Open Access Article
Open Access ArticleThe properties and catalytic performance of PtSn/Mg(x-Ga)AlO catalysts for ethane dehydrogenation†
Shuqi Fanga,
Keting Zhang a,
Chenguang Wang*b,
Longlong Mab,
Qi Zhangb,
Qiying Liub,
Lungang Chenb,
Lingpeng Chenbc,
Qiao Zhangb and
Zhipeng Tianbc
a,
Chenguang Wang*b,
Longlong Mab,
Qi Zhangb,
Qiying Liub,
Lungang Chenb,
Lingpeng Chenbc,
Qiao Zhangb and
Zhipeng Tianbc
aSchool of Chemical Engineering and Energy, Zhengzhou University, Zhengzhou 450001, P. R. China
bKey Laboratory of Renewable Energy, Guangzhou Institute of Energy Conversion, Chinese Academy of Sciences, Guangzhou, 510640, P. R. China. E-mail: wangcg@ms.giec.ac.cn; Fax: +86 20 87057789
cUniversity of Chinese Academy of Sciences, Beijing 100049, P. R. China
First published on 25th April 2017
Abstract
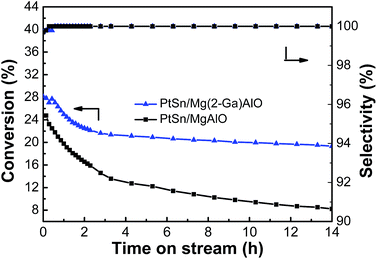
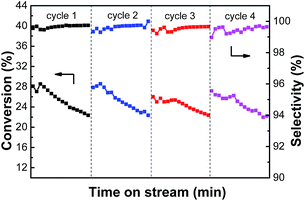
Mg(Ga)AlO hydrotalcite materials with different Ga contents are synthesized via the coprecipitation method and then used as supports for PtSn/Mg(x-Ga)AlO catalysts, in which Pt and Sn nanoparticles are deposited via the anion exchange method. The results indicate that a suitable content of Ga evidently enhances the performances and stability of ethane dehydrogenation for the PtSn/Mg(x-Ga)AlO catalysts. The SBET of the catalyst increases with the addition of Ga (2–4.5 wt%). The total acidity and strong acid sites decrease significantly when a small quantity of Ga is added. It is favorable for the catalysts to possess a smaller size and better distribution of Pt particles. Moreover, a suitable content of Ga enhances the interaction between Sn and the support and inhibits the reduction of oxidized Sn species, which is helpful to enhance the interaction between the Pt and Sn atoms. The PtSn/Mg(2-Ga)AlO catalyst exhibits the best performance of all the catalysts during the ethane dehydrogenation reaction, with an average ethane conversion of 25.4% for a period of 2 h. No evident activity decrease is observed after 4 cycles of 2 h testing.
1. Introduction
Recently, the demand for light olefins has gradually increased due to their wide use as building blocks in the chemical industry. Steam cracking and fluid catalytic cracking of crude-oil-derived naphtha and other oil byproducts are the traditional methods to obtain light olefins.1–4 Compared with the traditional methods, the catalytic dehydrogenation of light alkanes exhibits numerous advantages and is more economic and environmentally friendly.5 However, catalytic dehydrogenation is an endothermic process, and in order to obtain a high yield of olefin, the reaction must be carried out at a relatively high temperature, resulting in the sintering of Pt particles and undesirable side reactions, which are the main reasons for the deactivation of the catalyst.6,7 Therefore, the development of catalysts with high activity, stability and selectivity is very important.The performance of catalysts is greatly impacted by their support.8,9 Al2O3 is suggested as a potential support because of its unique physical properties, such as large surface area and tunable pore size over a wide range. However, since alumina is a type of acidic oxide, deactivation and low selectivity are unavoidable due to its intrinsic strong acid sites. A great amount of research has shown that non-acidic supports, including K–L zeolite, spinels, alkali doped alumina, and calcined hydrotalcite, can greatly enhance the desorption of alkenes and minimize coke formation.10–12 Among them, calcined hydrotalcite or hydrotalcite-like compounds are seen as ideal supports because they have moderately basic character, high thermal stability and high metal dispersion.13,14 MgAlO hydrotalcite is a type of layered double hydroxide (LDH), where in the hydrotalcite structure, some Mg2+ ions are replaced by Al3+ ions, forming positively charged layers. The positive charge is balanced by interlayer anions such as CO32−, which are situated between the brucite-like layers. Some divalent and trivalent metal cations can form the same structure as that of hydrotalcite, and is called a hydrotalcite-like compound.15 It is generally accepted that the ordered crystal structure of LDHs is formed by the supramolecular interactions between the host metal layers and the guest interlayer anions, and this determines the exchangeability of the interlayer anions.16,17 LDHs with different interlayer inorganic or organic anions can be obtained via the ion exchange method, which is useful for the precise synthesis of catalysts.18
Platinum is known as the most effective metal for the dehydrogenation of light alkanes.19 However, without modifiers monometallic Pt supported catalysts exhibit low olefin selectivity and deactivate quickly due to the sintering of Pt particles and rapid coke formation.20,21 To improve the catalyst performance, some bimetallic catalysts were synthesized using doping promoting elements such as Sn, In, Ga, Mn, Zn and Ca.22,23 Among them, Pt–Sn supported catalysts have been widely researched. The influence of Sn on Pt has been attributed to both geometric and electronic effects.6 It is well known that the addition of Sn is beneficial for Pt supported catalysts to form smaller Pt particles and a more uniform distribution, reducing the surface Lewis acid sites of the catalysts. Moreover, Sn can also change the interaction between Pt and the support and enhance catalytic stability.24,25
Although much attention has been given to Sn as a promoting element, studies have also found that Ga is an effective promoter for propane dehydrogenation.26,27 Jablonski et al.28 synthesized a PtGa/Al2O3 catalyst that exhibited higher activity and stability than the Pt/Al2O3 catalyst, and they also found that the catalyst deactivation and carbon deposition were restrained by the addition of Ga. Sun et al.29,30 synthesized a Pt/Mg(Ga)AlO catalyst using a hydrotalcite material (Mg(Ga)AlO) as the support, and they found that the performance of the catalyst was strongly influenced by the Ga content. Redekop et al.31 proved that Ga migrates from the surface of the support to the supported Pt nanoparticles to form Pt–Ga alloys, which can make the catalyst more selective and less prone to coking. Homs et al.32 studied a silica supported PtSn alloy doped with Ga, and observed the electronic modification of the platinum by the Pt–Ga interaction. Wang et al.33 researched the effect of Ga doping on Pt/CeO2–Al2O3 catalysts for propane dehydrogenation, and they found that Ga improves propylene desorption and greatly suppresses deep dehydrogenation and coke formation. Shao et al.34 studied the properties and catalytic performance of Ga2O3-based catalysts for the propane dehydrogenation reaction, where the initial propane conversion of the 5Ga2O3/ZSM-5 catalyst reached up to 78.1% at 620 °C and atmospheric pressure.
Since Sn and Ga both have distinct positive effects on the catalyst performance in the dehydrogenation of light alkanes and the synergistic effect between Pt, Sn and Ga is not well studied, herein, a series of hydrotalcite-like materials (Mg(Ga)AlO) is synthesized via the coprecipitation method with different contents of Ga. Pt and Sn nanoparticles are dispersed via the anion exchange method and then applied to the ethane dehydrogenation reaction after reduction. The synthesized catalysts are characterized via several analytical techniques. In addition, the influence of Ga loading on the catalytic performance of the PtSn/Mg(x-Ga)AlO catalysts for the ethane dehydrogenation reaction is investigated.
2. Experimental
2.1. Preparation of Mg(x-Ga)AlO supports
The hydrotalcite-like support was synthesized via the coprecipitation method.35 Briefly, an appropriate amount (Mg2+/Al3+ = 2; the loading of Ga was 0, 0.75, 2, 3 and 4.5 wt%) of Mg(NO)2·6H2O, Al(NO)3·9H2O and Ga(NO)3·9H2O were dissolved in 100 mL deionized water to form a mixed metal nitrate solution. Equal volumes of 1.0 M Na2CO3 and 2.0 M NaOH were mixed to form a mixed base solution. Then, these two solutions were mixed by dropwise addition to a reaction vessel containing 200 mL deionized water that was adjusted to pH 10.0 using the mixed base solution in advance. The reaction pH was maintained at 10.0, and the reaction was stirred heavily for the entire process. The entire procedure was carried out at room temperature. Once all of the mixed metal nitrate solution was consumed, an additional amount of 2.0 M NaOH was added to keep the reaction pH at 10.0 for the rest of the precipitation reaction. The mixture was then aged for 12 h at 100 °C with strong stirring. The resulting suspension was filtered, washed with deionized water to neutrality, and dried overnight in air at 100 °C. The dried material was described as hydrotalcite-like Mg(x-Ga)AlO support, where x represents the mass percentage of Ga in the catalysts.2.2. Preparation of PtSn/Mg(x-Ga)AlO catalysts
PtSn/Mg(x-Ga)AlO catalysts were prepared via the anion exchange method.36,37 In this method, appropriate amounts (depending on the Pt/Sn molar ratio and Pt wt%) of K2PtCl6 and Na2SnO3 were dissolved in 50 mL deionized water, and the mixed solution was heated at 70 °C. Next, 1 g dried hydrotalcite Mg(x-Ga)AlO was added to the mixed solution and stirred vigorously for 24 h at 70 °C to allow PtCl62− and SnO32− to sufficiently exchange with CO32−. After that, the suspension was filtered, washed and dried overnight in air at 100 °C. Then, the samples were reduced by hydrogen at 600 °C to obtain the PtSn/Mg(x-Ga)AlO catalysts. The contents of Pt and Sn were 0.5 wt% and 0.25 wt%.2.3. Catalyst characterization
2.4. Direct dehydrogenation of ethane
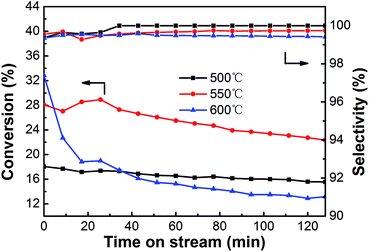
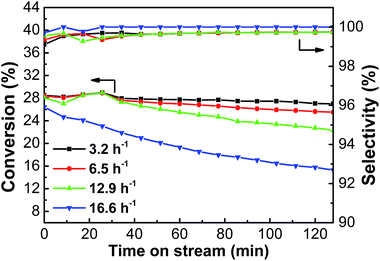
The ethane direct dehydrogenation reactions of the different catalysts were carried out in a fixed-bed quartz reactor with an inner diameter of 8 mm under atmospheric pressure. The catalyst (100 mg) was placed in the center of reactor and reduced in an atmosphere of 10% H2/N2 at 600 °C for 3 h. Then, a feed containing C2H6 and N2 (molar ratio of C2H6/N2 = 0.25, WHSV = 12.9 h−1) was used to determine the activity, selectivity and stability of all the PtSn/Mg(x-Ga)AlO catalysts. The ethane dehydrogenation reactions were carried out at 550 °C. The reaction products were analyzed online using a gas chromatography-mass spectrometer (GC-MS, Agilent 7890B). A flame ionization detector (FID) was used to quantify the concentrations of all organic compounds eluting from the capillary column. A thermal conductivity detector (TCD) was used to quantify H2, CO and N2. The ethane conversion and ethylene selectivity are defined as follows:
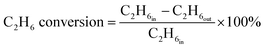 | (1) |
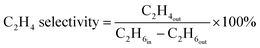 | (2) |
3. Results and discussion
3.1. Characterization of the samples
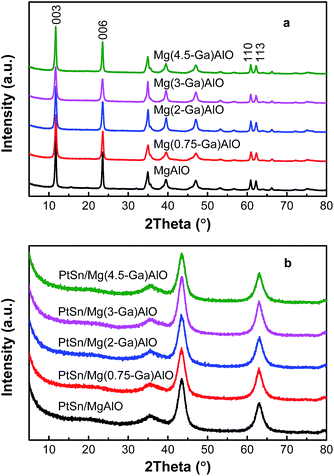 | ||
| Fig. 1 X-ray diffraction patterns for (a) hydrotalcite-like Mg(x-Ga)AlO and (b) PtSn/Mg(x-Ga)AlO catalysts. | ||
The XRD patterns of the catalysts are illustrated in Fig. 1b. As can be seen, all the characteristic diffraction peaks of the hydrotalcite material disappear and only characteristic diffraction peaks corresponding to MgO are observed. The reason for this is that the structure changes from a two-dimensional layered structure to a three-dimensional structure. The diffraction peaks of Al, Ga, Al2O3 or Ga2O3 species were not detected for all the catalysts because Al and Ga are highly dispersed in the LDHs during the synthetic process and located in the interstitial sites in the MgO framework after calcination;39 thus the crystal structure of MgO was not destroyed by the addition of Al and Ga. Moreover, the diffraction peaks of Pt and Sn species are also not detected since their small particle size and/or low concentration is lower than the XRD detection limitation.40
| Catalyst | Loadings (wt%) | SBET (m2 g−1) | Vp (cm3 g−1) | |
|---|---|---|---|---|
| Pt | Sn | |||
| PtSn/MgAlO | 0.34 | 0.19 | 212.7 | 0.80 |
| PtSn/Mg(0.75-Ga)AlO | 0.49 | 0.21 | 215.8 | 0.75 |
| PtSn/Mg(2-Ga)AlO | 0.47 | 0.21 | 276.4 | 0.96 |
| PtSn/Mg(3-Ga)AlO | 0.49 | 0.22 | 273.3 | 0.94 |
| PtSn/Mg(4.5-Ga)AlO | 0.46 | 0.20 | 278.2 | 0.96 |
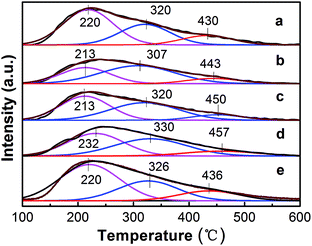 | ||
| Fig. 3 NH3-TPD profiles of the PtSn/Mg(x-Ga)AlO catalysts: (a) PtSn/MgAlO, (b) PtSn/Mg(0.75-Ga)AlO, (c) PtSn/Mg(2-Ga)AlO, (d) PtSn/Mg(3-Ga)AlO and (e) PtSn/Mg(4.5-Ga)AlO. | ||
| Catalyst | Peak temperature (°C) | Peak area fraction (%) | Total area (a.u.) | ||||
|---|---|---|---|---|---|---|---|
| Peak I | Peak II | Peak III | Peak I | Peak II | Peak III | ||
| PtSn/MgAlO | 220 | 320 | 430 | 55 | 30 | 15 | 785.46 |
| PtSn/Mg(0.75-Ga)AlO | 213 | 307 | 443 | 55 | 34 | 11 | 561.11 |
| PtSn/Mg(2-Ga)AlO | 213 | 320 | 450 | 45 | 45 | 10 | 650.7 |
| PtSn/Mg(3-Ga)AlO | 232 | 330 | 457 | 44 | 44 | 12 | 673.7 |
| PtSn/Mg(4.5-Ga)AlO | 220 | 326 | 436 | 55 | 30 | 15 | 1002.5 |
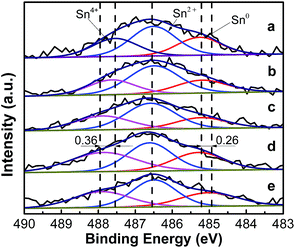 | ||
| Fig. 4 Sn 3d5/2 XPS profiles of PtSn/Mg(x-Ga)AlO catalysts: (a) PtSn/MgAlO, (b) PtSn/Mg(0.75-Ga)AlO, (c) PtSn/Mg(2-Ga)AlO, (d) PtSn/Mg(3-Ga)AlO, and (e) PtSn/Mg(4.5-Ga)AlO. | ||
| BEa (eV) | |||||
|---|---|---|---|---|---|
| PtSn/MgAlO | PtSn/Mg(0.75-Ga)AlO | PtSn/Mg(2-Ga)AlO | PtSn/Mg(3-Ga)AlO | PtSn/Mg(4.5-Ga)AlO | |
| a BE: binding energy. | |||||
| Sn0 | 485.27 (26.8%) | 485.20 (23.82%) | 485.22 (21.28%) | 485.29 (26.53%) | 485.01 (27.17%) |
| Sn2+ | 486.51 (45.45%) | 486.49 (51.44%) | 486.59 (49.34%) | 486.61 (36.73%) | 486.49 (41.25%) |
| Sn4+ | 487.55 (27.74%) | 487.63 (24.74%) | 487.88 (29.37%) | 487.86 (36.74%) | 487.91 (31.59%) |
3.2. Catalytic performances in ethane dehydrogenation
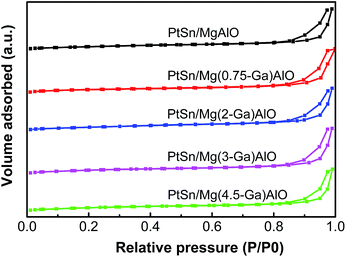
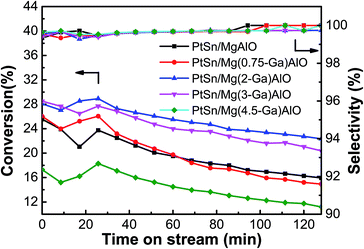
The results indicate that the presence of Ga has an evident impact on the ethane dehydrogenation performances of the PtSn/Mg(x-Ga)AlO catalysts. This can be explained as follows: first, according to the low temperature nitrogen adsorption–desorption experimental results in Table 2, we know that the addition of Ga can change the structure of the catalysts to some extent, leading to an increase in the SBET value of the PtSn/Mg(x-Ga)AlO catalysts, resulting in a better distribution of Pt particles, more Pt active reaction sites and better performances of ethane dehydrogenation.48
According to the NH3-TPD results, Ga can reduce the acidity of the catalysts, particularly the strong acid sites which is the main reason for side reactions. Therefore, it can effectively suppress the undesired side reactions for ethane dehydrogenation and improve the ethane conversion and ethylene selectivity. According to previous research, the PtSn/Mg(x-Ga)Al catalyst is bifunctional, and its two active centers (the metal particle and the acid site) work collaboratively. An optimum ratio exists between the number of metal active sites and the number of acid sites.49 The ratio between the support acid sites and the number of metal sites of the PtSn/Mg(x-Ga)Al catalyst may change by the addition of Ga so that a better catalytic performance can be obtained. Perhaps the PtSn/Mg(2-Ga)Al catalyst possesses an optimum ratio between the two types of sites.
According to previous research, the state of Sn has a significant influence on the performance of the catalytic properties of Pt. Metallic Sn has an evident inhibiting effect on the catalyst performance. However, when it exists in the oxidized state, it acts as a promoter.47 The XPS results indicate that the PtSn/Mg(2-Ga)Al catalyst has the lowest percent of tin metal and the highest percent of oxidation states of Sn species.
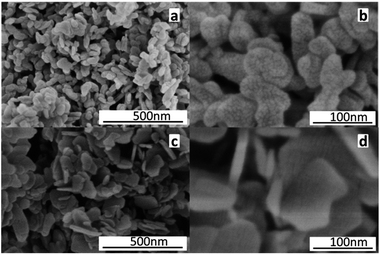
The micrographs of the hydrotalcite-like crystals obtained by SEM analysis are shown in Fig. 6, where great differences can be observed between MgAlO and Mg(2-Ga)AlO. It can be seen that the former exhibits mainly small tabular particles with a flake-like structure mixed in it, whereas the latter is formed mainly by a flake-like structure with a thickness of 10 nm; thus the addition of Ga causes hydrotalcite to form a more regular layer structure. The SEM image of the reduced PtSn/Mg(2-Ga)AlO catalyst is shown in Fig. S2,† in which some hydrotalcite layers are still observed. Therefore, it can be concluded that the structure of the catalysts was changed by the addition of Ga, which is in accordance with the BET results.
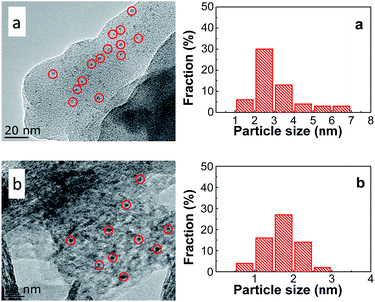
The morphology and metal particle size of PtSn/Mg(x-Ga)AlO were characterized by TEM in Fig. 7. It can be seen that the PtSn/Mg(2-Ga)AlO catalyst has a smaller average metal particle size (1.69 nm) than the 0.5PtSn/MgAlO catalyst (3.12 nm), and it exhibits a better distribution of Pt particles, which indicates that the addition of a certain amount of Ga is beneficial for the formation of small Pt particles and their uniform distribution. This can be explained as follows: on one hand, the presence of Ga has a dilution effect on platinum, which is conducive to decrease the size of the Pt particles.10 On the other hand, the addition of Ga can promote hydrotalcite to form more regular thin sheet particles, and thus a more thorough iron exchange can occur, which is beneficial for the uniform distribution of the Pt component on the support. According to previous research, smaller Pt particles and their better distribution can supply more Pt active reaction sites and inhibit the hydrogen and carbon deposition reaction at the same time. This is because hydrogen and carbon deposition are structure sensitive reactions that require big Pt particles.
4. Conclusions
Hydrotalcite Mg(x-Ga)AlO materials were synthesized via the coprecipitation method with a constant pH, and PtSn/Mg(x-Ga)AlO catalysts were prepared via the anion exchange method. The XRD, BET and SEM measurements show that the structure of the catalysts changed to some extent on the addition of Ga, but the crystal structure was not destroyed. The NH3-TPD results suggest that a moderate amount of Ga can markedly neutralize the acid sites of the catalysts and effectively suppress the undesired side reactions for ethane dehydrogenation. The TEM measurements show that the addition of Ga in the catalyst is beneficial for the formation of small Pt particles and their uniform distribution. The average particle size of the PtSn/Mg(2-Ga)AlO catalyst is 1.69 nm. The XPS results verify that the suitable Ga content can strengthen the Sn–support and Pt–Sn interactions, which can enhance the properties of the catalysts. The PtSn/Mg(2-Ga)AlO catalyst exhibits the best ethane dehydrogenation performance. The ethane conversion of the PtSn/Mg(2-Ga)AlO catalyst varies from 25.6% to 17.0% in a 14 h ethane dehydrogenation reaction. The catalyst performance has no evident decline after 4 reaction–regeneration cycles with selectivity above 99% for each cycle.Acknowledgements
This study was supported by the NSFC (Natural Science Foundation of China) project (51476175, 51536009), the National Key basic Research Program of China (973 program, 2013BC228105) and the Chinese Academy of Sciences “one hundred talented plan”.Notes and references
- N. A. Pakhomov, V. N. Kashkin, E. I. Nemykina, V. V. Molchanov, V. I. Nadtochiy and A. S. Noskov, Chem. Eng. J., 2009, 154, 185–188 CrossRef CAS.
- F. Cavani, N. Ballarini and A. Cericola, Catal. Today, 2007, 127, 113–131 CrossRef CAS.
- R. S. Vincent, R. P. Lindstedt, N. A. Malik, I. A. B. Reid and B. E. Messenger, J. Catal., 2008, 260, 37–64 CrossRef CAS.
- J. J. Sattler, J. Ruiz-Martinez, E. Santillan-Jimenez and B. M. Weckhuysen, Chem. Rev., 2014, 114, 10613 CrossRef CAS PubMed.
- L. Shi, G. M. Deng, W. C. Li, S. Miao, Q. N. Wang, W. P. Zhang and A. H. Lu, Angew. Chem., 2015, 54, 13994–13998 CrossRef CAS PubMed.
- K. Kumbilieva, N. A. Gaidai, N. V. Nekrasov, L. Petrov and A. L. Lapidus, Chem. Eng. J., 2006, 120, 25–32 CrossRef CAS.
- B. K. Vu, M. B. Song, I. Y. Ahn, Y.-W. Suh, D. J. Suh, W.-I. Kim, H.-L. Koh, Y. G. Choi and E. W. Shin, Appl. Catal., A, 2011, 400, 25–33 CrossRef CAS.
- J. Silvestre-Albero, J. C. Serrano-Ruiz, A. Sepúlveda-Escribano and F. Rodríguez-Reinoso, Appl. Catal., A, 2008, 351, 16–23 CrossRef CAS.
- Y. Zhang, Y. Zhou, J. Shi, S. Zhou, X. Sheng, Z. Zhang and S. Xiang, J. Mol. Catal. A: Chem., 2014, 381, 138–147 CrossRef CAS.
- S. A. Bocanegra, A. A. Castro, O. A. Scelza and S. R. de Miguel, Appl. Catal., A, 2007, 333, 49–56 CrossRef CAS.
- M. P. Lobera, C. Téllez, J. Herguido and M. Menéndez, Appl. Catal., A, 2008, 349, 156–164 CrossRef CAS.
- A. Virnovskaia, S. Morandi, E. Rytter, A. Giovanna Ghiotti and U. Olsbye, J. Phys. Chem. C, 2007, 111, 14732–14742 CAS.
- J. I. D. Cosimo, V. K. DíEz, M. Xu, E. Iglesia and C. R. ApesteguíA, J. Catal., 1998, 178, 499–510 CrossRef.
- J. H. Kwak, J. Hu, D. Mei, C.-W. Yi, D. H. Kim, C. H. F. Peden, L. F. Allard and J. Szanyi, Science, 2009, 325, 1670–1673 CrossRef CAS PubMed.
- R. L. Frost, S. J. Palmer and L.-M. Grand, J. Raman Spectrosc., 2010, 41, 1797–1802 CrossRef.
- J. T. Kloprogge, D. Wharton, L. Hickey and R. L. Frost, Am. Mineral., 2002, 87, 623–629 CrossRef CAS.
- S. Miyata, Clays Clay Miner., 1983, 31, 305–311 CAS.
- J. H. Lee, S. W. Rhee and D. Y. Jung, Chem. Mater., 2004, 16, 3774–3779 CrossRef CAS.
- B. Y. Jibril, A. Atta, K. Melghit, Z. El-Hadi and H. Ala'a, Chem. Eng. J., 2012, 193, 391–395 CrossRef.
- Y. Zhang, Y. Zhou, J. Shi, X. Sheng, Y. Duan, S. Zhou and Z. Zhang, Fuel Process. Technol., 2012, 96, 220–227 CrossRef CAS.
- P. Sun, G. Siddiqi, W. C. Vining, M. Chi and A. T. Bell, J. Catal., 2011, 282, 165–174 CrossRef CAS.
- J. Wu, Z. Peng and A. T. Bell, J. Catal., 2014, 311, 161–168 CrossRef CAS.
- C. Yu, H. Xu, Q. Ge and W. Li, J. Mol. Catal. A: Chem., 2007, 266, 80–87 CrossRef CAS.
- V. Galvita, G. Siddiqi, P. Sun and A. T. Bell, J. Catal., 2010, 271, 209–219 CrossRef CAS.
- K. Xia, W.-Z. Lang, P.-P. Li, L.-L. Long, X. Yan and Y.-J. Guo, Chem. Eng. J., 2016, 284, 1068–1079 CrossRef CAS.
- K. Searles, G. Siddiqi, O. V. Safonova and C. Copéret, Chem. Sci., 2017, 8, 2661–2666 RSC.
- R. Koirala, R. Buechel, F. Krumeich, S. E. Pratsinis and A. Baiker, ACS Catal., 2015, 5, 690–702 CrossRef CAS.
- E. Jablonski, A. Castro, O. Scelza and S. De Miguel, Appl. Catal., A, 1999, 183, 189–198 CrossRef CAS.
- P. Sun, G. Siddiqi, M. Chi and A. T. Bell, J. Catal., 2010, 274, 192–199 CrossRef CAS.
- G. Siddiqi, P. Sun, V. Galvita and A. T. Bell, J. Catal., 2010, 274, 200–206 CrossRef CAS.
- E. A. Redekop, V. V. Galvita, H. Poelman, V. Bliznuk, C. Detavernier and G. B. Marin, ACS Catal., 2014, 4, 1812–1824 CrossRef CAS.
- N. S. Homs, J. Llorca, M. Riera, J. Jolis, J.-L. G. Fierro, J. Sales and P. R. R. de la Piscina, J. Mol. Catal. A: Chem., 2003, 200, 251–259 CrossRef CAS.
- T. Wang, F. Jiang, G. Liu, L. Zeng, Z. J. Zhao and J. Gong, AIChE J., 2016, 62, 4365–4376 CrossRef CAS.
- C. T. Shao, W. Z. Lang, X. Yan and Y. J. Guo, Rsc. Adv., 2017, 7, 4710–4723 RSC.
- S. K. Yun and T. J. Pinnavaia, Chem. Mater., 1995, 7, 348–354 CrossRef CAS.
- O. Altuntasoglu, U. Unal, S. Ida, M. Goto and Y. Matsumoto, J. Solid State Chem., 2008, 181, 3257–3263 CrossRef CAS.
- L. P. Chen, Z. P. Tian, W. Lv, Z. Si, Q. Y. Liu, M. Y. Ding, L. G. Chen, L. L. Ma, Q. Zhang, T. J. Wang and C. G. Wang, J. Fuel Chem. Technol., 2016, 44, 597–606 CAS.
- Y. Lin, J. Wang, D. G. Evans and D. Li, J. Phys. Chem. Solids, 2006, 67, 998–1001 CrossRef CAS.
- J. A. van Bokhoven, J. Roelofs, K. P. de Jong and D. C. Koningsberger, Chem.–Eur. J., 2001, 7, 1258–1265 CrossRef CAS.
- Y. W. Zhang, Y. M. Zhou, X. L. Sheng, L. H. Wan, Y. J. Li, Y. M. Xiao, B. Yu and Z. Zeng, Fuel Process. Technol., 2012, 104, 23–30 CrossRef CAS.
- B. Xu, J. Long, H. Tian, Y. Zhu and X. Sun, Catal. Today, 2009, 147, S46–S50 CrossRef CAS.
- X. Liu, W.-Z. Lang, L.-L. Long, C.-L. Hu, L.-F. Chu and Y.-J. Guo, Chem. Eng. J., 2014, 247, 183–192 CrossRef CAS.
- C. Yu, Q. Ge, H. Xu and W. Li, Appl. Catal., A, 2006, 315, 58–67 CrossRef CAS.
- R. J. Balasamy, B. B. Tope, A. Khurshid, A. A. S. Al-Ali, L. A. Atanda, K. Sagata, M. Asamoto, H. Yahiro, K. Nomura, T. Sano, K. Takehira and S. S. Al-Khattaf, Appl. Catal., A, 2011, 398, 113–122 CrossRef CAS.
- F. B. Passos, D. A. Aranda and M. Schmal, J. Catal., 1998, 178, 478–488 CrossRef CAS.
- A. D. Ballarini, C. G. Ricci, S. R. de Miguel and O. A. Scelza, Catal. Today, 2008, 133, 28–34 CrossRef.
- J. Shi, Y. Zhou, Y. Zhang, S. Zhou, Z. Zhang, J. Kong and M. Guo, J. Mater. Sci., 2014, 49, 5772–5781 CrossRef CAS.
- K. Xia, W.-Z. Lang, P.-P. Li, X. Yan and Y.-J. Guo, Rsc. Adv., 2015, 5, 64689–64695 RSC.
- M. Larsson, M. Hulten, E. A. Blekkan and B. Andersson, J. Catal., 1996, 164, 44–53 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra00670e |
| This journal is © The Royal Society of Chemistry 2017 |