Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Heme protein-mediated synthesis of PEDOT:PSS: enhancing conductivity by inhibiting heme degradation†
J. J. Floresabc,
C. K. Payneab and
J. D. Morris *abc
*abc
aSchool of Chemistry and Biochemistry, Georgia Institute of Technology, Atlanta, Georgia 30332, USA
bPetit Institute for Bioengineering and Biosciences, Georgia Institute of Technology, Atlanta, Georgia 30332, USA
cSchool of Science and Technology, Georgia Gwinnett College, Lawrenceville, Georgia 30043, USA. E-mail: jmorris14@ggc.edu
First published on 20th February 2017
Abstract
Poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) (PEDOT:PSS) is a conducting polymer used in regenerative medicine, solar energy conversion, OLEDs, and biological sensing. PEDOT:PSS can be synthesized with a wide range of biomolecular oxidants including hemoglobin, catalase, horseradish peroxidase, soybean peroxidase, and laccase. Unfortunately heme proteins have been found to degrade during polymer synthesis, limiting their utility. We show that the peroxidase substrate, 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS), inhibits heme degradation during hemoglobin-mediated synthesis of PEDOT:PSS, measured by fluorescence emission. Four-point probe measurements show that films of PEDOT:PSS are more conductive when synthesized in the presence of ABTS. Characterization of the resulting PEDOT:PSS films using visible and near IR spectroscopy shows that ABTS produces a bipolaron rich polymer, as expected if heme degradation is inhibited. Conductivity is further enhanced (31 S cm−1) when an iron chelator, EDTA, is used in combination with ABTS.
Introduction
Poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) (PEDOT:PSS) is a conductive polymer1–3 that is transparent,4,5 biologically compatible,6–8 and water soluble9 allowing its use in antistatic agents,1 light-emitting diodes,10–13 solar cells,14–19 biological sensing,20–22 and regenerative medicine.23,24 Standard oxidative polymerizations of 3,4-ethylenedioxythiophene (EDOT) typically use small molecule iron compounds combined with an oxidant, such as sodium persulfate.25 This synthetic route produces a pristine polymer with relatively low conductivity (<1 S cm−1),1,25 that is then doped for higher-conductivity applications.26–28 Alternatively PEDOT:PSS can be synthesized with biomolecules.29–31 Other conductive polymers such as polyaniline32,33 and polyphenols34–36 have also been polymerized using biomolecular oxidants.Biomolecular synthesis utilizes the naturally occurring structural diversity of biomolecules to tune polymer properties and could allow for more environmentally friendly reaction conditions.29–31,37–39 Most biomolecules used for polymerization contain iron in a heme complex and are combined with hydrogen peroxide as an oxidant. Examples include catalase,40 cytochrome c,38 hemoglobin,41 horseradish peroxidase,37 and soybean peroxidase.38,42,43 Non-heme biomolecules used for synthesis include laccase44 and transferrin.40 As this list makes clear, enzymatic activity is not required for polymerization.40 The acidic conditions required for PEDOT:PSS synthesis (pH 1–2) means most biomolecules will be denatured.25 A notable exception is laccase, which can enzymatically polymerize PEDOT:PSS under more mild conditions (pH 3.5).44 Additionally, soybean peroxidase can enzymatically polymerize PEDOT:PSS at a pH of 5. In this case, however, terthiophene was added to the reaction as a radical initiator.43 Biomolecular synthesis of PEDOT:PSS results in conductivities ranging from 1 × 10−5 S cm−1 (catalase) to 2.8 S cm−1 (hemoglobin).40,41 A critical factor for heme proteins used in PEDOT:PSS synthesis is the relative concentration of heme-bound iron compared to unbound, free, iron.41 Heme iron, combined with hydrogen peroxide, generates a highly conductive polymer dominated by bipolarons.45 While free iron, also combined with hydrogen peroxide, generates a weakly conductive polymer dominated by polarons.25,45 Iron chelators, such as ethylenediaminetetraacetic acid (EDTA), can be used to prevent unbound iron from participating in the synthesis.45 For this reason, the addition of EDTA during hemoglobin mediated PEDOT:PSS polymerizations leads to a significant conductivity enhancement from 2.8 S cm−1 to 19.5 S cm−1.41
The balance of free iron versus heme iron is largely determined by the reaction of hydrogen peroxide with heme iron.38 Biomolecular oxidants require hydrogen peroxide for synthesis, but hydrogen peroxide also causes the degradation of the heme group and the release of free iron.46–48 While EDTA inhibits free iron from synthesizing PEDOT:PSS, it does not prevent the degradation of the heme group. We hypothesized that the conductivity of hemoglobin synthesized PEDOT:PSS would be enhanced by inhibiting heme degradation with 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), a peroxidase substrate.46,47,49,50
In this work we show that polymerization of PEDOT:PSS in the presence of ABTS inhibits heme degradation and significantly enhances conductivity. When ABTS is combined with EDTA during synthesis, we observe further enhancement of polymer conductivity to 31 S cm−1. To our knowledge, this represents a record conductivity for undoped PEDOT:PSS. We then investigate the underlying mechanism of this enhancement. We use visible and near IR spectroscopy to characterize the resulting PEDOT:PSS films. We find that the suppression of heme degradation by ABTS leads to bipolaron-dominated PEDOT:PSS.
PEDOT:PSS was synthesized by combining hemoglobin (4.8 μM by heme concentration), EDOT (50 mM) and PSS (25 mM, by monomer concentration) in an HCl–KCl buffer (pH 1.15) while stirring. The reaction was initiated by the addition of H2O2 (20 mM). The reaction was allowed to proceed for three hours followed by dialysis. For full experimental details, please see the ESI.†
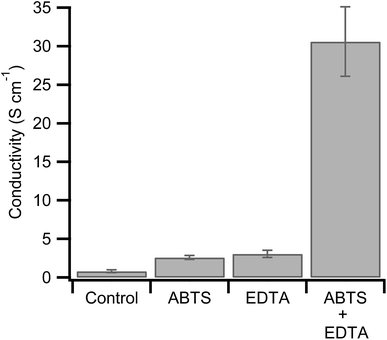
The combination of hemoglobin with hydrogen peroxide results in the degradation of the heme group and the release of free iron.46,47 In the context of hemoglobin-mediated synthesis of PEDOT:PSS, this leads to a less conductive polymer dominated by polarons.34 The peroxidase substrate, ABTS, however, inhibits the degradation of hemoglobin at neutral pHs.46,47,49,50 If heme degradation is inhibited under our reaction conditions (pH = 1.15), then the addition of ABTS during synthesis will enhance PEDOT:PSS conductivity. As a control, we synthesized hemoglobin without ABTS and used four point probe measurements to determine its conductivity (0.81 S cm−1, Fig. 1).
Next we synthesized PEDOT:PSS in the presence of ABTS. We found that 0.4 mM ABTS (Fig. S1, ESI†) led to the most promising polymer with a threefold conductivity enhancement to 2.6 S cm−1 (Fig. 1). This finding is consistent with our hypothesis that ABTS inhibits heme degradation during PEDOT:PSS synthesis. This is expected to increase conductivity for two reasons. First, in the absence of ABTS, the degradation of hemoglobin by hydrogen peroxide will result in a continual decrease in oxidant concentration, lowering yield and decreasing the final PEDOT:PSS ratio. The ratio of conductive PEDOT to insulating PSS critically determines the final conductivity of the resulting polymer films.25,51 Second, the degradation of hemoglobin results in the release of free iron, as discussed above. Any PEDOT oxidized by free iron and hydrogen peroxide is expected to have low conductivity (∼10−7 S cm−1).41
The conductivity of the polymer synthesized with hemoglobin and ABTS is relatively high for undoped PEDOT:PSS, but it remains lower than the previously reported combination of hemoglobin with EDTA (19.5 S cm−1).41 The present synthesis was done under slightly different conditions including a lower hemoglobin concentration, a lower hydrogen peroxide concentration, and a shorter reaction duration. Both the lower concentration of hemoglobin and the shorter reaction time will lower the final yield of PEDOT. Since the PSS template is already polymerized (MW = 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 kDa), this will lower the PEDOT:PSS ratio. As described above, this is expected to lower conductivity.25,51 For comparison with the previous result, we synthesized PEDOT:PSS with hemoglobin and EDTA under our reaction conditions. The resulting polymer has a conductivity of 3.06 S cm−1, similar to the polymerization in the presence of ABTS (Fig. 1).
000 kDa), this will lower the PEDOT:PSS ratio. As described above, this is expected to lower conductivity.25,51 For comparison with the previous result, we synthesized PEDOT:PSS with hemoglobin and EDTA under our reaction conditions. The resulting polymer has a conductivity of 3.06 S cm−1, similar to the polymerization in the presence of ABTS (Fig. 1).
While ABTS may prevent the release of free iron from the heme group, it does not prevent trace iron from participating in the polymerization. For this reason we polymerized PEDOT:PSS with hemoglobin in the presence of both ABTS and EDTA in the same polymerization. PEDOT:PSS synthesized under these conditions shows a conductivity of 31 S cm−1. This represents a 50% increase over the highest conductivity previously obtained from biomolecular synthesis and, to our knowledge, represents a record conductivity for undoped PEDOT:PSS.41
Besides the previously mentioned EDTA and hemoglobin polymerization, the next highest conductivity reported for undoped PEDOT:PSS is 10 S cm−1.52 For this synthesis, Qi, et al. formed an emulsion of EDOT in PSS. This allowed more EDOT to be dissolved in an aqueous solution and ultimately led to a higher PEDOT:PSS ratio. Investigations by Aleshin, et al. reported a conductivity of 20 S cm−1.53 In this case, however, commercial PEDOT:PSS was cast from low concentration HCl. It is well known that HCl, as well as other acids, are effective post-polymerization treatments to enhance the conductivity of PEDOT:PSS.54,55 Indeed films of PEDOT:PSS doped by acid can achieve conductivities as high as 4000 S cm−1.27,56 While films treated with organic solvents reach 1100 S cm−1.28 For a comprehensive discussion of PEDOT:PSS conductivity enhancements see the review by Shi, et al.26 Importantly, ABTS and EDTA do not alter PEDOT:PSS after polymerization, rather they optimize the oxidant used for polymerization. We are directly producing highly conductive PEDOT:PSS in a one-step, aqueous reaction.
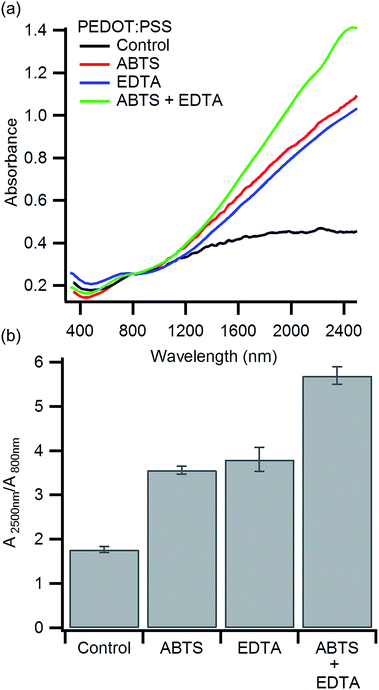
Previous work has shown that PEDOT:PSS oxidized by heme iron results in a higher bipolaron to polaron ratio compared to PEDOT:PSS oxidized with free iron.38,41,45 If ABTS limits heme degradation, we should see an increase in the bipolaron to polaron ratio. Polarons in PEDOT:PSS produce an absorption at ∼800 nm while bipolarons in PEDOT:PSS absorb at ∼3000 nm.25,45,57 To compare the bipolaron to polaron ratio, we characterized each polymer with visible and near IR spectroscopy from 350 nm to 2500 nm in a single spectra (Fig. 2a). These measurements were taken on polymer films cast on glass, as the water in aqueous solutions of PEDOT:PSS absorbs strongly in the IR.58 The measured film thicknesses of each polymer blend varied from 2 μm (hemoglobin alone) to 4 μm (hemoglobin and ABTS). For this reason, the absorbance at 800 nm was used to normalize each spectrum. This normalization allows us to compare the bipolaron to polaron ratio, but not absolute absorption. The control, synthesized using only hemoglobin, shows the lowest absorption at 2500 nm relative to the absorption at 800 nm (Fig. 2b). This indicates that the bipolaron to polaron ratio is the lowest for the control. The absorbance at 2500 nm, and hence the bipolaron to polar ratio, increases for PEDOT:PSS polymerized in the presence of ABTS or EDTA. The combination of ABTS and EDTA leads to the highest bipolaron to polaron absorption ratio. Since heme iron is expected to produce a bipolaron rich polymer, these observations are consistent with our hypothesis that ABTS inhibits heme degradation.45 To ensure this change is due to an interaction between heme and ABTS, we polymerized PEDOT:PSS with FeCl3 in the presence of ABTS. No significant change in the visible and near IR spectra was observed (Fig. S2, ESI†).
The impact of EDTA on the near IR absorbance is similar to ABTS, but for a different reason. EDTA removes free iron, while ABTS preserves more heme iron. Both effects lead to a bipolaron rich polymer. A detailed discussion of the impact of EDTA on the biomolecular synthesis of PEDOT:PSS has been published previously.41 Additionally, we find that higher bipolaron absorption in the near IR correlates with greater polymer conductivity. This suggests that the increased conductivity may arise in part due to an increase in oxidation state of the polymer.
It is well known that ABTS inhibits heme degradation at physiological pHs.46,47,49,50 To confirm this inhibition also occurs under our reaction conditions of pH 1.15, we directly monitored the impact of ABTS on the reaction of hemoglobin with hydrogen peroxide. The absorption of ABTS prevents monitoring heme degradation by UV-Vis spectroscopy due to spectral overlap (Fig. S3, ESI†). Fortunately, the degradation products from the reaction of hydrogen peroxide with hemoglobin are fluorescent.46,47 Although the chemical identity of these products is unclear, fluorescence has been used previously to observe the impact of ABTS on heme degradation.46,47 Hemoglobin was incubated with hydrogen peroxide in HCl–KCl (pH 1.15) for 60 minutes and then an emission scan was collected (excite: 320 nm, Fig. 3a). The fluorescence intensity of the heme degradation products is observed as a broad peak centered at 445 nm. In the absence of hemoglobin or H2O2, no peak is observed at 445 nm (Fig. S4, ESI†). Previous investigations, at neutral pH, show an emission peak at a slightly higher wavelength of 465 nm.46,47 This shift is likely due to the change in pH which can alter the wavelength of fluorescence emission.59 The fluorescence intensity at 445 nm is significantly reduced in the presence of ABTS indicating heme degradation is suppressed.
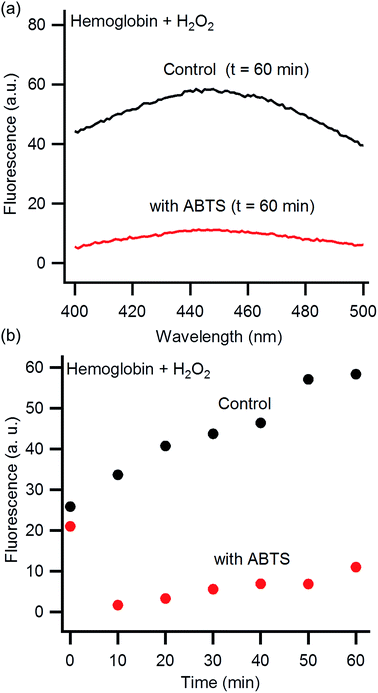 | ||
| Fig. 3 Fluorescence emission of hemoglobin combined with hydrogen peroxide at pH 1.15 (excite: 325 nm). (a) Representative fluorescence emission spectra of hemoglobin 60 minutes after exposure to hydrogen peroxide in the presence (red) and absence (black) of ABTS. (b) Kinetic plot of fluorescence emission intensity in the presence (red) and absence (black) of ABTS (emit: 445 nm). The initial (t = 0) high intensity of fluorescence in the presence of ABTS is from a fluorescence peak at 470 nm due to ABTS itself.60 This peak is no longer significant after 10 minutes of exposure to hydrogen peroxide (Fig. S5†). | ||
We then monitored the increase in fluorescence emission overtime at 445 nm (Fig. 3b). The fluorescence increases gradually overtime both with and without ABTS. The data imply heme degradation is reduced by ABTS, but not inhibited entirely. The initial (t = 0) high intensity of fluorescence in the presence of ABTS is from a fluorescence peak at 470 nm due to ABTS itself.60 This peak is no longer significant after 10 minutes of exposure to hydrogen peroxide (Fig. S5†).
To understand the mechanism by which ABTS inhibits heme degradation, it is important to consider the interaction of hydrogen peroxide and acid denatured hemoglobin. Under our reaction conditions, hemoglobin possesses ferrous iron (Fe(II)).41,61–63 When hydrogen peroxide reacts with ferrous hemoglobin, it can generate ferrylhemoglobin (Fe(IV)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O).64 The subsequent reaction of ferrylhemoglobin with another equivalent of hydrogen peroxide, produces superoxide and methemoglobin (Fe(III)).46 Superoxide then degrades the heme group and releases free iron. ABTS inhibits this degradation by reducing ferrylhemoglobin to methemoglobin.46 Methemoglobin can still react with another equivalent of hydrogen peroxide, but in this case, it produces the radical oxoferrylhemoglobin (˙Fe(IV)
O).64 The subsequent reaction of ferrylhemoglobin with another equivalent of hydrogen peroxide, produces superoxide and methemoglobin (Fe(III)).46 Superoxide then degrades the heme group and releases free iron. ABTS inhibits this degradation by reducing ferrylhemoglobin to methemoglobin.46 Methemoglobin can still react with another equivalent of hydrogen peroxide, but in this case, it produces the radical oxoferrylhemoglobin (˙Fe(IV)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). Hydrogen peroxide reacting oxoferrylhemoglobin still produces methemoglobin but with oxygen, instead of superoxide. In this way, ABTS protects hemoglobin from degradation. For more details on this process see the work by Nagababu, et al.46
O). Hydrogen peroxide reacting oxoferrylhemoglobin still produces methemoglobin but with oxygen, instead of superoxide. In this way, ABTS protects hemoglobin from degradation. For more details on this process see the work by Nagababu, et al.46
Conclusions
We have demonstrated that ABTS can be used in hemoglobin-mediated PEDOT:PSS synthesis to enhance polymer conductivity (Fig. 1). When EDTA is also added during synthesis, the conductivity is further enhanced to 31 S cm−1. These increases in conductivity are correlated with an increased bipolaron to polaron absorption ratio in the near IR (Fig. 2). This suggests the conductivity is enhanced due to an increase in PEDOT:PSS oxidation state. Finally, we confirmed that ABTS inhibits heme degradation under our acidic reaction conditions (Fig. 3). These experiments confirm our hypothesis that inhibiting heme degradation during PEDOT:PSS synthesis increases polymer conductivity. This work highlights the importance of understanding the underlying mechanistic details of biomolecular polymer synthesis to synthesize high conductivity PEDOT:PSS in a one-step, aqueous reaction.Acknowledgements
This work was supported by a Vasser Woolley Faculty Fellowship to CKP, an NSF REU Grant (CHE-1560335) to Georgia Tech, and by the School of Science and Technology at Georgia Gwinnnett College through its STEC 4500 research program.Notes and references
- B. L. Groenendaal, F. Jonas, D. Freitag, H. Pielartzik and J. R. Reynolds, Adv. Mater., 2000, 12, 481–494 CrossRef.
- S. Kirchmeyer and K. Reuter, J. Mater. Chem., 2005, 15, 2077–2088 RSC.
- K. Sun, S. Zhang, P. Li, Y. Xia, X. Zhang, D. Du, F. Isikgor and J. Ouyang, J. Mater. Sci.: Mater. Electron., 2015, 26, 4438–4462 CrossRef CAS.
- M. Dietrich, J. Heinze, G. Heywang and F. Jonas, J. Electroanal. Chem., 1994, 369, 87–92 CrossRef CAS.
- G. Heywang and F. Jonas, Adv. Mater., 1992, 4, 116–118 CrossRef CAS.
- X. Cui and D. C. Martin, Sens. Actuators, B, 2003, 89, 92–102 CrossRef CAS.
- K. A. Ludwig, J. D. Uram, J. Yang, D. C. Martin and D. R. Kipke, J. Neural. Eng., 2006, 3, 59–70 CrossRef PubMed.
- T. F. Otero and J. G. Martinez, J. Mater. Chem. B, 2016, 4, 2069–2085 RSC.
- F. Jonas, W. Krafft and B. Muys, Macromol. Symp., 1995, 100, 169–173 CrossRef.
- W. H. Kim, A. J. Makinen, N. Nikolov, R. Shashidhar, H. Kim and Z. H. Kafafi, Appl. Phys. Lett., 2002, 80, 3844–3846 CrossRef CAS.
- C. Zhong, C. Duan, F. Huang, H. Wu and Y. Cao, Chem. Mater., 2010, 23, 326–340 CrossRef.
- C. A. Zuniga, S. Barlow and S. R. Marder, Chem. Mater., 2011, 23, 658–681 CrossRef CAS.
- F. Chen, Q. Lin, H. Wang, L. Wang, F. Zhang, Z. Du, H. Shen and L. S. Li, Nanoscale Res. Lett., 2016, 11, 376 CrossRef PubMed.
- W. J. Hong, Y. X. Xu, G. W. Lu, C. Li and G. Q. Shi, Electrochem. Commun., 2008, 10, 1555–1558 CrossRef CAS.
- G. Li, V. Shrotriya, J. Huang, Y. Yao, T. Moriarty, K. Emery and Y. Yang, Nat. Mater., 2005, 4, 864–868 CrossRef CAS.
- F. L. Zhang, A. Gadisa, O. Inganäs, M. Svensson and M. R. Andersson, Appl. Phys. Lett., 2004, 84, 3906–3908 CrossRef CAS.
- Ö. Yagci, S. S. Yesilkaya, S. A. Yüksel, F. Ongül, N. M. Varal, M. Kus, S. Günes and O. Icelli, Synth. Met., 2016, 212, 12–18 CrossRef.
- F. Ongul, S. A. Yuksel, M. Kazici, S. Bozar, A. Gunbatti and S. Gunes, Polym. Adv. Technol., 2015 DOI:10.1002/pat.3677.
- E. P. Tomlinson, M. E. Hay and B. W. Boudouris, Macromolecules, 2014, 47, 6145–6158 CrossRef CAS.
- K. C. Donavan, J. A. Arter, R. Pilolli, N. Cioffi, G. A. Weiss and R. M. Penner, Anal. Chem., 2011, 83, 2420–2424 CrossRef CAS PubMed.
- J. A. Arter, D. K. Taggart, T. M. McIntire, R. M. Penner and G. A. Weiss, Nano Lett., 2010, 10, 4858–4862 CrossRef CAS PubMed.
- N. K. Guimard, N. Gomez and C. E. Schmidt, Prog. Polym. Sci., 2007, 32, 876–921 CrossRef CAS.
- S. M. Richardson-Burns, J. L. Hendricks and D. C. Martin, J. Neural. Eng., 2007, 4, L6–L13 CrossRef PubMed.
- S. M. Richardson-Burns, J. L. Hendricks, B. Foster, L. K. Povlich, D. H. Kim and D. C. Martin, Biomaterials, 2007, 28, 1539–1552 CrossRef CAS PubMed.
- A. Elschner, S. Kirchmeyer, W. Lovenich, U. Merker and K. Reuter, PEDOT: Principles and Applications of an Intrinsically Conductive Polymer, CRC Press, Boca Raton, Fl, 2010 Search PubMed.
- H. Shi, C. Liu, Q. Jiang and J. Xu, Adv. Electron. Mater., 2015, 1, 150017 Search PubMed.
- C. Yeon, S. J. Yun, J. Kim and J. W. Lim, Adv. Electron. Mater., 2015, 1, 1500121 CrossRef.
- K. Lim, S. Jung, S. Lee, J. Heo, J. Park, J.-W. Kang, Y.-C. Kang and D.-G. Kim, Org. Electron., 2014, 15, 1849–1855 CrossRef CAS.
- S. Kobayashi, H. Uyama and S. Kimura, Chem. Rev., 2001, 101, 3793–3818 CrossRef CAS PubMed.
- S. Kobayashi, J. Polym. Sci., Part A: Polym. Chem., 1999, 37, 3041–3056 CrossRef CAS.
- B. Ryan, K. Akshay, R. Sethumadhavan, N. Subhalakshmi, K. Jayant, A. S. Lynne, F. B. Ferdinando and N. Ramaswamy, in Green Polymer Chemistry: Biocatalysis and Biomaterials, American Chemical Society, 2010, vol. 1043, ch. 23, pp. 315–341 Search PubMed.
- W. Liu, J. Kumar, S. Tripathy, K. J. Senecal and L. Samuelson, J. Am. Chem. Soc., 1999, 121, 71–78 CrossRef CAS.
- K. S. Alva, J. Kumar, K. A. Marx and S. K. Tripathy, Macromolecules, 1997, 30, 4024–4029 CrossRef CAS.
- W. Liu, S. Bian, L. Li, L. Samuelson, J. Kumar and S. Tripathy, Chem. Mater., 2000, 12, 1577–1584 CrossRef CAS.
- J. S. Dordick, M. A. Marletta and A. M. Klibanov, Biotechnol. Bioeng., 1987, 30, 31–36 CrossRef CAS PubMed.
- H. Uyama, H. Kurioka, I. Kaneko and S. Kobayashi, Chem. Lett., 1994, 23, 423–426 CrossRef.
- V. Rumbau, J. A. Pomposo, A. Eleta, J. Rodriguez, H. Grande, D. Mecerreyes and E. Ochoteco, Biomacromolecules, 2007, 8, 315–317 CrossRef CAS PubMed.
- J. D. Morris, K. M. Wong, C. D. Penaherrera and C. K. Payne, Biomater. Sci., 2016, 4, 331–337 RSC.
- R. A. Gross, A. Kumar and B. Kalra, Chem. Rev., 2001, 101, 2097–2124 CrossRef CAS PubMed.
- S. M. Hira and C. K. Payne, Synth. Met., 2013, 176, 104–107 CrossRef CAS.
- J. D. Morris, D. Khanal, J. A. Richey and C. K. Payne, Biomater. Sci., 2015, 3, 442–445 RSC.
- A. Tewari, A. Kokil, S. Ravichandran, S. Nagarajan, R. Bouldin, L. A. Samuelson, R. Nagarajan and J. Kumar, Macromol. Chem. Phys., 2010, 211, 1610–1617 CrossRef CAS.
- S. Nagarajan, J. Kumar, F. F. Bruno, L. A. Samuelson and R. Nagarajan, Macromolecules, 2008, 41, 3049–3052 CrossRef CAS.
- G. Shumakovich, G. Otrokhov, I. Vasil'eva, D. Pankratov, O. Morozova and A. Yaropolov, J. Mol. Catal. B: Enzym., 2012, 81, 66–68 CrossRef CAS.
- J. D. Morris and C. K. Payne, Org. Electron., 2014, 15, 1707–1710 CrossRef CAS.
- E. Nagababu and J. M. Rifkind, Biochemistry, 2000, 39, 12503–12511 CrossRef CAS PubMed.
- E. Nagababu and J. M. Rifkind, Biochem. Biophys. Res. Commun., 1998, 247, 592–596 CrossRef CAS PubMed.
- J. M. C. Gutteridge, FEBS Lett., 1986, 201, 291–295 CrossRef CAS PubMed.
- R. E. Childs and W. G. Bardsley, Biochem. J., 1975, 145, 93–103 CrossRef CAS PubMed.
- M. B. Arnao, M. Acosta, J. A. del Rio and F. García-Cánovas, Biochim. Biophys. Acta, 1990, 1038, 85–89 CrossRef CAS.
- A. M. Nardes, M. Kemerink, R. A. J. Janssen, J. A. M. Bastiaansen, N. M. M. Kiggen, B. M. W. Langeveld, A. J. J. M. van Breemen and M. M. de Kok, Adv. Mater., 2007, 19, 1196–1200 CrossRef CAS.
- Z. Qi and P. G. Pickup, Chem. Commun., 1998, 2299–2300, 10.1039/A805322G.
- A. N. Aleshin, S. R. Williams and A. J. Heeger, Synth. Met., 1998, 94, 173–177 CrossRef CAS.
- Y. Xia and J. Ouyang, ACS Appl. Mater. Interfaces, 2010, 2, 474–483 CAS.
- Y. Xia, K. Sun and J. Ouyang, Adv. Mater., 2012, 24, 2436–2440 CrossRef CAS PubMed.
- N. Kim, S. Kee, S. H. Lee, B. H. Lee, Y. H. Kahng, Y.-R. Jo, B.-J. Kim and K. Lee, Adv. Mater., 2014, 26, 2268–2272 CrossRef CAS PubMed.
- F. L. E. Jakobsson, X. Crispin, L. Lindell, A. Kanciurzewska, M. Fahlman, W. R. Salaneck and M. Berggren, Chem. Phys. Lett., 2006, 433, 110–114 CrossRef CAS.
- R. F. Goddu and D. A. Delker, Anal. Chem., 1960, 32, 140–141 CAS.
- J. R. Lakowicz, Principles of fluorescence spectroscopy, Springer Science & Business Media, 2013 Search PubMed.
- C. Lee and J. Yoon, J. Photochem. Photobiol., A, 2008, 197, 232–238 CrossRef CAS.
- M. F. Perutz, J. K. M. Sanders, D. H. Chenery, R. W. Noble, R. R. Pennelly, L. W. M. Fung, C. Ho, I. Giannini, D. Poerschke and H. Winkler, Biochemistry, 1978, 17, 3640–3652 CrossRef CAS PubMed.
- E. A. Rachmilewitz, J. Peisach and W. E. Blumberg, J. Biol. Chem., 1971, 246, 3356–3366 CAS.
- B. L. Horecker, J. Biol. Chem., 1943, 148, 173–183 CAS.
- C. Giulivi and K. J. A. Davies, in Methods Enzymol., Academic Press, 1994, vol. 231, pp. 490–496 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Materials and methods, near IR spectra of hemoglobin–PEDOT:PSS and FeCl3–PEDOT:PSS as a function of ABTS concentration, UV-Vis spectra of hemoglobin and ABTS in HCl–KCl, fluorescence emission spectra of ABTS, ABTS with hydrogen peroxide, and hemoglobin in HCl–KCl. See DOI: 10.1039/c7ra00887b |
| This journal is © The Royal Society of Chemistry 2017 |