Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Thiourea catalysed reduction of α-keto substituted acrylate compounds using Hantzsch ester as a reducing agent in water†
Guanglin Weng‡
a,
Xiaobo Ma‡a,
Dongmei Fangb,
Ping Tana,
Lijiao Wanga,
Linlin Yanga,
Yuanyuan Zhanga,
Shan Qian*a and
Zhouyu Wang *a
*a
aDepartment of Chemistry, Xihua University, Chengdu, 610039, China. E-mail: zhouyuwang77@gmail.com; qians33@163.com; Fax: +86-28-8772-3006; Tel: +86-28-8772-9463
bChengdu Institute of Biology, Chinese Academy of Sciences, Chengdu, 610041, China
First published on 26th April 2017
Abstract
The first method for the reduction of α-keto substituted acrylate compounds by Hantzsch ester in water under the catalysis of thiourea has been developed. The products were isolated in moderate to high yields (38–95%). These products are important intermediates in the synthesis of a series of natural products and other biologically active molecules.
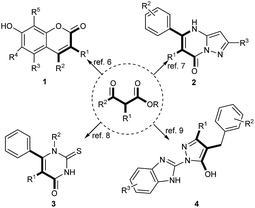
β-Keto esters are important intermediates in organic synthesis,1–5 and they have been extensively used in the synthesis of a series of natural products and other biologically active molecules such as coumarin derivatives 1 (ref. 6) (potent TNF-α inhibitors), pyrazolo[1,5-a]pyrimidin-7(4H)-ones 2 (ref. 7) (potent inhibitors of hepatitis C virus polymerase), cambinol analogues 3 (ref. 8) (isoenzyme inhibitors of the sirtuin family of protein deacetylases), and 4 (ref. 9) (potent prostate cancer antigen-1 inhibitors) (Fig. 1).
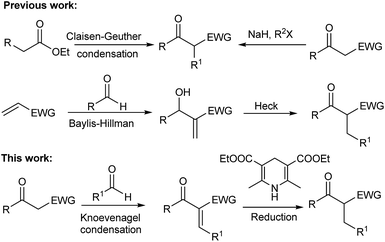
Many efforts have been made to achieve efficient preparation of these scaffolds.10–21 Two general syntheses of β-keto esters include (i) acylation of ketones or carboxylic esters by acyl halides or esters22 and (ii) acylation of acetylacetone or ethyl acetoacetate and their substituted derivatives by halides or esters followed by base promoted cleavage of a carbonyl group.23,24 Recently, Heck reaction between Baylis–Hillman adducts and various aryl sources has been developed as a very attractive alternative method for syntheses of β-keto esters.25–27 In principle, the reduction of Knoevenagel condensation adducts could be an efficient method for the preparation of β-keto esters. However, it has been barely reported (Scheme 1).
In recent years, Hantzsch esters and their related organic hydride donors have been widely utilized in the reduction of a variety of compounds containing C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C
C, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and C
N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O unsaturated groups.28,29 We reported the first example of catalyst free transfer hydrogenation of C
O unsaturated groups.28,29 We reported the first example of catalyst free transfer hydrogenation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C with Hantzsch ester in water.30 A series of alkenes conjugated with strong electron-withdrawing groups such as nitrile, ester and nitro could be reduced to the corresponding products in water with excellent yields. More recently, we reported a base promoted cascade protocol for the preparation of reduced Knoevenagel condensation products in water.31 However, the α-keto substituted acrylate compounds could not be reduced for high yield (35%) under the existing reaction conditions. Here in, we wish to report a simple and efficient synthetic method for the reduction of the α-keto substituted acrylate compounds by Hantzsch ester in water under the catalysis of thiourea catalysts.
C with Hantzsch ester in water.30 A series of alkenes conjugated with strong electron-withdrawing groups such as nitrile, ester and nitro could be reduced to the corresponding products in water with excellent yields. More recently, we reported a base promoted cascade protocol for the preparation of reduced Knoevenagel condensation products in water.31 However, the α-keto substituted acrylate compounds could not be reduced for high yield (35%) under the existing reaction conditions. Here in, we wish to report a simple and efficient synthetic method for the reduction of the α-keto substituted acrylate compounds by Hantzsch ester in water under the catalysis of thiourea catalysts.
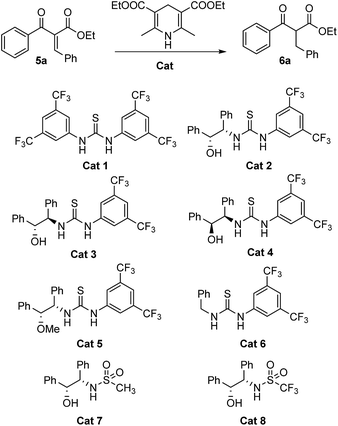
Initially, we found acetic acid could be used as catalyst in the reaction to get a moderate yield at 100 °C (Table 1, entry 2). However, no improvement could be achieved when a series of acids with different pKa were used in the reaction (Table 1, entries 3–4). Fortunately, 56% yield could be obtained when 20% of thiourea was used in the reaction under the otherwise identical conditions (Table 1, entry 6). Thus, thiourea derivatives Cat 1–8 were synthesized. The yield of the reduction product could be improved to 72% when 20% of Cat 1 was used (Table 1, entry 7). The Cat 2 has the best performance among these new catalysts, and 93% yield could be achieved when 20% of Cat 2 was used (Table 1, entry 8). Its diastereomer Cat 3 and enantiomer Cat 4 gave lower yield (Table 1, entries 9 and 10). The hydroxyl and the thiourea parts of Cat 2 seemed very important for the activity of the catalyst. When the hydroxyl protected Cat 5 and the sulfonamides Cat 7 and 8 were used in the reaction, 80%, 62% and 65% yields could be obtained, respectively (Table 1, entries 11–14).
| Entry | Solvent | Catalyst | Cat. (eq.) | Temp (C) | ee (%) | Yieldb (%) |
|---|---|---|---|---|---|---|
| a Unless specified otherwise, reactions were carried out with 1.0 mmol of 5a and 1.0 mmol of Hantzsch ester for 24 hours.b Isolated yield.c The reaction time is 12 hours. | ||||||
| 1 | H2O | — | — | 100 | — | 35 |
| 2 | H2O | CH3COOH | 0.2 | 100 | — | 60 |
| 3 | H2O | CF3COOH | 0.2 | 100 | — | 40 |
| 4 | H2O | CF3SO3H | 0.2 | 100 | — | 38 |
| 5 | H2O | Urea | 0.2 | 100 | — | 40 |
| 6 | H2O | Thiourea | 0.2 | 100 | — | 56 |
| 7 | H2O | Cat 1 | 0.2 | 100 | — | 72 |
| 8 | H2O | Cat 2 | 0.2 | 100 | <5 | 93 |
| 9 | H2O | Cat 3 | 0.2 | 100 | <5 | 82 |
| 10 | H2O | Cat 4 | 0.2 | 100 | <5 | 90 |
| 11 | H2O | Cat 5 | 0.2 | 100 | <5 | 80 |
| 12 | H2O | Cat 6 | 0.2 | 100 | — | 85 |
| 13 | H2O | Cat 7 | 0.2 | 100 | <5 | 62 |
| 14 | H2O | Cat 8 | 0.2 | 100 | <5 | 65 |
| 15 | H2O | Cat 2 | 0.2 | 80 | <5 | 75 |
| 16 | H2O | Cat 2 | 0.1 | 100 | <5 | 82 |
| 17c | H2O | Cat 2 | 0.2 | 100 | <5 | 71 |
| 18 | PhMe | Cat 2 | 0.2 | 100 | <5 | 48 |
| 19 | DCM | Cat 2 | 0.2 | 40 | <5 | 13 |
| 20 | EtOH | Cat 2 | 0.2 | 78 | <5 | 59 |
| 21 | MeCN | Cat 2 | 0.2 | 84 | <5 | 12 |
| 22 | THF | Cat 2 | 0.2 | 70 | <5 | 18 |
| 23 | — | Cat 2 | 0.2 | 100 | <5 | 25 |
We next found 100 °C is the most suitable temperature for the reaction, only 75% yield could be obtained when the reaction temperature was decreased to 80 °C (Table 1, entry 15). Decreasing the equivalent of the catalyst to 10%, the yield decreased to 82% (Table 1, entry 16). Shorten the reaction time to 12 hours, the yield dropped to 71% (Table 1, entry 17). Water was found to be the best solvent for the current reduction system since only trace or small amounts of products could be isolated when the reactions were set up in organic solvents such as toluene, dichloromethane, acetonitrile and tetrahydrofuran (Table 1, entries 18–22). Solvent-free made the reaction proceed poorly (Table 1, entry 23). As a result, the best reaction conditions in which the reduction is performed are using 20 mol% of Cat 2, Hantzsch ester (1.0 equiv.) in water at 100 °C for 24 h.
Unfortunately, the products are almost racemate with the catalysis of chiral catalysts Cat 2–5 and Cat 7–8. Two reasons maybe responsible for the results. One is that the β-keto esters are easy to racemize. The other is the high reaction temperature.
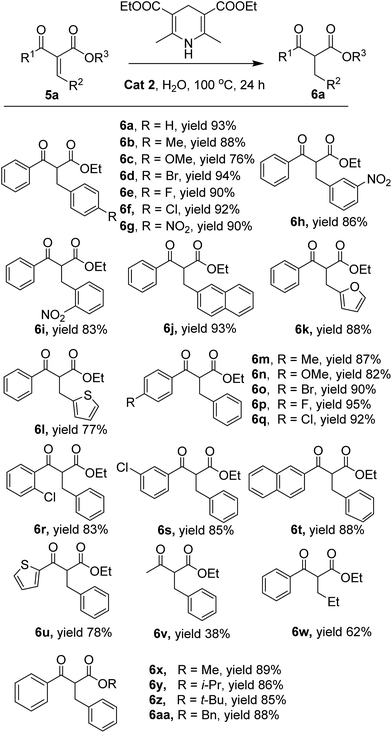
With the optimized reaction conditions in hand, the scope of the thiourea catalyst catalysed reduction was next investigated. A variety of α-keto arylacrylate compounds could be reduced to the corresponding products. When the R2 in 5 were p-substituted phenyls, all the tested substrates could be reduced to the desired products with 76–94% yields. The substrates with an electron withdrawn group on the para position of the phenyl rings comparing with the substrates with an electron donating groups were easier to be reduced and a higher yield could be obtained (Fig. 2, 6a–g). When the R2 in 5 were o/m-substituted phenyls, the substrates could be reduced equally at the existing reaction conditions, and almost identical yields were obtained (Fig. 2, 6h and 6i). When the R2 in 5 were bulky groups such as 2-naphthyl, the substrate could also be reduced with a 93% yield (Fig. 2, 6j). The 2-furanyl and 2-thiophenyl could also be used as R2 in the substrate 5, 88% and 77% yields were obtained when these substrates were used, respectively (Fig. 2, 6k and 6l). The R1 in 5 could be the para electron donating and withdrawn groups substituted phenyl rings, 82–95% yields were obtained when these substrates were reduced under the existing conditions (Fig. 2, 6m–q). The o/m-choro substituted phenyls could also be used as R1 in 5, and they could be reduced with 83% and 85% yields, respectively (Fig. 2, 6r–s). The relatively bulky rings and the hetero aromatic ring were tolerated also when they were used as R1 in 5, and the corresponding products could be isolated with 75–88% yields (Fig. 2, 6t–u). Besides Et, other groups such as Me, i-Pr, and Bn could also be used as R3 in 5. 85–89% yields could be obtained when these substrates were reduced under the existing reaction conditions (Fig. 2, 6x–aa). What's more, the α-keto alkylacrylate was also can be reduced in the protocol and the ethyl 2-benzoylpentanoate was obtained with 62% yield (Fig. 2, 6w). However, alkyl group used as R1 in 5 leads to only 38% yield of the desired product 6v.
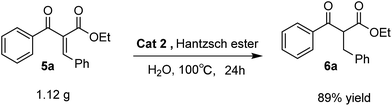
Additionally, a gram-scale synthesis of ethyl 2-benzyl-3-oxo-3-phenylpropanoate was conducted in the presence of Cat 2 (Scheme 2). The pure product was obtained through column chromatograph with 89% yield.
Regarding the mechanism of the reduction, we assume that the substrate, Hantzsch ester and the catalyst could be dissolved in water at 100 °C. Both the two carbonyl groups of the substrate 5 and hydrogen of the Hantzsch ester could be activated by the two NH of the thiourea and the OH of catalyst Cat 2, respectively, under the solvent affection of water.
Conclusions
In summary, we have developed a novel method for the reduction of α-keto arylacrylate and alkylacrylate compounds by Hantzsch ester in water under the catalysis of thiourea catalysts. A series of α-alkyl-β-ketoesters were isolated with moderate to high yields. Although the chiral products were fail to obtained, the obvious advantages of this method are high chemoselectivity, water tolerance and mild experimental conditions over the traditional methods such as hydrogenation. Especially, functional groups such as keto, ester, nitro, fluoro, chloro, bromo, furanyl and thienyl are all tolerated by the reaction conditions employed. We believe that this simple, green catalytic protocol provides an alternative way to prepare α-alkyl-β-ketoesters.Acknowledgements
We are grateful for the financial supports from the Spring Plan of Ministry of Education (Z2016162), the founds of Sichuan Province (2017JQ0023, 2017NZ0048), the founds of Chengdu City (2015-HM01-00366-SF), the Undergraduate Scientific and Technological Innovation Project, the National Undergraduate Innovation and Entrepreneurship Training Programs of China and The Innovation Fund Of Postgraduate, Xihua University.Notes and references
- J. Yang, W. Li, Z. Jin, X. Liang and J. Ye, Org. Lett., 2010, 12, 5218–5221 CrossRef CAS PubMed.
- C. Yin, W. Cao, L. Lin, X. Liu and X. Feng, Adv. Synth. Catal., 2013, 355, 1924–1930 CrossRef CAS.
- S. Kotha and E. Manivannan, ARKIVOC, 2003, 67–76 CAS.
- H. J. Cristau, X. Marat, J. P. Vors and J. L. Pirat, Tetrahedron Lett., 2003, 44, 3179–3181 CrossRef CAS.
- S. Dhiman and S. S. V. Ramasastry, J. Org. Chem., 2013, 78, 10427–10436 CrossRef CAS PubMed.
- J. F. Cheng, M. Chen, D. Wallace, S. Tith, T. Arrhenius, H. Kashiwagi, Y. Ono, A. Ishikawa, H. Sato, T. Kozono, H. Sato and A. M. Nadzan, Bioorg. Med. Chem. Lett., 2004, 14, 2411–2415 CAS.
- Y. Deng, G. W. Shipps Jr, T. Wang, J. Popovici-Muller, K. E. Rosner, M. A. Siddiqui, J. Duca, A. B. Cooper and M. Cable, Bioorg. Med. Chem. Lett., 2009, 19, 5363–5367 CrossRef CAS PubMed.
- F. Medda, T. L. Joseph, L. Pirrie, M. Higgins, A. M. Z. Slawin, S. Lain, C. Verma and N. J. Westwood, MedChemComm, 2011, 2, 611–615 RSC.
- S. Nakao, M. Mabuchi, T. Shimizu, Y. Itoh, Y. Takeuchi, M. Ueda, H. Mizuno, N. Shigi, I. Ohshio, K. Jinguji, Y. Ueda, M. Yamamoto, T. Furukawa, S. Aoki, K. Tsujikawa and A. Tanaka, Bioorg. Med. Chem. Lett., 2014, 24, 1071–1074 CrossRef CAS PubMed.
- M. Yoshida, K. Fujikawa, S. Sato and S. Hara, ARKIVOC, 2003, 36–42 CAS.
- J. Christoffers, H. Oertling, P. Fischer and W. Frey, Tetrahedron, 2003, 59, 3769–3778 CrossRef CAS.
- T. Aoyama, T. Takido and M. Kodomari, Tetrahedron Lett., 2004, 45, 1873–1876 CrossRef CAS.
- C. V. Galliford and K. A. Scheidt, Chem. Commun., 2008, 1926–1928 RSC.
- H. F. Cui, K. Y. Dong, J. Nie, Y. Zheng and J. A. Ma, Tetrahedron Lett., 2010, 51, 2374–2377 CrossRef CAS.
- W. Li, J. Wang, X. Hu, K. Shen, W. Wang, Y. Chu, L. Lin, X. Liu and X. Feng, J. Am. Chem. Soc., 2010, 132, 8532–8533 CrossRef CAS PubMed.
- J. Xu, Y. Hu, D. Huang, K. H. Wang, C. Xu and T. Niu, Adv. Synth. Catal., 2012, 354, 515–526 CrossRef CAS.
- Y. K. Kang, H. H. Kim, K. O. Koh and D. Y. Kim, Tetrahedron Lett., 2012, 53, 3811–3814 CrossRef CAS.
- M. Vellakkaran, M. M. S. Andappan and N. Kommu, Eur. J. Org. Chem., 2012, 4694–4698 CrossRef CAS.
- J. Luo, W. Wu, L. W. Xu, Y. Meng and Y. Lu, Tetrahedron Lett., 2013, 54, 2623–2626 CrossRef CAS.
- W. B. Liu, C. M. Reeves and B. M. Stoltz, J. Am. Chem. Soc., 2013, 135, 17298–17301 CrossRef CAS PubMed.
- Y. Zhao, X. J. Wang, Y. Lin, C. X. Cai and J. T. Liu, Tetrahedron, 2014, 70, 2523–2528 CrossRef CAS.
- B. O. Linn and C. R. Hauser, J. Am. Chem. Soc., 1956, 78, 6066–6070 CrossRef CAS.
- S. M. McElvain and K. H. Weber, J. Am. Chem. Soc., 1941, 63, 2192–2197 CrossRef CAS.
- H. E. Zaugg, R. J. Michaels and E. J. Baker, J. Am. Chem. Soc., 1968, 90, 3800–3808 CrossRef CAS.
- J. M. Kim, K. H. Kim, T. H. Kim and J. N. Kim, Tetrahedron Lett., 2008, 49, 3248–3251 CrossRef CAS.
- H.-S. Kim, S. J. Lee, B. Choi and C. M. Yoon, Synthesis, 2012, 44, 3161–3164 CrossRef CAS.
- K. B. Raju, V. Mari and K. Nagaiah, Synthesis, 2013, 45, 2867–2874 CrossRef CAS.
- For reviews about Hantzsch esters: (a) C. Zheng and S. L. You, Chem. Soc. Rev., 2012, 41, 2498–2518 RSC; (b) M. Rueping, J. Dufour and F. R. Schoepke, Green Chem., 2011, 13, 1084–1105 RSC; (c) Z. Y. Wang and Z. J. Jiang, Asian J. Chem., 2010, 22, 4141–4149 CAS; (d) S. L. You, Chem.–Asian J., 2007, 2, 820–827 CrossRef CAS PubMed; (e) S. G. Ouellet, A. M. Walji and D. W. MacMillan, Acc. Chem. Res., 2007, 40, 1327–1339 CrossRef CAS PubMed.
- For recent representative paper about Hantzsch esters: (a) L. Qi and Y. Y. Chen, Angew. Chem., Int. Ed., 2016, 55, 13312–13315 CrossRef CAS PubMed; (b) K. H. Kim, T. Akiyama and C. H. Cheon, Chem.–Asian J., 2016, 11, 274–279 CrossRef CAS PubMed; (c) J. Jung, J. Kim, G. Park, Y. You and E. J. Cho, Adv. Synth. Catal., 2016, 358, 74–80 CrossRef CAS.
- Q. He, Z. H. Xu, D. H. Jiang, W. S. Ai, R. H. Shi, S. Qian and Z. Y. Wang, RSC Adv., 2014, 4, 8671–8674 RSC.
- T. He, R. H. Shi, Y. M. Gong, G. Y. Jiang, M. Liu, S. Qian and Z. Y. Wang, Synlett, 2016, 1864–1869 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra00995j |
| ‡ These authors contributed equally to this work and should be considered co-first authors. |
| This journal is © The Royal Society of Chemistry 2017 |