 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Thermodynamic stabilization of semiclathrate hydrates by hydrophilic group
S. Muromachi*a,
R. Kamob,
T. Abeb,
T. Hiakib and
S. Takeyaa
aNational Institute of Advanced Industrial Science and Technology (AIST), 16-1 Onogawa, Tsukuba 305-8569, Japan. E-mail: s-muromachi@aist.go.jp
bCollege of Industrial Technology, Nihon University, 1-2-1 Izumicho, Narashino 275-8575, Japan
First published on 28th February 2017
Abstract
Introducing hydrophilic groups into carboxylates is a way to modify semiclathrate hydrate frameworks and change the properties of the hydrates. In this study, we report the characterization of semiclathrate hydrates formed by tetra-n-butylammonium (TBA) 2-hydroxybutyrate (2HB). In addition, TBA lactate and the TBA 2HB salt formed stable hydrate crystals, which basically had rectangular columnar shapes. We performed equilibrium measurements and calorimetry. The melting temperature and fusion heat of the TBA 2HB hydrate crystals were 285.3 K and 177 kJ kg−1, respectively. A comparison with other carboxylate anions showed that the substitution of hydrogen atom at the 2-position in the carbon chain by a hydrophilic hydroxy group stabilizes the hydrates more than that by hydrophobic methyl group, which is the case for alcohols in clathrate hydrates. The phase equilibrium data for a number of semiclathrate hydrates were compared. A rough trend of temperature depending on type of guest anions was observed, but it is unclear if there are other correlating factors.
Introduction
Semiclathrate hydrates are host–guest compounds that are analogous crystalline materials to clathrate hydrates.1,2 Ionic guest substances, which are necessary to form semiclathrate hydrates, form hydrogen bonds with water molecules in their structures. With various ionic guest substances, the semiclathrate hydrates have a wide melting temperature range such as 270–300 K.1–3 The stabilities of semiclathrate hydrates can be modified with the choice of ionic guest cation and counter anion, which are both incorporated in the hydrate structure with different coordination to the hydrate cages. This structure also provides unique properties of melting temperatures and gas capture.3–12 By the combination of ions,3–5 control of functional properties of these materials, such as melting temperature, latent heat, gas selectivity, and gas capacity, is expected. Above all, semiclathrate hydrates have attracted considerable attention as one of phase change materials for thermal storage.6Ionic guest substances are ions of which cations usually have suitably-sized alkyl chains. Tetra-n-butylammonium (TBA) and tetra-n-butylphosphonium (TBP) cations are used widely as ionic guest molecules because of their good availability, as recently known for ionic liquids.7 The options for the counter anions vary: halogens and organic acids, such as carboxylates and hydroxycarboxylates.3,4 In the structures of semiclathrate hydrates of TBA and TBP salts, water molecules form a cage-like network, such as 512, 51262 and 51263 cages, where 512 denotes a 12-hedron composed of pentagonal faces. The TBA and TBP cations occupy a four-cage fused cage by the four butyl chains. Ionic guest anions usually form hydrogen-bonds with the host water molecules.1,2 The melting temperatures of semiclathrate hydrates may represent overall suitability of the anions including size, shape and affinity for water. With halogen anions, the melting temperatures of hydrates have clear tendencies, which decrease with increasing anion size.3,13 In the case of carboxylate anions, contrary to halogens, there is a suitable alkyl chain length for stabilization of the hydrate structure because propionate and acrylate anions provide the highest temperature.3,4,9 This is because the carboxy group of the carboxylate anions forms hydrogen-bonds with cage water molecules and occupies the hydrate cages.14–16 In the case of propionate and acrylate anions, the hydrophobic alkyl chain is directed at the cage centre and stabilizes the 512 cage.14,16 With a longer alkyl chain, the carboxylate anion occupies a larger cage, such as a valerate anion incorporated in a 51262 cage,12 where 51262 denotes a 14-hedron composed of 12 pentagonal faces and 2 hexagonal faces.
In addition to the canonical clathrate hydrates,17,18 a side chain of the guest is a key to stabilizing the semiclathrate hydrates mainly because of its size and shape effects.1–3 For semiclathrate hydrates, substitutions by a methyl group at the 2-position of propionate and butyrate anions, i.e., 2-methylpropionate and 2-methylbutyrate, destabilizes the melting temperatures.3,4 With the substitution of a side chain by a hydrophilic group, the functional properties of the semiclathrate hydrates may be changed. In a previous study, we reported the incorporation of a lactate anion in the hydrate cages, which was a hydroxycarboxylate with a hydroxy group substituting a hydrogen at the 2-position in propionate.10
(Fig. 1) It was found that both of the carboxy and hydroxy groups in the lactate anion formed hydrogen bonds with the water molecules of the 512 cage. This supports the fact that hydroxy group can stabilize the semiclathrate hydrate. Owing to this coordination, the methyl group in the lactate anion showed off centred orientation in the distorted 512 cage (Fig. 1). This is different from propionate and acrylate anions, of which the carbon chains are located in the centre of the 512 cage.14,16 With a longer alkyl chain, hydroxycarboxylates may further stabilize the hydrate structure. For thermal storage applications of semiclathrate hydrates, the selection criteria of potential or promising guest molecule of semiclathrate hydrates are necessary. In this respect, knowledge of the melting temperature tendency depending on the type of guest anions is valuable. Herein, we report semiclathrate hydrates of the TBA 2-hydroxybutyrate (2HB). The 2HB anion has one additional alkyl chain to the lactate anion, which may stabilize the 512 cage due to its size (Fig. 1). The thermodynamic stability of the TBA 2HB hydrate is discussed based on a comparison of the melting temperatures of the propionate and butyrate classes.
 | ||
| Fig. 1 Possible coordination structure of the hydroxybutyrate anions in the 512 cage based on the ref. 10. The structure was visualized by the VESTA program.19 The red, grey and white colours indicate oxygen, carbon and hydrogen atoms or bonds, respectively. In the 512 hydrate cage, the atoms of the lactate anions are shown as spheres. The lattice water is only shown by bonds, and the hydrogen atoms are omitted for clarity. Herein, the methyl group of 2HB anion (dashed line) may be encaged. | ||
Experimental
Materials
We used DL-2-hydroxybutyric acid (Tokyo Chemical Industry Co., Ltd., >95.0%) and tetra-n-butylammonium hydroxide (40 mass% solution in water, Alfa Aesar Inc.) for the synthesis of TBA 2HB. Water with a resistivity of 18.2 MΩ cm at 298 K and less than 5 ppb of total organic content was used. Tetra-n-butylammonium bromide (Sigma-Aldrich, Co., >99.0%) and c-hexane (Wako Pure Chemical Industries, Ltd., 99.8%) was used as received from the provider. The TBA lactate was the same as used in a previous study.10 For the nuclear magnetic resonance (NMR) measurements, deuterium oxide (Wako Pure Chemical Industries, Ltd., 99.9%) and deuteriochloroform (Wako Pure Chemical Industries, Ltd., 99.8% containing 0.05 vol% tetramethylsilane) were used.Synthesis and characterization of TBA 2HB
TBA 2HB aqueous solution was synthesized by the neutralization of DL-2-hydroxybutyric acid by equimolal tetra-n-butylammonium hydroxide. About 1 g of the synthesized solution was dried with the aid of an evaporator, and the water content was determined based on the mass difference between dry and wet conditions. The dried salt was mixed with a solvent and subjected to solution-state 1H and 13C NMR measurements to determine the ratio of the TBA cation and the 2HB anion. We used an NMR spectrometer (Avance 500, Bruker Biospin, Bruker Co.). The measurements were made at temperature (∼295 K), and the number of acquisitions was 16 and 1000 for the 1H and 13C measurements, respectively. The spectra showed an equivalent cation to anion ratio in the synthesized solution.Phase equilibrium measurements
We employed the optical observation method for the phase equilibrium measurements.10 The samples of TBA 2HB aqueous solutions were prepared with 14-different concentrations in the range of TBA 2HB mole fractions (x) between 0.0029 and 0.0424. Furthermore, 2 g of the solutions was injected in a glass tube sealed with a lid, and the glass tube was set in a temperature-controlled water bath. Once the hydrate crystals were grown at a low temperature, the system temperature was increased stepwise with a step of 0.1 K. At each temperature step, we observed the hydrate crystals using a microscope equipped with a CCD camera. After we visually confirmed that the hydrate crystals did not melt further, we raised the system temperature. This waiting process took more than week in the longest case. The temperature before the temperature at which we observed complete dissociation of the hydrate crystals was determined to be the phase equilibrium temperature. The measurement uncertainties of the concentration and temperature were 0.0012 in mole fraction of TBA 2HB and 0.1 K, respectively.DSC measurements
The samples for the DSC measurements were aqueous solutions of TBA lactate and TBA 2HB, which have compositions close to their hydrate crystals. After the phase equilibrium measurements, we formed TBA 2HB hydrate crystals in the aqueous solution with x = 0.0255 at 282.9 K. For TBA lactate hydrates, we formed crystals at 282.7 K from the aqueous solution with x = 0.0228. These crystals were melted and the solution was subjected to the DSC measurements. The compositions of the solutions were determined by the measurements of the water content by evaporation. An aqueous solution of 12–18 mg was injected in an aluminium sealing pan of which the volume was 24 μL. The mass of the cell was measured before and after sealing, and the mass difference was the mass of the sample. An electronic mass balance (AUW220D, Shimadzu Co.) was used, and the mass of the sample solution injected in the cell was determined within 0.0004 mg of uncertainty.We used a differential scanning calorimeter (DSC-60, Shimadzu Co.). Nitrogen gas was made to flow to the measurement chamber. An empty reference cell, which was the same as sealing-type aluminium one, was used. To crystallize the sample in the first attempt, supercooling to ∼250 K was usually needed, and at this temperature, ice could also form. Therefore, we employed the annealing process20 to avoid the formation of ice in the cell. After the first crystallization, the cell temperature was raised near the melting point of the hydrate, i.e., ∼283 K, and the cell was cooled again to a temperature just over 273 K. After repeating this process a few times, we obtained good DSC curves without an ice peak. We employed a temperature increasing rate of 1 K min−1 for c-hexane and 2 K min−1 for the other samples. The measurements were implemented three times for a sample, and average value was obtained. The standard deviation of each measurement was less than 0.06 J.
We used c-hexane for the reference, and heat of fusion was calibrated from the value 31.84 kJ kg−1 reported by Domalski.21 To check the reliability of our measurements by a comparison with the reference data, we performed DSC measurements for TBA Br hydrate. We determined the accuracy of the measurements to be 6 kJ kg−1.
Results and discussion
Results of phase equilibrium measurements are shown in Table 1 and plotted in Fig. 2. With x = 0.0029, the equilibrium temperature was lower than the freezing point of ice. The equilibrium temperatures increased with increasing concentration. The highest equilibrium temperature, which may be equivalent to the melting temperature of the TBA 2HB hydrate, was 285.3 K at x = 0.0331 and 0.0360. The equilibrium temperature decreased slightly at a higher concentration, i.e., x = 0.00424. The concentrations having the highest equilibrium temperature suggest that the hydrate crystals have a hydration number of approximately 26 to 30, which corresponds to the semiclathrate hydrate structures of cubic or tetragonal. The melting points of TBA 2HB hydrates are slightly higher than those of the TBA lactate hydrates. These results suggest that the difference in carbon chain length barely affects the melting temperature.| Mole fraction (x) (×10−2) | Mass fraction | Temperature (K) |
|---|---|---|
| 0.29 | 0.052 | 272.4 |
| 0.60 | 0.104 | 273.6 |
| 0.96 | 0.157 | 278.7 |
| 1.36 | 0.209 | 281.5 |
| 1.62 | 0.240 | 282.7 |
| 1.90 | 0.271 | 283.5 |
| 2.11 | 0.292 | 284.1 |
| 2.32 | 0.313 | 284.4 |
| 2.55 | 0.334 | 284.8 |
| 2.78 | 0.355 | 284.9 |
| 3.04 | 0.375 | 285.0 |
| 3.31 | 0.397 | 285.3 |
| 3.60 | 0.417 | 285.3 |
| 4.24 | 0.459 | 284.8 |
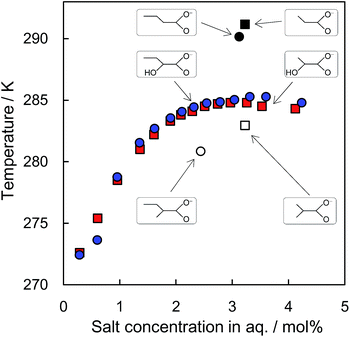 | ||
| Fig. 2 Phase equilibrium data of TBA 2HB hydrates in comparison with the other TBA salt hydrates. ○ with blue, TBA 2HB hydrate (this study); ○ with black, TBA butyrate;3 ○ with white, TBA 2-methylbutyrate;3 □ with red, TBA lactate hydrate;3 □ with black, TBA propionate;3 □ with white, TBA 2-methylpropionate.3 | ||
In the literature, characterizations of semiclathrate hydrates formed with TBA propionate (291.2 K)3 and TBA butyrate (290.2 K)3 were reported. The relationship of the melting point between the TBA 2HB hydrate and TBA lactate hydrate is similar to that between the hydrates of TBA propionate and TBA butyrate. The side chains of the both anions are substituted by a methyl group, which is in contrast to the relationship between lactate and 2-hydroxybutyrate anions.3,4 Fig. 2 compares the effects of the side chain substitution on the melting temperature and the hydrate composition. In the class of propionate, the side methyl group decreases the melting temperature with ∼8 K, i.e., 291.2 K with propionate and 283.0 K with 2-methylpropionate. The anion substituted with a hydroxy group, i.e., lactate anion, recovers the melting temperature with ∼2 K to (284.8 K). The butyrate class shows a different trend. The melting temperature with 2-methylbutyrate anion (280.9 K) is ∼10 K lower than that with butyrate (290.2 K). The hydration numbers also differ, i.e., 40 with 2-methylbutyrate and 31 with butyrate, which suggests that the substituted methyl group modifies and destabilizes the hydrate structure.3 As is the case in the propionate class, the presently used 2HB anion recovered the melting temperature to 285.3 K, but with a larger degree, i.e., ∼4 K, which is the same level as the melting temperature of TBA lactate hydrate.
Two types of crystal morphologies of the TBA 2HB hydrates were observed during the phase equilibrium measurements: rectangular–columnar crystals and block crystals at ∼283 K. With x = 0.0211, two different morphologies, i.e., thin and thick columnar crystals, were observed (Fig. 3A). With x = 0.0232, thick rectangular–columnar crystals were obtained (Fig. 3B). With x = 0.0255, columnar–rectangular crystals and block crystals both formed (Fig. 3C). With x = 0.0360, both crystals were observed, but the block type crystal appeared dominant in this system (Fig. 3D).
 | ||
| Fig. 3 Crystal morphologies of TBA 2HB hydrates. (A) x = 0.0211 at 283.2 K; (B) x = 0.0232 at 283.2 K; (C) x = 0.0255 at 282.9 K; (D) x = 0.0360 at 283.0 K. | ||
We performed single crystal X-ray diffraction on the same crystal as that for the DSC measurements by the method used in our earlier study.10 The present preliminary structure analysis showed the tetragonal structure with unit cell parameters for a = 23.3850 Å and c = 24.4019 Å and a possible space group P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) . This unit cell is twice the size of that of the TBA lactate hydrate with a = 23.361 and c = 12.248.10 The present diffraction data were not sufficient for structure refinement analysis, but they showed the tetragonal hydrate structure. This means that the 2HB anion slightly modified host water cages from those of the TBA lactate hydrate due to the longer carbon chain. Specifically, the cell extended 0.1% in the direction of the a-axis and shortened 0.4% in the direction of c-axis due to the change from TBA lactate to TBA 2HB.
. This unit cell is twice the size of that of the TBA lactate hydrate with a = 23.361 and c = 12.248.10 The present diffraction data were not sufficient for structure refinement analysis, but they showed the tetragonal hydrate structure. This means that the 2HB anion slightly modified host water cages from those of the TBA lactate hydrate due to the longer carbon chain. Specifically, the cell extended 0.1% in the direction of the a-axis and shortened 0.4% in the direction of c-axis due to the change from TBA lactate to TBA 2HB.
Table 2 summarizes the results of DSC measurements. Fig. 4 shows that the DSC data for TBA Br hydrate with x = 0.0370 (0.408 in mass fraction) was 193 kJ kg−1. Although there may be a minor peak before the major peak, which suggests the sample was a bi-phase, the fusion heat of the TBAB hydrates agreed well with the ref. 20, 22 and 23. The DSC curves of TBA 2HB hydrate and TBA lactate hydrate had similar peak depths to those of the TBA Br hydrate, but the heats of fusion for TBA 2HB and TBA lactate were significantly different, i.e., 177 and 191 kJ kg−1, respectively.
| Ionic guest | Ionic guest concentration in hydrate crystal (mass fraction) | Heat of fusion | |
|---|---|---|---|
| kJ kg−1 | kJ kg(water)−1 | ||
| a Determined from the masses between wet and dry conditions with the aid of evaporator.b Gravimetrically prepared to be the same composition as reported in the ref. 20, 22 and 23. | |||
| TBA 2HB | 0.396a | 177 | 294 |
| TBA lactate | 0.369a | 191 | 303 |
| TBA Br | 0.408b | 193 | 323 |
 | ||
| Fig. 4 DSC curves for the TBA lactate and TBA 2HB hydrates. ○ with blue, TBA 2HB hydrate; □ with red, TBA lactate; △, TBA Br hydrate. | ||
The mass fractions of the TBA 2HB solutions determined by evaporation were 0.396. This suggests a hydration number of approximately 30, which agreed with the value suggested by the phase equilibrium data. The dried sample analysed by 1H NMR spectroscopy showed that the ratios of the cation to the anion corresponded to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 both in the hydrate crystals of TBA 2HB and TBA lactate.
1 both in the hydrate crystals of TBA 2HB and TBA lactate.
The results obtained in this study suggest that an extraordinarily large and heavy anion can be incorporated in the hydrate cage by introducing a hydrophilic hydroxy group. The difference in carbon chain length of the ionic guest anion modified the melting temperature. This is not the case with clathrate hydrates, such as hydrocarbons and alcohols, which basically destabilize the structure.18,24 Further studies on ionic guests will give a variety of the properties of semiclathrate hydrates.
Conclusions
In this study, we investigated the modification of the thermodynamic stability of tetra-n-butylammonium (TBA) salt hydrates by hydroxycarboxylate anions. The 2-hydroxybutyrate (2HB) anion, which has one additional methyl group to lactate, formed ionic clathrate hydrates with the tetra-n-butylammonium cation. The crystal morphologies suggested mainly two different crystal structures. The melting temperature of the TBA 2HB hydrate was 285.3 K, which is close to that of the TBA lactate hydrate. In contrast, the heat of fusion was significantly lower than that of the TBA lactate hydrate and TBA Br hydrate. The single crystal X-ray diffraction analysis for the TBA 2HB hydrate showed the modification of the unit cell from that of TBA lactate hydrate. A comparison with 2-methylcarboxylate anions showed that the introduction of hydroxy group to the ionic guest anion recovered the melting temperatures of the hydrates, and hence, the stability, from substitution by a hydrophobic methyl group. The present study showed a way to modify the thermodynamic properties of semiclathrate hydrates by the functional group of an ionic guest.Acknowledgements
SM thanks Grant-in-Aid for Young Scientists (B) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (No. 26820069). We thank Takuma Nukazawa for his help on the DSC measurements, Dr Motoi Oshima and Michika Ohtake for their kind advices on DSC measurements, and Dr Yoshitaka Yamamoto for discussion.Notes and references
- Y. A. Dyadin and K. A. Udachin, J. Struct. Chem., 1987, 28, 75 CrossRef CAS.
- G. A. Jeffrey, in Inclusion Compounds, ed. J. L. Atwood, J. E. D. Davies and D. D. MacNicol, Academic Press, London, 1984, vol. 1, ch. 5 Search PubMed.
- H. Nakayama and S. Torigata, Bull. Chem. Soc. Jpn., 1984, 57, 171 CrossRef CAS.
- Y. A. Dyadin, L. A. Gaponenk, L. S. Aladko and S. V. Bogatyryova, J. Inclusion Phenom., 1984, 2, 259 CrossRef CAS.
- S. Muromachi and S. Takeya, Fluid Phase Equilib. DOI:10.1016/j.fluid.2017.01.008.
- S. Wenji, X. Rui, H. Chong, H. Shihui, D. Kaijun and F. Ziping, Int. J. Refrig., 2009, 32, 1801 CrossRef CAS; P. Zhang, Z. W. Ma and R. Z. Wang, Renew. Sustain. Energy Rev., 2010, 14, 598 CrossRef; H. Kumano, T. Hirata and T. Kudoh, Int. J. Refrig., 2011, 34, 1963 CrossRef; J. Douzet, M. Kwaterski, A. Lallemand, F. Chauvy, D. Flick and J.-M. Herri, Int. J. Refrig., 2013, 36, 1616 CrossRef.
- N. V. Plechkova and K. R. Seddon, Chem. Soc. Rev., 2008, 37, 123 RSC.
- A. Chapoy, R. Anderson and B. Tohidi, J. Am. Chem. Soc., 2007, 129, 746 CrossRef CAS PubMed; N. H. Duc, F. Chauvy and J.-M. Herri, Energy Convers. Manage., 2007, 48, 1313 CrossRef; D. Zhong and P. Englezos, Energy Fuels, 2012, 26, 2098 CrossRef; N. Ye and P. Zhang, J. Chem. Eng. Data, 2014, 59, 2920 CrossRef.
- H. Sakamoto, K. Sato, K. Shiraiwa, S. Takeya, M. Nakajima and R. Ohmura, RSC Adv., 2011, 1, 315 RSC.
- S. Muromachi, T. Abe, Y. Yamamoto and S. Takeya, Phys. Chem. Chem. Phys., 2014, 16, 21467 RSC.
- S. Muromachi, K. A. Udachin, K. Shin, S. Alavi, I. L. Moudrakovski, R. Ohmura and J. A. Ripmeester, Chem. Commun., 2014, 50, 11476 RSC.
- S. Muromachi, K. A. Udachin, S. Alavi, R. Ohmura and J. A. Ripmeester, Chem. Commun., 2016, 52, 5621 RSC.
- T. Kobori, S. Muromachi, T. Yamasaki, S. Takeya, Y. Yamamoto, S. Alavi and R. Ohmura, Cryst. Growth Des., 2015, 15, 3862 CAS.
- V. Y. Komarov, T. V. Rodionova and K. Suwinska, J. Struct. Chem., 2012, 53, 768 CrossRef CAS.
- T. Rodionova, V. Komarov, J. Lipkowski and N. Kuratieva, New J. Chem., 2010, 34, 432 RSC.
- S. Muromachi, M. Kida, S. Takeya, Y. Yamamoto and R. Ohmura, Can. J. Chem., 2015, 93, 954 CrossRef CAS.
- K. Tezuka, K. Murayama, S. Takeya, S. Alavi and R. Ohmura, J. Phys. Chem. C, 2013, 117, 10473 Search PubMed; K. Yasuda, Ind. Eng. Chem. Res., 2009, 48, 9335 CrossRef CAS; O. S. Rouher and A. J. Barduhn, Desalination, 1969, 6, 57 CrossRef; S. Takeya, H. Fujihisa, A. Hachikubo, H. Sakagami and Y. Gotoh, Chem.–Eur. J., 2014, 20, 17207 CrossRef PubMed; M. Kida, H. Sakagami, M. Watanabe, Y. Jin, N. Takahashi and J. Nagao, Chem. Eng. Sci., 2016, 140, 10 CrossRef; H. Youn, M. Cha and H. Lee, ChemPhysChem, 2015, 16, 2876 CrossRef PubMed.
- K. A. Udachin, S. Alavi and J. A. Ripmeester, J. Chem. Phys., 2011, 134, 121104 CrossRef PubMed.
- K. Momma and F. Izumi, J. Appl. Crystallogr., 2011, 44, 1272 CrossRef CAS.
- M. Oshima, M. Kida, Y. Jin and J. Nagao, J. Chem. Thermodyn., 2015, 90, 277 CrossRef CAS.
- E. S. Domalski and E. D. Hearing, J. Phys. Chem. Ref. Data, 1996, 25, 1 CrossRef CAS.
- W. Lin, D. Dalmazzone, W. Fürst, D. Anthony, L. Fournaison and P. Clain, Fluid Phase Equilib., 2014, 372, 63 CrossRef CAS.
- H. Oyama, W. Shimada, T. Ebinuma, Y. Kamata, S. Takeya, T. Uchida, J. Nagao and H. Narita, Fluid Phase Equilib., 2005, 234, 131 CrossRef CAS.
- S. Alavi, R. Ohmura and J. A. Ripmeester, J. Chem. Phys., 2011, 134, 054702 CrossRef CAS PubMed; K. Shin, K. A. Udachin, I. L. Moudrakovski, D. M. Leek, S. Alavi, C. I. Ratcliffe and J. A. Ripmeester, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 8437 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2017 |
