Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Preparation of sponge-like porous SiO2 antireflective coatings with excellent environment-resistance by an acid-catalysed sol–gel method
Bibo Xiaa,
Jianhui Luobc,
Yuanyang Lia,
Bowen Yanga,
Shuming Zhanga and
Bo jiang *a
*a
aKey Laboratory of Green Chemistry & Technology, College of Chemistry, Sichuan University, Chengdu 610064, China. E-mail: jiangbo@scu.edu.cn
bResearch Institute of Petroleum Exploration & Development (RIPED), PetroChina, China
cKey Laboratory of Nano Chemistry (KLNC), CNPC, P.O.Box 910, 20# Xueyuan Road, Haidian District, Beijing 100083, P. R. China
First published on 19th May 2017
Abstract
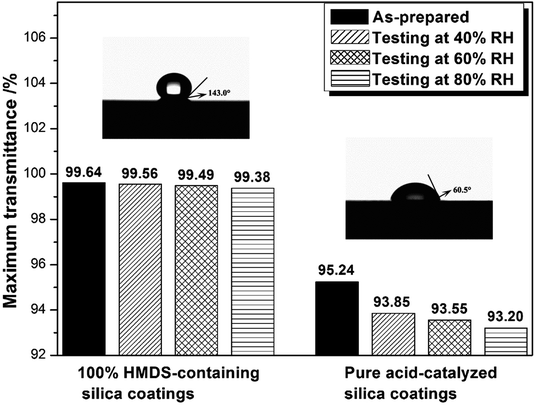
Silica coatings with high-transmittance and good environment-resistance properties were prepared by an acid-catalyzed sol–gel method without template. Hexamethyldisilazane (HMDS) was added into an acid-catalyzed silica sol which acted not only as the catalyst but also as a blocking agent. The effect of different contents of HMDS has been investigated. The sponge-like silica coating was obtained when the weight ratio of HMDS to SiO2 was higher than 90%. The addition of HMDS enabled these silica antireflective coatings to possess excellent hydrophobicity properties. When the weight ratio of HMDS to SiO2 was 100%, the silica coating exhibited transmittance as high as 99.64% at a given wavelength and static water contact angle of 143.0°. After 48 hours of environment aging testing at 40%, 60% and 80% RH, these coatings showed a minute decrease upon maximum transmittance, from 99.64% to 99.56%, 99.49% and 99.38%, respectively. The sponge-like HMDS-containing coatings exhibited easy-to-clean properties, which would have great potential application in laser systems.
1. Introduction
Antireflective (AR) coatings have been widely used in optical devices such as solar cells, laser systems and cathode ray tubes.1–3 The reflective index of coating (nc) and actual thickness of coating (dc) are decisive factors for obtaining an ideal homogeneous AR coating which achieves effectively 100% transmittance at a specific wavelength, that is, nc is equal to (nans)0.5, where na and ns are the refractive indices of the air and the substrate, respectively; and dc should be λ/4nc, where λ is the wavelength of incident light.4,5 A typical glass has a refractive index between 1.45 and 1.65 in the visible spectral region, which implies that the refractive index of a matched AR coating must be between 1.20 and 1.25. For instance, BK-7 (n = 1.52) requires a refractive index as low as 1.23. Dielectric materials such as silica, alumina, and titania with refractive indices of 1.45, 1.65, and 2.3, respectively, were widely used as AR coatings. The refractive index can be lowered by introducing nanopores (n = 1) into AR coatings.6 Several methods have been applied to generate nanoporous AR coatings, including etching,7,8 oblique-angle deposition,9,10 reactive twin-magnetron sputtering,11 phase separation and self-assembly of block copolymers,12 the layer-by-layer deposition method,13 and the sol–gel process.14 Compared with other methods, sol–gel-derived AR coating has many advantages, such as low cost, simple operation process and controllable structure.15Sol–gel acid-catalyzed silica coatings, possessing remarkable abrasion-resistance, have attracted tremendous attention in both academic and industrial areas.16–18 The growth of silica gel with acid catalyzed sol–gel process results in a structure of linear chains which leads to a strong adhesive force on the substrate and a high refractive index about 1.42.19 Therefore, an ideal AR coating cannot be obtained by just depositing single pure acid-catalyzed film on typical glass. Generation of micro pore in the silica film by porogen, which can be post-removed, is an efficient method to create micropore. Low molecular weight compound,20 surfactant16,21 and polymer22,23 acting as porogen had been studied widely. Nonetheless, high calcination temperature is undesirable for certain polymer substrate during the removal of additional porogen.
Here we report a new approach to generate manopore in the acid-catalyzed silica film so as to prepare low refractive index acid-catalyzed silica coating without porogen. When added into the acid-catalyzed silica sol, on the one hand, HMDS releases ammonia which can act as catalyzer; on the other hand, the volume effect of tri-methylsilyl group can hinder the condensation reaction. The importance of these two effects depends on the content of HMDS in the acid sol. Addition of HMDS changes deeply the morphology of the resulting acid-catalyzed silica film, by transforming the chain-silica to sponge-like porous silica. As the tri-methylsilyl group in the HMDS can afford the film good hydrophobicity,24,25 by adjusting the content of HMDS, sponge-like porous coatings with high-transmittance and prominent environmental resistance properties can be obtained.
2. Experimental section
2.1. Materials
Tetraethylorthoxylsilicane (TEOS, Si(OC2H5)4, 98%) and hexamethyldisilazane (HMDS, (CH3)3SiNHSi(CH3)3, 98%) were purchased from Acros and Alfa Aesar. Ethyl alcohol (C2H5OH, 99.7%) and concentrated hydrochloric acid (HCl, 37%) were purchased from Kelong Chemical Reagents Factory (Chengdu City, Sichuan Province, China). Ethyl alcohol was distilled twice before use. The water was deionized. All chemicals were used without further purification.2.2. Preparations of sponge-like porous coatings
The sol–gel HMDS-containing acid-catalyzed coatings were fabricated as follows: a solution of tetraethylorthoxylsilicane, ethyl alcohol, H2O and HCl was first prepared and stirred for 2 hours at 30 °C. The molar ratio of TEOS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O
H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C2H5OH
C2H5OH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HCl was 1
HCl was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.01
4.01![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 36.83
36.83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.16 × 10−3 and the final concentration of SiO2 was 3% by weight. The sol was aged in a water-bathing at 25 °C for 7 days and then refluxed for 24 hours to remove the catalyst (HCl), filtered through a 0.22 μm polyvinylidene fluoride (PVDF) filter. The resultant sol (named acid-catalyzed silica sol) was stored at room temperature for use. Hexamethyldisilazane was mixed in acid-catalyzed silica sol with the weight ratio of HMDS to SiO2 (WH/S) varying from 0% to 200%, and then stirred for 2 hours at 30 °C, the prepared sol was diluted twice prior to use. The borosilicate glass substrates (BK-7) were cleaned by ultrasonication in acetone for 10 min, and then wiped carefully with cleanroom wipers. Afterwards the silica sols were deposited on substrates by dip-coating at the withdrawal rate of 30 mm min−1 at 40 Relative Humidity (RH). The coatings were treated at 160 °C for 2 hours.
4.16 × 10−3 and the final concentration of SiO2 was 3% by weight. The sol was aged in a water-bathing at 25 °C for 7 days and then refluxed for 24 hours to remove the catalyst (HCl), filtered through a 0.22 μm polyvinylidene fluoride (PVDF) filter. The resultant sol (named acid-catalyzed silica sol) was stored at room temperature for use. Hexamethyldisilazane was mixed in acid-catalyzed silica sol with the weight ratio of HMDS to SiO2 (WH/S) varying from 0% to 200%, and then stirred for 2 hours at 30 °C, the prepared sol was diluted twice prior to use. The borosilicate glass substrates (BK-7) were cleaned by ultrasonication in acetone for 10 min, and then wiped carefully with cleanroom wipers. Afterwards the silica sols were deposited on substrates by dip-coating at the withdrawal rate of 30 mm min−1 at 40 Relative Humidity (RH). The coatings were treated at 160 °C for 2 hours.
2.3. Characterization
To determine particle size and distribution, the silica sols were analyzed by dynamic light scattering (DLS, Malvern Nano-ZS, wavelength of 632.8 nm) at 25 °C. The transmission spectra were measured with a UV-vis spectrophotometer (Mapada, UV-3200PC), with wavelength ranging from 300–1100 nm. Before transmission spectra measurement, the film thicknesses of AR coatings were optimized by varying the withdrawal rate and sol concentration. The structures of the HMDS-containing colloids were investigated with a transmission electron microscope (JEOL, JEM-100CX) operated at 200 kV. The samples were prepared on a holey carbon coated copper grid by placing a drop of the colloidal suspension used for coatings. SEM (JSM-5900LV) was used to observe the surface profile and cross section structure of coatings at low vacuum conditions. Static water contact angles of the coatings were performed using a contact angle meter (Krüss DSA100 Germany). To investigate the environment aging resistance of silica coatings, the prepared coatings were put into an unventilated cabin for 48 hours, whose humidity were 40%, 60% and 80% RH and temperature was 25 °C. Ammonia hardening experiment: HMDS-containing acid-catalyzed coatings were put into an unventilated cabin saturated with ammonia for 12 hours at 60 °C.3. Results and discussion
Particle size and its distribution were investigated by DLS and are shown in Fig. 1. For an AR coating prepared from silica sol, the sol particle structure is important factor which affect the values of the refractive index of the AR coating. The condensation reaction of TEOS under acid catalyzed conditions is an electrophilic reaction, as a result, chain-structured silica were formed.26 The particle size of pure acid-catalyzed silica sol is under 5 nm.26 When HMDS was added and the WH/S was lower than 90%, gelation occurred during the aging process of the HMDS-containing acid-catalyzed silica sol. The number particle size of different content of HMDS-modified acid-catalyzed silica sol was shown in Fig. 1. The average diameter of particle was 18.5 nm, when WH/S was 100%. While the WH/S increased to 150% and 200%, the corresponding particle size was 10.1 nm and 8.1 nm. It is indicated that a bigger size of silica particle was formed on account of the addition of HMDS.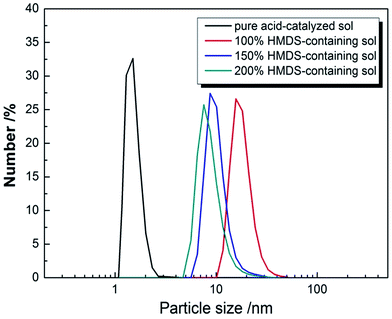 | ||
| Fig. 1 Particle size number of pure, 100%, 150% and 200% HMDS-containing acid-catalyzed silica sols. | ||
When added into an acid-catalyzed silica sol, HMDS reacted with chain-structured silica by substituting hydroxyl group with tri-methylsilyl group and released ammonia as catalyst. Under ammonia conditions, intermolecular hydroxyl group or intramolecular hydroxyl groups aggregated with each other through condensation reaction. Silica chains gathered together and formed the net silica structure. Meanwhile HMDS played another role on supplying the tri-methylsilyl which took the place of exterior hydroxyl group, hence hindered the condensation. Addition of HMDS resulted two competition effects, the release ammonia catalyst accelerated the condensation reaction of chain-structured acid-catalyzed silica, and tri-methylsilyl group prevented the silica structures from further condensation reaction. When the content of HMDS was small, there were not enough tri-methylsilyl to substitute with exterior hydroxyl group to counterbalance the effect of ammonia catalyst, the silica sol would polymerize excessively and gelation of the sol occurred during aging. When the WH/S was too high, the redundant tri-methylsilyl would substitute the most hydroxyl group, the polymerization of the silica sol would decrease dramatically, and then there would not be enough silica with certain size and shape. Only when the content of HMDS was appropriate, the effects of catalyst ammonia and tri-methylsilyl's hindering counterbalanced and silica nanoparticle with diameter ranging from 10 nm to 20 nm were obtained. It explains the particle size decreased as the WH/S increased from 100% to 200%. The TEM of 100% HMDS-containing acid-catalyzed silica coatings are shown in Fig. 2(a). The HMDS-modified acid-catalyzed silica agglomerated easily and turned into floccules structure. However, it is easily found that net-work structure generated by chain-structured silica appeared at the edge of TEM image. After deposited on silica wafer, the surface profile SEM and cross section SEM of 100% HMDS-containing acid-catalyzed silica coatings were depicted in Fig. 2(b) and (c). It is obvious that the 100% HMDS-containing acid-catalyzed coating showed as porous structure with uneven nanopore size. As the shape of HMDS-containing silica nanoparticle was irregular and the nanoparticle size was not uniform, after randomly stacking on substrate, the nanopore size of HMDS-containing coatings was also uneven. This porous structure with uneven nanopore size was called “sponge-like structure”.
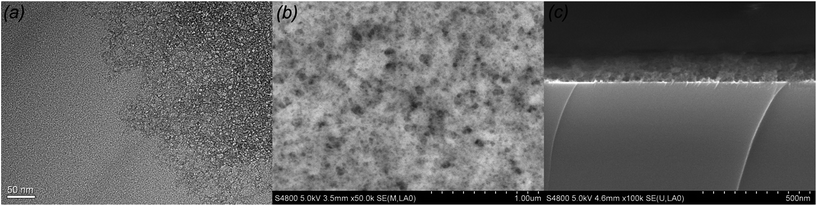 | ||
| Fig. 2 TEM of 100% HMDS-containing acid-catalyzed coating (a), surface SEM of 100% HMDS-containing acid-catalyzed coating (b) and cross section SEM of 100% HMDS-containing acid-catalyzed coating (c). | ||
Owing to the discrepancy of refractive indices between air and optical substrate (BK-7, n = 1.52), the transmittance of optical substrate in visible spectral region is about 91.8%. So, it is necessary to deposit coatings with corresponding refractive index on optical element to enhance the transmittance. Pure acid-catalyzed silica coating, which is composed of chain-structured silica, possesses a refractive index as high as 1.42. By depositing one-layer acid-catalyzed silica coating on BK-7 merely, it only brings an improvement of 3.5% upon transmittance as shown in Fig. 3. The addition of HMDS changed the morphology of acid-catalyzed silica, and decreased the refractive index of coating, hence increased transmittance of resulted coating. When the WH/S was 100%, HMDS-containing silica coating achieved a transmittance as high as 99.64%, bringing a significant improvement upon transmittance. When the WH/S increased to 150% or 200%, the transmittance of HMDS-containing silica coatings were 98.82% or 97.56%, probably due to a higher packing of a smaller silica particles during gel process.
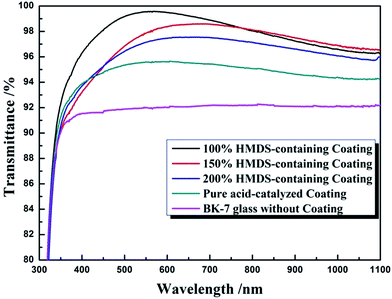 | ||
| Fig. 3 Spectral transmittance of borosilicate glass, acid-catalyzed coating and HMDS-containing coatings. | ||
Kim et al. suggested that the hydrophobic nature of a silica thin film was closely linked to the AR durability of the AR coating.27 Hydrophobicity can be monitored by the water contact angle (WCA). As shown in Fig. 4, pure acid-catalyzed silica coating was hydrophilic with a WCA of 60.5°. Because of the addition of HMDS, the WCA of containing silica coating enhanced conspicuously. When the WH/S was 100%, 150% and 200%, the coating's WCA reached to 143.0°, 145° and 148°, respectively. The environmental resistance properties of pure and HMDS-modified acid-catalyzed silica coating were investigated by putting them in saturated containers with 40%, 60% and 80% RH for 48 hours at 25 °C. We measured the maximum transmittance of as-prepared coatings and processed coatings. The change in maximum transmittance as a function of relative humidity after testing for 48 hours was shown in Fig. 4. It was found that after testing for 48 hours the pure acid-catalysed silica coating showed striking decrease on maximum transmittance, from 95.24% to 93.85%, 93.55% and 93.20% for 40%, 60% and 80% RH, respectively. However, 100%-HMDS acid-catalysed silica coatings only showed minute decrease upon maximum transmittance, from 99.64% to 99.56%, 99.49% and 99.38%, respectively. So these HMDS-modified acid-catalysed silica coatings could be considered to possess excellent environment aging resistance properties.
The growth of silica gel with acid catalyzed sol–gel process results in a structure of linear chains which leads to a strong adhesive force on the substrate and good abrasion resistance, however, the random assembly of sponge-like nanoparticle endowed the HMDS-containing coatings with poor mechanical durability, in other words, the easy-to-clean properties. A lot of works have been reported to address the mechanical durability of nanoparticle silica coatings,28,29 inspired by that, the ammonia hardening experiment had been applied to enhance the mechanical durability of the HMDS-containing acid-catalyzed coatings. Unfortunately, after ammonia modification, the HMDS-containing coating didn't show significant improvement on abrasion resistance properties. So, it was concluded that there almost remained no residual hydroxyl group in 100% HMDS-containing acid-catalyzed coating. These antireflective coatings with excellent environment aging resistance properties and easy-to-clean properties would have greatly potential application in laser systems.30
4. Conclusion
Antireflective silica coatings with environment resistance properties and easy-to-clean performance derived from acid-catalyzed silica were prepared via sol–gel process. We presented a new method to decrease the refractive index of acid-catalyzed silica coating by changing the morphology of chain-structured silica. The addition of HMDS into acid-catalyzed silica sol not only accelerated the polymerization but also introduced excellent hydrophobicity properties. These coatings with excellent performance may have a wide range of application in optical systems.Acknowledgements
The authors gratefully acknowledge the support from China National Petroleum & gas Corporation science and technology development project “Nano intelligent chemical flooding agent” (2014A-1001)Notes and references
- B. G. Prevo, E. W. Hon and O. D. Velev, J. Mater. Chem., 2007, 17, 791–799 RSC.
- L. Zhang, Z. Qiao, M. Zheng, Q. Huo and J. Sun, J. Mater. Chem., 2010, 20, 6125–6130 RSC.
- P. Prené, J. J. Priotton, L. Beaurain and P. Belleville, J. Sol-Gel Sci. Technol., 2000, 19, 533–537 CrossRef.
- B. G. Prevo, Y. Hwang and O. D. Velev, Chem. Mater., 2005, 17, 3642–3651 CrossRef CAS.
- W. T. Wang, N. Lu, J. Y. Hao, H. B. Xu, D. P. Qi and L. F. Chi, J. Phys. Chem. C, 2010, 114, 1989–1995 CAS.
- H. Hattori, Adv. Mater., 2001, 12, 51–54 CrossRef.
- B. Dudem, J. W. Leem and J. S. Yu, RSC Adv., 2016, 6, 3764–3773 RSC.
- M. Ibn-Elhaj and M. Schadt, Nature, 2001, 410, 796–799 CrossRef CAS PubMed.
- J. Q. Xi, M. F. Schubert, J. K. Kim, E. F. Schubert, M. Chen, S. Y. Lin, W. Liu and J. A. Smart, Nat. Photonics, 2007, 1, 176–179 CAS.
- J. Q. Xi, J. K. Kim and E. F. Schubert, Nano Lett., 2005, 5, 1385–1387 CrossRef CAS PubMed.
- J. Szczyrbowski, G. Bräeuer, M. Ruske, G. Teschner and A. Zmelty, J. Non-Cryst. Solids, 1997, 218, 262–266 CrossRef CAS.
- X. Li, L. Xue and Y. Han, J. Mater. Chem., 2011, 21, 5817–5827 RSC.
- J. Hiller, J. D. Mendelsohn and M. F. Rubner, Nat. Mater., 2002, 1, 59–63 CrossRef CAS PubMed.
- Y. Du, L. E. Luna, W. S. Tan, M. F. Rubner and R. E. Cohen, ACS Nano, 2010, 4, 4308–4316 CrossRef CAS PubMed.
- D. R. Uhlmann, T. Suratwala, K. Davidson, J. M. Boulton and G. Teowee, J. Non-Cryst. Solids, 1997, 218, 113–122 CrossRef CAS.
- L. Yao and J. He, J. Mater. Chem. A, 2014, 2, 6994 CAS.
- H. Ye, X. Zhang, Y. Zhang, L. Ye, B. Xiao, H. Lv and B. Jiang, Sol. Energy Mater. Sol. Cells, 2011, 95, 2347–2351 CrossRef CAS.
- H. K. Raut, A. S. Nair, S. S. Dinachali, V. A. Ganesh, T. M. Walsh and S. Ramakrishna, Sol. Energy Mater. Sol. Cells, 2013, 111, 9–15 CrossRef CAS.
- G. Wu, J. Wang, J. Shen, T. Yang, Q. Zhang, B. Zhou, Z. Deng, B. Fan, D. Zhou and F. Zhang, Mater. Sci. Eng., B, 2000, 78, 135–139 CrossRef.
- D. Zhao, Q. Huo, J. Feng, B. F. Chmelka and G. D. Stucky, J. Am. Chem. Soc., 1998, 120, 6024–6036 CrossRef CAS.
- L. Ye, S. Zhang, Q. Wang, L. Yan, H. Lv and B. Jiang, RSC Adv., 2014, 4, 35818–35822 RSC.
- B. Xia, Q. Zhang, S. Yao, Y. Zhang, B. Xiao and B. Jiang, J. Sol-Gel Sci. Technol., 2014, 71, 291–296 CrossRef CAS.
- Y. Li, H. Lv, L. Ye, L. Yan, Y. Zhang, B. Xia, H. Yan and B. Jiang, RSC Adv., 2015, 5, 20365–20370 RSC.
- M. Manca, A. Cannavale, L. D. Marco, A. S. Aricò, R. Cingolani and G. Gigli, Langmuir, 2009, 25, 6357–6362 CrossRef CAS PubMed.
- X. X. Zhang, S. Cai, D. You, L. H. Yan, H. B. Lv, X. D. Yuan and B. Jiang, Adv. Funct. Mater., 2013, 23, 4361–4365 CrossRef CAS.
- A. Vincent, S. Babu, E. Brinley, A. Karakoti, S. Deshpande and S. Seal, J. Phys. Chem. C, 2007, 111, 8291–8298 CAS.
- S. Kim, J. Cho and K. Char, Langmuir, 2007, 23, 6737–6743 CrossRef CAS PubMed.
- P. F. Belleville and H. G. Floch, Proc. SPIE, 1994, 2288, 25–32 CrossRef CAS.
- I. S. Bayer, Coatings, 2017, 7, 12 CrossRef.
- D. Glöβ, P. Frach, C. Gottfried, S. Klinkenberg, J.-S. Liebig, W. Hentsch, H. Liepack and M. Krug, Thin Solid Films, 2008, 516, 4487–4489 CrossRef.
| This journal is © The Royal Society of Chemistry 2017 |