010 CAS.
- Q. Lin, A. Armin, R. Chandra, R. R. C. R. Nagiri, P. L. Burn, P. Meredith, G. E. Eperon, S. D. Stranks, C. Menelaou, M. B. Johnston, L. M. Herz, H. J. Snaith, N. J. Jeon, J. H. Noh, Y. C. Kim, W. S. Yang, S. Ryu and S. Il Seok, Nat. Mater., 2014, 13, 1–7 Search PubMed.
- Q. Lin, A. Armin, R. Chandra, R. R. C. R. Nagiri, P. L. Burn and P. Meredith, Nat. Photonics, 2014, 9, 106–112 CrossRef.
- D. Shi, V. Adinolfi, R. Comin, M. Yuan, E. Alarousu, A. Buin, Y. Chen, S. Hoogland, A. Rothenberger, K. Katsiev, Y. Losovyj, X. Zhang, P. A. Dowben, O. F. Mohammed, E. H. Sargent and O. M. Bakr, Science, 2015, 347, 519–522 CrossRef CAS PubMed.
- S. D. Stranks, S. D. Stranks, G. E. Eperon, G. Grancini, C. Menelaou, M. J. P. Alcocer, T. Leijtens, L. M. Herz, A. Petrozza and H. J. Snaith, Science, 2014, 342, 341–344 CrossRef PubMed.
- Y. Liu, Y. Luo, A. A. Elzatahry, W. Luo, R. Che, J. Fan, K. Lan, A. M. Al-enizi, Z. Sun, B. Li, Z. Liu, D. Shen, Y. Ling and C. Wang, ACS Cent. Sci., 2015, 1, 400–440 CrossRef CAS PubMed.
- H. R. Xia, J. Li, W. T. Sun and L. M. Peng, Chem. Commun., 2014, 50, 13695–13697 RSC.
- X. Hu, X. Zhang, L. Liang, J. Bao, S. Li, W. Yang and Y. Xie, Adv. Funct. Mater., 2014, 24, 7373–7380 CrossRef CAS.
- X. Hu, K. Wang, C. Liu, T. Meng, Y. Dong, S. Liu, F. Huang, X. Gong and Y. Cao, J. Mater. Chem. C, 2014, 2, 9592–9598 RSC.
- Y. Zhang, J. Du, X. Wu, G. Zhang, Y. Chu, D. Liu, Y. Zhao, Z. Liang and J. Huang, ACS Appl. Mater. Interfaces, 2015, 21634–21638 CAS.
- C. Bao, W. Zhu, J. Yang, F. Li, S. Gu, Y. Wang, T. Yu, J. Zhu, Y. Zhou and Z. Zou, ACS Appl. Mater. Interfaces, 2016, 8, 23868–23875 CAS.
- H.-R. Xia, W. Sun and L.-M. Peng, Chem. Commun., 2015, 51, 13787–13790 RSC.
- A. Marchioro, J. Teuscher, D. Friedrich, M. Kunst, R. van de Krol, T. Moehl, M. Gratzel and J.-E. Moser, Nat. Photonics, 2014, 8, 250–255 CrossRef CAS.
- C. Wehrenfennig, G. E. Eperon, M. B. Johnston, H. J. Snaith and L. M. Herz, Adv. Mater., 2014, 26, 1584–1589 CrossRef CAS PubMed.
- G. Liu, H. G. Yang, J. Pan, Y. Q. Yang, G. Q. M. Lu and H. M. Cheng, Chem. Rev., 2014, 114, 9559–9612 CrossRef CAS PubMed.
- Y. Bai, F. D. Angelis, J. Bisquert and P. Wang, Chem. Rev., 2014, 114, 10131–10176 CrossRef PubMed.
- R. Asahi, T. Morikawa, H. Irie and T. Ohwaki, Chem. Rev., 2014, 114, 9824–9852 CrossRef CAS PubMed.
- F. De Angelis, C. Di Valentin, S. Fantacci, A. Vittadini and A. Selloni, Chem. Rev., 2014, 114, 9708–9753 CrossRef CAS PubMed.
- K. Bourikas, C. Kordulis and A. Lycourghiotis, Chem. Rev., 2014, 114, 9754–9823 CrossRef CAS PubMed.
- Y. Ma, X. L. Wang, Y. S. Jia, X. B. Chen, H. X. Han and C. Li, Chem. Rev., 2014, 114, 9987–10043 CrossRef CAS PubMed.
- Y. Bai, I. Mora-Seró, F. De Angelis, J. Bisquert and P. Wang, Chem. Rev., 2014, 114, 10095–10130 CrossRef CAS PubMed.
- M. I. Dar, F. J. Ramos, Z. Xue, B. Liu, S. Ahmad, S. A. Shivashankar, M. K. Nazeeruddin and M. Gratzel, Chem. Mater., 2014, 26, 4675–4678 CrossRef CAS.
- H. R. Xia, J. Li, W. T. Sun and L. M. Peng, Chem. Commun., 2014, 50, 13695–13697 RSC.
- H. G. Yang, C. H. Sun, S. Z. Qiao, J. Zou, G. Liu, S. C. Smith, H. M. Cheng and G. Q. Lu, Nature, 2008, 453, 638–641 CrossRef CAS PubMed.
- X. Zhao, W. Jin, J. Cai, J. Ye, Z. Li, Y. Ma, J. Xie and L. Qi, Adv. Funct. Mater., 2011, 21, 3554–3563 CrossRef CAS.
- J. S. Chen, C. Chen, J. Liu, R. Xu, S. Z. Qiao and X. W. Lou, Chem. Commun., 2011, 47, 2631–2633 RSC.
- J. Yu, J. Low, W. Xiao, P. Zhou and M. Jaroniec, J. Am. Chem. Soc., 2014, 136, 8839–8842 CrossRef CAS PubMed.
- J. Y. Zheng, S. H. Bao, Y. Guo and P. Jin, ACS Appl. Mater. Interfaces, 2014, 6, 5940–5946 CAS.
- Z. Wang, K. Lv, G. Wang, K. Deng and D. Tang, Appl. Catal., B, 2010, 100(1–2), 378–385 CrossRef CAS.
- K. Lv, B. Cheng, J. Yu and G. Liu, Phys. Chem. Chem. Phys., 2012, 14(16), 5349 RSC.
- J. Wang, G. Lian, H. Si, Q. Wang, D. Cui and C. P. Wong, ACS Nano, 2016, 10, 405–412 CrossRef CAS PubMed.
- T. Tachikawa, S. Yamashita and T. Majima, J. Am. Chem. Soc., 2011, 133, 7197–7204 CrossRef CAS PubMed.
- X. H. Yang, Z. Li, G. Liu, J. Xing, C. Sun, H. G. Yang and C. Li, CrystEngComm, 2011, 13, 1378 RSC.
- J. Yu, J. Low, W. Xiao, P. Zhou and M. Jaroniec, J. Am. Chem. Soc., 2014, 136, 8839–8842 CrossRef CAS PubMed.
- N. Roy, Y. Sohn and D. Pradhan, ACS Nano, 2013, 7, 2532–2540 CrossRef CAS PubMed.
- X. H. Yang, Z. Li, C. Sun, H. G. Yang and C. Li, Chem. Mater., 2011, 23, 3486–3494 CrossRef CAS.
- J. Yu, J. Fan and K. Lv, Nanoscale, 2010, 2, 2144–2149 RSC.
- J. Min, Z. Zhang, Y. Hou, C. O. Ramírez Quiroz, T. Przybilla, C. Bronnbauer, F. Guo, K. Forberich, H. Azimi, T. Ameri, E. Spiecker, Y. Li and C. J. Brabec, Chem. Mater., 2015, 27(1), 227–234 CrossRef CAS.
- B. Bouthinon, R. Clerc, J. Vaillant, J. M. Verilhac, J. Faure-Vincent, D. Djurado, I. Ionica, G. Man, A. Gras, G. Pananakakis, R. Gwoziecki and A. Kahn, Adv. Funct. Mater., 2015, 25(7), 1090–1101 CrossRef CAS.
- J. Qi, X. Zhou, D. Yang, W. Qiao, D. Ma and Z. Y. Wang, Adv. Funct. Mater., 2014, 24(48), 7605–7612 CrossRef CAS.
- Y. S. Lee, J. Heo, S. C. Siah, J. P. Mailoa, R. E. Brandt, S. B. Kim, R. G. Gordon and T. Buonassisi, Energy Environ. Sci., 2013, 6(7), 2112–2118 CAS.
- Y. S. Lee, J. Heo, S. C. Siah, J. P. Mailoa, R. E. Brandt, S. B. Kim, R. G. Gordon and T. Buonassisi, Energy Environ. Sci., 2013, 6(7), 2112–2118 CAS.
- F. Hao, C. C. Stoumpos, Z. Liu, R. P. H. Chang and M. G. Kanatzidis, J. Am. Chem. Soc., 2014, 136, 16411–16419 CrossRef CAS PubMed.
- Y. Li, Y. Zhao, Q. Chen, Y. Yang, Y. Liu, Z. Hong, Z. Liu, Y. T. Hsieh, L. Meng, Y. Li and Y. Yang, J. Am. Chem. Soc., 2015, 137, 15540–15547 CrossRef CAS PubMed.
- L. F. Yang, X. G. Yu, M. S. Xu, H. Z. Chen and D. R. Yang, J. Mater. Chem. A, 2014, 2, 16877–16883 CAS.
- W. Nie, H. Tsai, R. Asadpour, J.-C. Blancon, A. J. Neukirch, G. Gupta, J. J. Crochet, M. Chhowalla, S. Tretiak, M. A. Alam, H.-L. Wang and A. D. Mohite, Science, 2015, 347(6221), 522–525 CrossRef CAS PubMed.
- H. Zhou, Q. Chen, G. Li, S. Luo, T. Song, H.-S. Duan, Z. Hong, J. You, Y. Liu and Y. Yang, Science, 2014, 345(6196), 542–547 CrossRef CAS PubMed.
- W. Chen, Y. Wu, Y. Yue, J. Liu, W. Zhang, X. Yang, H. Chen, E. Bi, I. Ashraful, M. Grätzel and L. Han, Science, 2015, 350, 944–948 CrossRef CAS PubMed.
- H. S. Jung and N.-G. Park, Small, 2014, 1–16 Search PubMed.
- M. I. Saidaminov, M. A. Haque, M. Savoie, A. L. Abdelhady, N. Cho, I. Dursun, U. Buttner, E. Alarousu, T. Wu and O. M. Bakr, Adv. Mater., 2016, 1–6 Search PubMed.
- H.-S. Kim, J.-W. Lee, N. Yantara, P. P. Boix, S. A. Kulkarni, S. Mhaisalkar, M. Grätzel and N.-G. Park, Nano Lett., 2013, 13, 2412–2417 CrossRef CAS PubMed.
- O. Malinkiewicz, A. Yella, Y. H. Lee, G. M. M. Espallargas, M. Graetzel, M. K. Nazeeruddin and H. J. Bolink, Nat. Photonics, 2014, 8, 128–132 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra01073g |
| ‡ These authors contributed equally to the work. |
| This journal is © The Royal Society of Chemistry 2017 |